
References
J Blood Disord. 2014;1(4): 1018.
Arsenic Trioxide for Non Acute Promyelocytic Leukemia Hematological Malignancies: A New Frontier
LiLi Zhou1, JianHou1, Godfrey Chi-Fung Chan2 and Daniel Man-Yuen Sze3*
1Department of Hematology, Shanghai Changzheng Hospital, Affiliated Hospital to the Second Military Medical University, China
2Department of Pediatrics & Adolescent Medicine, Queen Mary Hospital, The University of Hong Kong, HKSAR, China
3School of Medical Sciences and Health Innovations Research Institute (HiRi), RMIT University, Australia
*Corresponding author: Daniel Man-Yuen Sze, School of Medical Sciences, RMIT University, PO Box 71, Bundoora, Vic 3083 Australia.
Received: November 14, 2014; Accepted: December 25, 2014; Published: December 31, 2014
Abstract
Arsenic trioxide (As2O3) has been confirmed to be effective in the treatment of Acute Promyelocytic Leukemia (APL). Also, encouraging results have been reported in preclinical studies and pilot clinical trials of As2O3 in other hematological malignancies such as Multiple Myeloma (MM), Myeloplastic syndromes (MDS), T-cell leukemia-lymphoma and Chronic Myelogenous Leukemia (CML). However, conflicting findings have been reported in non-APL Acute Myeloid Leukemia (AML). The mechanisms of As2O3 activity are complex, with multiple modifications of cell growth and apoptosis control regulations of pro-survival and cell defense molecules, cell cycle arrest, glutathione redox system, p53-dependent apoptotic signals; telomerase activity and caspase pathway. It is now known that other mechanisms are also involved including the immunomodulatory and angiogenesis regulation. Recently there is a trend of investigating whether tetra-arsenic tetra-sulphide will be a better alternative for the arsenic trioxide. In summary this review describes emerging information that provides new insights for As2O3 as a broad spectrum chemotherapeutic agent in the treatment of hematological malignancies beyond APL.
Keywords: Arsenic trioxide; Leukemia; Mechanisms; Treatment
Introduction
Arsenic trioxide (As2O3) is a traditional folk medicine that has been used for over two thousand years in China [1]. During the 1970s, researchers working in China proposed As2O3 as part of the treatment for acute promyelocytic leukemia (APL) [2,3]. It has since been demonstrated that As2O3 can achieve complete remission rates that range from 70% to 90% in both newly diagnosed and relapsed APL patients [3-5]. Hence in the last decade, As2O3 has been widely accepted as an effective agent in both newly diagnosed and relapsed patients with cytogenetically confirmed APL. This effectiveness has been confirmed with a high Complete Remission rate (CR) and a low adverse effect rate with minimal myelosuppression [5].
The remarkable success of As2O3 in APL provides an impetus for the exploration of a possible role of As2O3 as an anti-cancer agent in other hematological cancers. Clinical studies of As2O3, singularly or in combination with other anti-tumor agents, revealed the potential wide applicability of this agent in patients with hematological malignancies beyond APL. These studies are supported by preclinical laboratory investigations indicating that As2O3 exhibits anti-tumor effects against cell lines derived from Multiple Myeloma (MM), Myelodysplastic Syndrome (MDS), non-APL Acute Myeloid Leukemia (AML), and lymphoma.
The first section of this review will discuss the various As2O3 anticancer mechanisms which are in common for many hematological malignancies. This will be followed by the second section of the tabulation of related clinical trials.
Mechanisms of action
The array of mechanisms that As2O3 achieves anti-cancer actions comprises induction of apoptosis; anti-proliferation; anti-angiogenic activity and immunomodulation as highlighted in Figure 1. The understanding of these As2O3 - related mechanisms is developed through many preclinical studies that provide valuable insights on the molecular and genetic processes involved in these pathways.
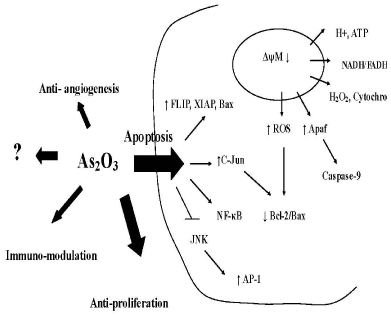
Figure 1: Arsenic targets at different levels of cellular control including: 1) induction of apoptosis via both intrinsic and extrinsic pathway; 2) antiproliferation by inducing G1/S & G2/M phase arrest; 3) anti-angiogenesis by suppressing VEGF release; and 4) immunomodulation in a dose dependent manner.
