
Case Report
J Blood Disord. 2014;1(3): 4.
Burkitt’s Lymphoma of the Stomach: A Case Report and Review of the Literature
Neil Watson1,2, Vincenzo Cassibba1, Marco Casini1, Andrea Mega3, Martina Tauber4, Andrea Iori3, Raimondo Di Bella1 and Andrea Piccin1*
1Haematology Department, San Maurizio Regional Hospital, Italy
2University of Edinburgh, United Kingdom
3Gastroenterology Department, San Maurizio Regional Hospital, Italy
4Pathology Department, San Maurizio Regional Hospital, Italy
*Corresponding author: Andrea Piccin, Haematology Department and Bone Marrow Transplant Unit, San Maurizio Regional Hospital, Bolzano/Bozen, South Tyrol, Italy.
Received: September 18, 2014; Accepted: October 02, 2014; Published: November 07, 2014
Abstract
Sporadic Burkitt’s Lymphoma (BL) is a subtype of Non-Hodgkins Lymphoma (NHL). In contrast to other types of NHL, sporadic Burkitt’s lymphoma usually affects the ileocaecal area of small bowel. We report a case of sporadic BL affecting the stomach, which represents an under-reported manifestation of the disease. This is followed by a review of the literature on gastric Burkitt’s lymphoma.
Keywords: Burkitt lymphoma; Gastric présentation; Helycobacter pylori; Gastric resection
Introduction
Sporadic Burkitt Lymphoma (BL) is an aggressive subtype of B cell Non-Hodgkin Lymphoma (NHL) which is rare in adults. The disease typically manifests in the Gastrointestinal (GI) tract, usually at the ileocaecal area [1]. The pathogenetic hallmark of BL is rearrangement of the c-MYC oncogene, an important regulator of cell proliferation and apoptosis. While the stomach is the commonest extra nodal site of presentation for other types of NHL [2], gastric presentation of sporadic BL is rare. The true prevalence of gastric BL is unclear due to a paucity of reports, and it may be under-recognized by clinicians. Correct diagnosis of BL is crucial, as prompt management is imperative and outcomes differ from other types of NHL. We report a case of sporadic BL presenting with gastric erosion in an adult male and we compare our findings with previous reports.
Case Description
Case #1
A 23 year old man presented in January 2013 to the Accident and Emergency Department of San Maurizio Regional Hospital of Bolzano, Italy, complaining of a three months history of abdominal pains, and increasing asthenia, fever, night sweats and weight loss of 10Kg. Examination revealed diffuse pallor, tachycardia, and marked epigastria pain. There was no lymphadenopathy, hepatosplenomegaly or palpable masses. Baseline full blood count revealed hemoglobin 7.5 g/dl (12-18), white cell count 8.7 x109/L (4.8-10.8), neutrophils 60.2% (40-74), lymphocytes 24.7% (19-48%), monocytes 11.7 (0.2-1), eosinophils 2.5 % (0-0.7), basophils 0.9% (0-0.2%), platelet count 496 x109/L (130-400). Other results included Erythrocyte Sedimentation Rate (ESR) 46mm (<25), prothrombin time 0.95 (<1.2), creatinine 0.58mg/dl (0.7-1.2), LDH 1121 IU (<225), protein 5.1g/dl (6.6-8.3), triglycerides 204 mg/dl (30-150). Serological studies shown: CMV IgG+ IgM-, HSV1/HSV2 IgG+ IgM-, VZV IgG+ IgM-, HAV IgG+ IgM-, HCV Ab-, HIV Ag/Ab-, HBs-Ag -ve, HBsAb+, HbcAb+, HbeAg-, HbeAb+, HBV DNA not elevated, EBV Ig G– IgM-.
An Oesophago-Gastroduodenoscopy (OGD) demonstrated a large gastric ulceration involving two-thirds of the body of the stomach and duodenum (Figure 1 OGD).