Induction of apoptosis: As2O3 displays direct cytotoxic effects via caspase-9 and caspase-3 activation in MM cell lines U266 and MM1.S [6]; and via caspase 8 activation in leukemic cell lines [7]. This As2O3 -induced cytotoxcity has been shown to be related to both caspase-dependent and caspase-independent death signals in various myeloma cell lines [8]. Other than the caspase cascade system, p53 has also been shown to be involved. For myeloma cells with mutated p53, As2O3 induced rapid and extensive (more than 90%) apoptosis in a time- and dose-dependent manner concomitant with arrest of cells in G2/M phase of the cell cycle [9]. This is in contrast to the wildtype p53 cells which are relatively resistant to As2O3 with maximal apoptosis of only about 40% concomitant with partial arrest of cells in G1 and up-regulation of p21 [9].
There are lines of evidence from various model systems delineating the effects of arsenic altering the function of several enzymes and signaling molecules, thus markedly influencing gene expression of apoptosis and proliferation. For instance, the transcription factor AP-1 is activated simultaneously with JNKs, which in turn phosphorylates many transcription factors that enhance expression of immediate early genes. As2O3 stimulates JNK activity by inhibiting constitutive JNK phosphates, of which the role in un-stimulated cells is to maintain low basal JNK activity.
Sustained activation of JNK played a critical role in As2O3- induced MM cell lines apoptosis [10]. As2O3 up-regulated Bax / Bim and 3 other pro-apoptotic BH3-only proteins (Noxa, Bmf, and Puma) and down-regulated 2 anti-apoptotic proteins Mcl-1 and Bcl-X (L) leading to apoptosis. Silencing of Bmf, Noxa, and Bim but not Puma significantly protected cells from As2O3-induced apoptosis. This data suggested that Noxa, Bmf, and Bim are necessary for As2O3-induced cell death in myeloma [11].
Other than myeloma, As2O3 has been reported to induce apoptosis in marrow cells from patients with Myelodysplastic Syndrome (MDS). It has been shown that As2O3 interferes with NF-κB; the protein that correlates with disease stage and flow scores [12]. The transcription of NF-κB-dependent anti-apoptotic proteins, such as FLIP Long, Bcl-xl, Bcl-2, and XIAP, can lead to the down-regulation of NF-κBdependent anti-apoptotic genes. Constitutive NF-κB activity and deregulated FLIP levels may confer resistance to As2O3 in advancedstage MDS. So in patients with more advanced MDS and high levels of FLIP, As2O3 was recommended to be used in combination with other agents capable of blocking FLIP amplification [13,14].
Furthermore, As2O3-induced apoptosis appears to be associated with the mitochondrial or intrinsic apoptotic pathway. It has been shown As2O3 relates to reduced mitochondrial transmembrane potential, enhanced generation of intracellular Reactive Oxygen Species (ROS), release of cytochrome c and Apoptosis-Inducing Factor (AIF) from mitochondria into cytoplasm. This in turn relates to the activation of caspases, and up-regulation of Bax and Bim expression. Exogenous glutathione (GSH) in a dose-dependent manner can reverse the As2O3-induced apoptosis, by enhancing oxidative stress [15,16].
As2O3 was also found to be active against acute megakaryocytic leukemia (AMKL, FAB-M7) Lam et al. [17] demonstrated that As2O3 is a potent agent against AMKL by activating the intrinsic (mitochondrial) pathway of apoptosis, which involved disrupting mitochondrial membrane potential, increased Bax/Bcl-2 ratio and caspase-9 activation, as well as the extrinsic apoptotic pathway mediated by Fas and caspase-8 activation. The multiple-signaling mechanism of As2O3 warrants it as a potential agent for the treatment of AMKL.
It has been reported that As2O3 can lead to the targeted degradation of AML1/MDS1/EVI1 oncoprotein [18]. It also can effectively overcome some known drug resistances in-vitro by showing significant cytotoxic effects on Daunorubicin (DNR)-resistant cell line K562/D1-9 which over expresses P-glycoprotein (Pgp); DNR and 1-beta-D-arabinofuranosylcytosine (Ara-C) double-resistant cell line HL60/AD which over expresses Multidrug Resistance-associated Protein (MRP1); and pre-B lineage leukemia cell line 697/Bcl-2 which was Bcl-2-transfected [19]. But higher As2O3 concentrations and combination treatments are required to reverse the resistance and suppress leukemia cells proliferation [19,20].
Anti-proliferative activity: As2O3 induces growth inhibition in a number of malignant hematological cell lines [21,22]. These effects appear to act through the cyclin and cyclin dependent kinases involved in the cell cycle control.
Our group has previously demonstrated that the inhibition of proliferation in myeloma cells by As2O3 is associated with demethylation of p15 and p16 and induction of the p21 cyclindependent kinase inhibitor protein [22]. As2O3 induced most of HSSultan cells, arrest at G0/G1 phase with a small fraction at G2/M phase and apoptosis occurred mainly in S phase. There was no expression of p15 and p16 mRNA in untreated HS-Sultan cells and 1.0 μM/L As2O3 could make them expressed after being exposed for 24 or 48 hours respectively. Expression of p12 mRNA was obviously elevated by As2O3 comparing with that of the control. The pharmacological mechanisms of As2O3 are partly due to the expression of CDKI p15, p16 and p21, and consequently affect cell proliferation cycle. In addition, G2/M phase arrest with disruption of the microtubule polymerization process is another possible mechanism in inhibiting cell proliferation by As2O3 [23].
Anti-angiogenic activity: As2O3 has also been shown to inhibit angiogenesis. Roboz et al [24] treated Human Umbilical Vein Endothelial Cells (HUVECs) with a variety of concentrations of As2O3 and showed that in a reproducible dose- and time-dependent sequence of events marked by change to an activated morphology, up-regulation of endothelial cell adhesion markers, and apoptosis of the HUVECs. In addition, treatment with As2O3 also caused inhibition of VEGF production in the leukemic cell line HEL, but not HL60 cell line which has previously been shown to be refractory to the effects of As2O3 [25]. Thus, the researchers postulated that the anti-angiogenesis effect of hematological malignancies may be due to As2O3interrupting the reciprocal stimulatory loop between the leukemic cells and endothelium through inducing apoptosis of the endothelium and inhibiting VEGF production by leukemic cells. Using the Matrigel-induced capillary tube formation assay, the same research group continued to show that incubation of HUVECs with As2O3 prevented capillary tubule and branch formation in an in vitro endothelial cell-differentiation assay. This As2O3-induced down regulation of VEGF production has been shown in CML [26] and myeloma [6].
It is important to note that the ability of As2O3 to induce apoptosis or cause proliferation of the endothelium depends on the concentration of arsenite used. Barchowsky et al. [27] showed that incubation of second passage vascular endothelial cells with less than 5μ Marsenite for 4 hours increased incorporation of [3H] thymidine into genomic DNA, while higher concentrations failed to stimulate or inhibit DNA synthesis. The researchers showed that within this 1 hour following addition of non-cytotoxic concentrations of arsenite, oxidants accumulated and thiol status increased; together with increased nuclear retention of NF-κB binding proteins and nuclear translocation of NF-κB. The same group has further shown that this arsenite-induced proliferation of primary porcine aortic endothelial cells MAPK-independent pathway for phenotypic change and proliferation in vascular cells [28].
Immuno-modulation: It is known that chronic arsenic poisoning affects the human immune function [29] with a decreased resistance against viral and bacterial infection associated with a decline in IL-1β and TNF-α release [30]. It also induced apoptosis on macrophages [31] and CD3+, CD56+ but not CD14+ cells [32]. This section aims to summarize the preclinical immunologic mechanisms of the effects of As2O3 in hematological malignancies other than APL.
As2O3 induced a marked increase in lymphocyte-activated killers mediated killing, which is possibly through the up-regulation of the CD38/CD31 and CD11a/CD54 receptor-ligand systems that increase recognition, adhesion, and lysis of targeted myeloma cells [33]. This data supported the notion that As2O3 has a role in the management of relapsed/refractory myeloma via the immunomodulatory mechanisms.
Natural Killer (NK) cells are important effect or immunological cells against tumor mediating through specific activating and inhibitory receptor-ligand pairs. It is now known that the induction of NKG2D ligands on tumor cells by various stresses will render them more sensitive to NK cell-mediated killing. As2O3 has been shown to up regulate NKG2D ligands on CML, APL and breast cancer cells and increase their susceptibility to NK cells induced lysis [34]. This increase in cytotoxicity was abolished by the addition of a blocking NKG2D monoclonal antibody, indicating that the action is mediated through up-regulation of NKG2D ligands. This study further supports that the immunomodulatory feature of As2O3.