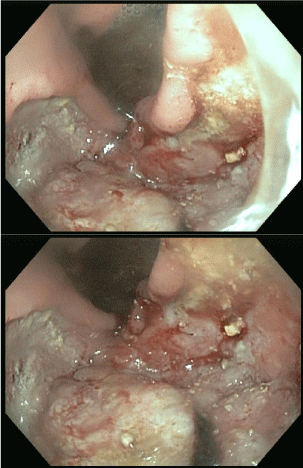
Figure 1: OGD 29/01/2013.
Biopsies of the lesions were performed. On histological sections an extensive atypical lymphoid infiltrate was observed. The tumor shows a diffuse monotonous pattern of growth and invades the glandular tissue in the lamina propria and deeply the sub mucosa. The cells were medium-sized cells with rounded nuclei and scanty cytoplasm. Multiple apoptotic bodies were observed (Figure 2 ABC Histology).
A: Gastric musocsa with diffuse infiltration by medium-sized neoplastic lymphoid cells, HE, 20x.
B: gastric mucosa with findings as in picture A shown enhanced, HE, 60x.
C: bone marrow biopsy with extensive infiltration by the tumour cells with starry sky pattern, HE, 20x.
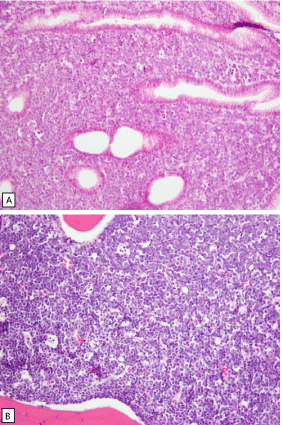
Figure 2: Histology.
A: Gastric musocsa with diffuse infiltration by medium-sized neoplastic
lymphoid cells, HE, 20x.
B: gastric mucosa with findings as in picture A shown enhanced, HE, 60x.
C: bone marrow biopsy with extensive infiltration by the tumour cells with starry sky pattern, HE, 20x.
Immunohistochemically the tumor cells were positive for CD20, CD 10, Bcl-6, MUM-1 and variable weakly for Bcl-2. The proliferation index (MIB/Ki67) was close to 100%. Staining for CD3, cytokeratin PAN (epithelial), CD56, synaptophysin, CD34, TDT, EBV and BCL1 were all negative. No EBV or EBV-encoded RNA (EBER) was detected. The FISH analsysis revealed a translocation of the MYC locus (8q24) using break apart (Dakocytomation). Helicobacter pylori were not detected in the biopsies. The bone marrow biopsy demonstrated an atypical infiltration with medium-sized cells and “starry sky” appearance in about 65% of the cellularity. A final histological diagnosis of Burkitt lymphoma with infiltration of the bone marrow was made.
The proliferative index (MIB1/Ki67) was 95% (Figure 3 ABCD immuneshistochemistry). Staining for CD3, cytokeratin PAN (epithelial), CD56, synaptophysin, CD34, TDT and BCL1 were all negative. Fluorescent In-Situ Hybridization (FISH) revealed a translocation of the MYC locus (8q24). A CT-scan (31.01.13) of thorax was unremarkable, while CT-scan of abdomen showed thickening of gastric walls with ulcers on the greater curve and liver lesions suggestive for neoplastic infiltrations on the IVa and the VII liver segments. An Ann Arbour stage IVE+B was therefore given. The patient was transferred to the haematology ward and started on the BFM German protocol GMALL-B-ALL/NHL 2002. In February 2013 he received the first a course of chemotherapy (prephase: cyclophosphamide 200mg/m2 i.v. days 1-5; prednisolone 60mg/m2 PO on day 1-5), followed by a II° course of chemotherapy (cycle A1: Rituximab© 375mg/m2 i.v. day 7; dexamethasone 10mg/mg/m2 PO days 8-12; vincristine 2mg iv day 8; ifosfamide 800mg/m2 iv days 8-12; methotrexate 1500mg/m2 iv day 8; etoposide 100mg/m2 i.v. day 11-12; cytarabine 2x150 mg/m2 iv day 11-12; cytarabin 40mg and methotrexate 15mg intrathecal day 8 and 12), which was well tolerated.