Clinical trials on the use of arsenic trioxide in non-apl hematological malignancies
Multiple myeloma (MM): MM is a plasma cell neoplasm characterized with skeletal destruction, renal failure, anemia, and malignant proliferation of bone marrow plasma cells. Recent advances in the use of As2O3 as a single agent or in combination with other agents such as Ascorbic Acid (AA), melphalan, Bortezomib, or conventional regimens, are promising in patients with relapsed/ refractory MM [6-9]. A significant number of preclinical experiments and clinical trials have been initiated based on various mechanisms (Table 1). We will first describe the clinical trials that use As2O3 as a single agent; then followed by a range of combinational therapeutics together with As2O3.
Reference
Study Design & Regimen
Patientsí
Characteristics
Outcome 1: Complete/ Partial Objective Response(%)
Outcome 2:†† Minor Objective Response(%)
Outcome 2:
Stablization (%)
Single agent
Rousselot et al [36]
median duration 38 days (9-54); 0.15 mg/kg/day.
Relapsed or refractory (N=10)
0 (0%)
3 (30%)
4 (40%)
Hussein et al [77]
0.25 mg/kg/day for 2week on/ 2week off at least 1 4-wk cycle
Relapsed or refractory (N=24)
0 (0%)
8 (33%)
6 (25%)
Berenson et al [71]
twice-weekly, 0.25-
0.35 mg/kg
Relapsed or refractory (N=11)
0 (0%)
3 (27%)
Munshi et al [72]
APL induction regimen of 0.15 mg/kg for 60 days
Relapsed or refractory (N=14)
0 (0%)
1 (7.1%)
2 (14.2%)
Combination regimens
Berenson et al [35]
As2O3+AA+MEL
Relapsed or refractory (N=65)
2 CR+15 PR (26%)
14 (22%)
Bahlis et al [37]
As2O3+AA
Relapsed (N=6)
1PR (17%)
1 (17%)
4 (67%)
Abou-Jawde et al [39]
As2O3+AA+DEX
relapsed or refractory (N=20)
2 NCR + 4 PR (30%)
10 (50%)
Hofmeister et al [43]
As2O3 +DVD
relapsed or refractory (N=11)
4 PR (36%)
6 (55%)
Berenson et al [44]
As2O3+ bortezomib +AA
22
2 PR (9%)
4 (18%)
9 (41%)
PL: acute Promyelocytic Leukemia; As2O3: Arsenic trioxide; AA: Ascorbic Acid; MEL: Melphalan; MDS: Myelodysplastic syndrome; Dex: Dexamethasone; DVD: liposomal doxorubicin, vincristine and dexamethasone. The inclusion and exclusion criteria of the patients in these studies please refer to each paper respectively.
Table 1: Clinical trials on the responses of As2O3 in the treatment of multiple myeloma.
In a multi-center phase II trial [35] eight relapsed and sixteen refractory MM cases (median age 63 yrs) received As2O3 0.25 mg/ kg intravenously, 5 days per week for 2 weeks followed by no therapy for 2 weeks, in repeated 4-week cycles [24]. There are 9 of 21 (43%) evaluable patients had an objective response as measured by a >25% decrease in serum M-protein concentrations including one patient with refractory disease with a 50% decrease in size of the plasmacytoma. Eight patients had stable disease and four had progressive disease at the first evaluation visit. However, for advanced MM patients it was reported that As2O3 as a single agent did not produce any significant response [36].
In comparison to the use of As2O3 as a single agent, there are more clinical trials of combination chemotherapeutics with As2O3 in MM. For instance, in a phase I/II trial, six patients with stage IIIA relapsed/refractory myeloma were treated with As2O3 (0.25 mg/kg/ day) + AA (1,000 mg/day) for 25 days (over a 35-day period) without dose-limiting toxicity [37]. Two patients (both with thalidomiderefractory disease) had partial responses; four patients had stable disease, suggesting that As2O3 + AA has acceptable toxicity and promising activity in refractory/relapsed myeloma. In a prospective multi-centre and phase II clinical trial [35] of MAC regimen (melphalan 0.1 mg/kg p.o., As2O3 0.25 mg/kg i.v. and AA 1 g i.v), objective responses occurred in 31 of 65 (48%) patients, including two complete, 15 partial and 14 minor responses. Median Progression- Free Survival (PFS) and Overall Survival (OS) were 7 and 19 months respectively, indicating that the steroid-free regimen was effective and well tolerated in heavily pretreated MM.