A: gastric biopsy with medium-sized tumour cells, HE, 60x.
B: tumour cells are postitive for CD20, 40X.
C: tumour cells are positive for CD10, 40X.
D: nearly 100% of tumour cells are Ki67 positive, 20x.
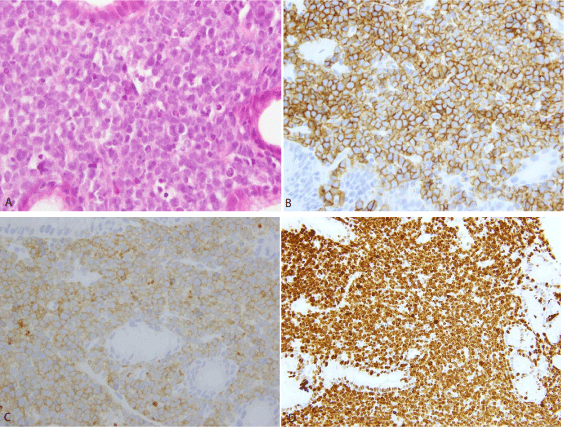
Figure 3: Immunohistochemistry, Immunohistochemical features.
A: gastric biopsy with medium-sized tumour cells, HE, 60x.
B: tumour cells are postitive for CD20, 40X.
C: tumour cells are positive for CD10, 40X.
D: nearly 100% of tumour cells are Ki67 positive, 20x.
In March2013 he received the III° course of chemotherapy (cycle B1 BFM: Rituximab© 375mg/m2 i.v. day 28; dexamethasone 10mg/m2 PO day 29; vincristine 2mg i.v. day 29; cyclophosphamide 200mg/m2 i.v. day 29 -33; methotrexate 1500mg/m2 iv day 29; adriamycin 25mg/ m2 i.v. day 32-33; cytarabin 40mg and methotrexate 15mg intrathecal 29,33). Prophylactic lamivudine was also administered because positive hepatitis B serology (see above).
On day +22 (30.4.13) of cycle III, the patient developed hematemesis leading to hypovolaemic shock. He was transfused with 2 units of RBC, 1 unit of PLT, and 600 mls of fresh frozen plasma to correct a prolonged PT (1.33). A new OGD demonstrated a 30mm gastric oozing ulcer at the lesser curvature of the stomach (Figure 4). However, the extent of the lesion had largely improved on endoscopy according to the gastro-enterologist. Haemostasis was achieved and the patient returned to the ward.
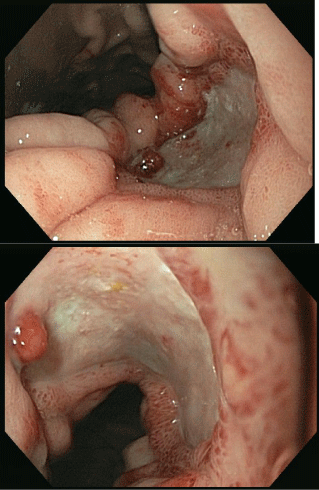
Figure 4: OGD 30/4/2013. Ongoing GI bleed.
At the moment the patient is undergoing an IV° cycle of chemotherapy with peripheral blood stem-cell collection in case an autologous transplantation becomes necessary. The possibility of performing an allogeneic transplantation is being evaluated. Gastrectomy was not considered because the bleeding settled after the second OGD.
Discussion
Burkitt´s lymphoma is an aggressive form of B-cell NHL. The US incidence is 0.3 per 100,000 [3]. While BL is the commonest NHL in childhood, it accounts for >5% of adult lymphomas, and is thought to be under-researched in adults [4]. The sporadic (non-endemic) form encountered outside of Africa usually presents with an abdominal mass, most commonly localized at the ileocaecal region; it is rare for BL to manifest primarily in the stomach or duodenum [1,5]. The prevalence of this is unknown due to a paucity of published literature, consisting mostly of case reports and small case series (summarized in Table 1). Reviews are outdated by two decades. Cases display much heterogeneity in disease presentation, gastric site, patient age, and associated features such as infection. Gastric BL is prone to developing complications such as perforation and haematemesis, with some patients requiring surgery [6-8].
The aetiology of sporadic BL is unclear. A number of reports have demonstrated BL arising in association with H. Pylori infection [7-10]. Notably, one group demonstrated concomitant remission of gastric BL with HP eradication alone (no chemotherapy was initiated) [9]. In our case no HP was detected. HP has been established as a causative agent in MALT lymphoma where its eradication leads to lymphoma regression [11-13], and has also been associated with DLBCL, both through evolution from MALT, and spontaneous DLBCL; here eradication also promotes healing [14]. The low incidence of gastric BL means that insufficient data exists to establish any causative association. Such a link might lead to improved management of BL in a similar manner as in MALT and DLBCL.
Our patient´s serology showed prior resolved acute HBV infection. This is significant as HBV has been implicated in studies as an oncogenic agent in various subtypes of NHL [15-17] and cases have reported BL associated with HBV infection [18,19]. Causative links have not been firmly established. It has been shown that HBV can persist in tissues, undetectable by serology, and therefore the presence of ongoing HBV infection in NHL cases may be underrecognized [15,20]. Further exploration of the role of the virus in NHL development is necessary to establish a causative role. EBV is the causative agent in endemic BL, and is present in a minority of sporadic BL cases. Our patient´s pathology samples were negative for EBV suggesting this was not a causative agent.
Chemotherapy is highly effective in BL and is the mainstay of management. The role of surgery is restricted to managing complications. Three of the cases of gastric BL previously reported required surgical resection of the stomach, and other published cases required management of GI perforation [21,22]. Our case presents a dilemma, as radical surgery on a BL patient has risks due to the chemotherapy-induced neutropaenia, and the damaged oozing mucosa. Furthermore, gastrectomy carries several complications and impairs the quality of life of a young patient, so where possible we believe that a conservative approach restricted to chemotherapy should be followed. Published studies on this condition are lacking, therefore more reports should be encouraged to identify patients’ characteristics and dictate the best management. Serological screening including HP should be carried out and, if positive, eradication therapy should be prescribed.
Conclusion
BL affecting the stomach remains rare and likely under-reported. No up to date review articles exist in the literature and while sporadic reports show associations with EBV, HP and HBV, no causal relationship is established (Table 1). Such a link would improve understanding of pathogenesis, and potentially offer additional treatment options as has been shown for DLBCL and MALT tumours. Finally, there is no clear guidance on when to perform surgery for gastro duodenal BL. Published cases vary; generally operating in emergency circumstances (for instance, several had perforations). We believe that this is an appropriate role, as data are lacking on curative resections. Further case reports are warranted to improve our understanding and management of this disease.
Study
Age
Gender
Site
Appearance
Notable features
Surgery
Outcome
Angotti et al 2012 [23]
4
M
Stomach anterior and posterior walls
Ulcerated masses
No
Undisclosed
Ziade et al 2012 [24]
15
F
Stomach, duodenum, jejunum
Raised, ulcerated tumours
EBV +ve; Krukenberg tumour
No
Remission
Colovic et al 2011 [6]
30
M
Antrum
Ulcero-infiltrative lesion
Subtotal distal gastrectomy
Remission
Ergun Met et al 2011 [25]
42
F
Mid portion of ant wall
Large Ulcer
Subsequent perforation
Closure
Remission
Kesik et al 2010 [7]
7
M
Antrum
Ulcerated masses
HP positive.
EBV/HBV -vePerforation
Semi-total gastrectomy for perforation; oesophago-jejunostomy
Remission
Baumgartner et al 2009 [9]
30
F
Lesser curvature of antrum
Ulcer 5x5cm
Remission with HP eradication
No
Remission
Chogle et al 2009 [26]
11
M
Body of somach
Deep ulcers
HIV positive
No
Death
Chieng et al 2009 [27]
9
M
Greater curve of gastric body, duodenum
Multiple raised ulcerated tumours
Presented as protein losing enteropathy
No
Remission
Grewal et al 2007 [28]
12
M
Proximal lesser curvature
Ulceration, thickened walls
HP infection
No
Sharma et al 2001 [29]
35
M
Fundus, body and pylorus
Thick folds, lack of distensibility
Nil
No
Death
Shannon et al 2000 [30]
53
M
anterior wall of duodenum
Ulcer, perforation
HP +ve, perforation
Partial gastrectomy
Table 1: Previous case reports on Gastric Burkitt’s Lymphoma.
Acknowledgement
We acknowledge the contribution of Dr Gamper Anna and Dr Pagani Elisabetta, from the Microbiology Dept of San Maurizio Regional Hospital for performing the microbiological testing. We are grateful to the society “The Butterfly” from South Tyrol Region, Italy, for their kind financial support. Specifically we would like to thank Ms Wirth Birgit and Ms Ulrike Negri. Also we are deeply grateful to the -mayor Gertrud Benin Bernard and all the people from the city of Caldaro/Kaltern for supporting our research. Similarly we would like to thank the Associazione CHERNOBYL ALTO ADIGE/ SUDTIROL, their President Sara Endrizzi and the always great supporter Max Goller.
Authors Contribution
NW designed the study, reviewed the literature and wrote the manuscript; AP, VC, MC and SC were involved in patient management and reviewed the manuscript. AM performed OGD studies, GN performed histological studies.
References
- Ferry JA. Burkitt's lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 2006; 11: 375-383.
- Park YH, Kim WS, Kang HJ, Na II, Ryoo BY, Yang SH, et al. Gastric Burkitt lymphoma is a distinct subtype that has superior outcomes to other types of Burkitt lymphoma/leukemia. Ann Hematol. 2006; 85: 285-290.
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006; 107: 265-276.
- Divine M, Casassus P, Koscielny S, Bosq J, Sebban C, Le Maignan C, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005; 16: 1928-1935.
- Brooks JJ, Enterline HT. Primary gastric lymphomas. A clinicopathologic study of 58 cases with long-term follow-up and literature review. Cancer. 1983; 51: 701-711.
- Colovic N, Radovanović N, Vidović A, Colović M. [Primary Burkitt's lymphoma of the stomach]. Srp Arh Celok Lek. 2011; 139: 523-526.
- Kesik V, Safali M, Citak EC, Kismet E, Koseoglu V. Primary gastric Burkitt lymphoma: a rare cause of intraabdominal mass in childhood. Pediatr Surg Int. 2010; 26: 927-929.
- Shannon C, Vickers C, Field A, Ward R. Burkitt's lymphoma associated with Helicobacter pylori. J Gastroenterol Hepatol. 2000; 15: 99-103.
- Baumgaertner I, Copie-Bergman C, Levy M, Haioun C, Charachon A, Baia M, et al. Complete remission of gastric Burkitt's lymphoma after eradication of Helicobacter pylori. World J Gastroenterol. 2009; 15: 5746-5750.
- Grewal SS, Hunt JP, O'Connor SC, Gianturco LE, Richardson MW, Lehmann LE. Helicobacter pylori associated gastric Burkitt lymphoma. Pediatr Blood Cancer. 2008; 50: 888-890.
- Witkowska M, Smolewski P. Helicobacter pylori infection, chronic inflammation, and genomic transformations in gastric MALT lymphoma. Mediators Inflamm. 2013; 523170.
- Owens SR, Smith LB. Molecular Aspects of H. pylori-Related MALT Lymphoma. Patholog Res Int. 2011; 193149.
- Ferreri AJ, Govi S, Raderer M, Mule A, Andriani A, Caracciolo D, et al. Helicobacter pylori eradication as exclusive treatment for limited-stage gastric diffuse large B-cell lymphoma: results of a multicenter phase 2 trial. Blood. 2012; 120: 3858-3860.
- Kuo SH, Yeh KH, Wu MS, Lin CW, Hsu PN, Wang HP, et al. Helicobacter pylori eradication therapy is effective in the treatment of early-stage H pylori-positive gastric diffuse large B-cell lymphomas. Blood. 2012; 119: 4838-4844.
- Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011; 117: 1792-1798.
- Kim YM, Jeong SH, Kim JW, Lee SH, Hwang JH, Park YS, et al. Chronic hepatitis B, non-Hodgkin's lymphoma, and effect of prophylactic antiviral therapy. J Clin Virol. 2011; 51: 241-245.
- Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. 2010; 11: 827-834.
- Lin H, Sun XF, Zhen ZJ, Xia Y, Xiang XJ, Ling JY, et al. [Clinical analysis of 69 cases of Burkitt's lymphoma]. Ai Zheng. 2008; 27: 425-428.
- Huang CB, Eng HL, Chuang JH, Cheng YF, Chen WJ. Primary Burkitt's lymphoma of the liver: report of a case with long-term survival after surgical resection and combination chemotherapy. J Pediatr Hematol Oncol. 1997; 19: 135-138.
- Yuki N, Nagaoka T, Yamashiro M, Mochizuki K, Kaneko A, Yamamoto K, et al. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology. 2003; 37: 1172-1179.
- Finch DA, Wilson MS, O'Dwyer ST. Successful Management of Jejunal Perforation in Burkitt's Lymphoma: A Case Report. Case Rep Surg. 2012; 2012: 230538.
- Goldberg SR, Godder K, Lanning DA. Successful treatment of a bowel perforation after chemotherapy for Burkitt lymphoma. J Pediatr Surg. 2007; 42: 1-3.
- Angotti R, Marini M, Giannotti G, Burgio A, Meucci D, Pavone M, et al. Gastric Burkitt's lymphoma in a child: A rare case. Oncol Lett. 2012; 4: 802-804.
- Ziade F, von der Weid N, Beck-Popovic M, Nydegger A. Burkitts's lymphoma--an atypical presentation. BMC Pediatr. 2012; 12: 113.
- Ergun M, Cindoruk M, Akyurek N, Akyol G, Unal S. A case of primary gastric Burkitt-like lymphoma with chemotherapy-induced perforation. Turk J Gastroenterol. 2012; 23: 299-300.
- Chogle A, Nguyen K, Lazare F, Guzman M, Anderson V, Treem WR. Gastric Burkitt lymphoma: A rare cause of upper gastrointestinal bleeding in a child with HIV/AIDS. J Pediatr Gastroenterol Nutr. 2009; 48: 237-239.
- Chieng JH, Garrett J, Ding SL, Sullivan M. Clinical presentation and endoscopic features of primary gastric Burkitt lymphoma in childhood, presenting as a protein-losing enteropathy: a case report. J Med Case Rep. 2009; 3: 7256.
- Grewal SS, Hunt JP, O'Connor SC, Gianturco LE, Richardson MW, Lehmann LE. Helicobacter pylori associated gastric Burkitt lymphoma. Pediatr Blood Cancer. 2008; 50: 888-890.
- Sharma A, Raina V, Gujral S, Kumar R, Tandon R, Jain P. Burkitt's lymphoma of stomach: A case report and review of literature. Am J Hematol. 2001; 67: 48-50.
- Shannon C, Vickers C, Field A, Ward R. Burkitt's lymphoma associated with Helicobacter pylori. J Gastroenterol Hepatol. 2000; 15: 99-103.