Based on preclinical results [38], a phase II trial of As2O3 combined with dexamethasone and AA was conducted in 20 relapsed/refractory MM patients [39]. Median PFS was 316 days in all the patients and 584 days in those with a response, similar to the result of Wu [40], showing the clinical efficacy and tolerability of the combination of As2O3, dexamethasone and ascorbic acid.
As2O3 has also been shown to enhance the sensitivity of MM cells to Bortezomib in five myeloma cell lines and primary cells from MM patients [41]. The synergistic activity of As2O3 and Bortezomib, melphalan and Ascorbic Acid (AA) has been shown by in vitro and in vivo using a Severe Combined Immunodeficient (SCID)-hum urine myeloma model [42]. In human trials, ABC regimen (AS2O3/ bortezomib/ascorbic acid) showed 27% objective response rate in heavily pretreated MM cases [43]. However, the addition of As2O3 to liposomal Doxorubicin, Vincristine and Dexamethasone (DVD) did not demonstrate improvement in newly diagnosed patients [44]. As2O3 has shown to inhibit the proliferation of myeloma cells by the down-regulation of Jag2 and Hes1 gene, as well as the up-regulation of tumor suppressor gene PTEN [45].
In summary, As2O3 as a single agent could not produce significant response in advanced MM patients, while the combination of As2O3 with AA, dexamethasone + AA or MAC regimen have shown promising activity with acceptable toxicity in heavily pretreated refractory/relapsed MM. The As2O3 +DVD regimen was not recommended with the availability of more effective front line therapies. Optimal design and combination are still needed to improve the clinical effect of As2O3 in MM.
Myelodysplastic syndrome (MDS): MDS is a heterogeneous bone marrow disorder predominantly affecting older adults, for whom the only curative therapy, BMT, is rarely an option. As2O3 was found to have therapeutic efficacy in patients with MDS (Table 2). The deregulation of apoptosis is one of the central events in the pathophysiology of MDS. As2O3 induces apoptosis through several mechanisms. Preliminary clinical results indicate that As2O3 has activity in both lower- and higher-risk MDS [46-49]. Approximately, a third of patients experienced hematological improvement, with few complete or partial remissions.
Reference
Treatment
Number of Patients
Response in hematolotical improvement (HI)
Transfusion independence
Vey et al [47]
As2O3 0.3 mg/kg/d x 5d,0.25 mg/kg 2/w x 15w
114
13/50 (26%)LR
11/64 (17%)HR
12/75 (16%) became RBC: transfusion-independent; 8/28 (29%) became Platelet transfusion-independent;
List et al [49]
As2O3 0.25 mg/kg/d
2w on, 2w off
46
8/21(38%)LR
4/25(16%)HR
4/35 (11%) became transfusion-independent;† and transfusion requirements in 3 others decreased by
50%
Schiller et al [50]
As2O3 0.25 mg/kg/d
2w on, 2w off
121 (1 cycle: N=70; 2 or more cycles of therapy N=51)
For 1 cycle: Patients with HI (major and minor responses) 11/32 (34%) for LR and 2/36 (6%) for HR
For 2 cycles or more: Patients with HI (major and minor responses) 11/28 (39%) for LR and 2/22 (9%) for HR
33% became RBC: transfusion-independent;
Raza et al [51]
As2O3 + Thalidomide
28
7/28 (25%) of LR+4 HR
6/28 (21%) total transfusion independence and two had 50% reduction in PRBC transfusions.
Zheng et al [52]
As2O3 + AA + Thal
21 (only HR)
10/21 (48%) including 1CR, 1 PR
5/21 (24%) total SRBC transfusion independence
As2O3=Arsenic trioxide; AAL: Ascorbic Acid; MDS: Myelodysplastic Syndrome; LR: Lower-Risk Group; HR: Higher-Risk Group; CR: Complete Remission; PR: Part Remission. The inclusion and exclusion criteria of the patients in these studies please refer to each paper respectively.
Table 2: Clinical responses of either As2O3 alone or As2O3 in combination with other agents for treatment of MDS.
In an updated phase II clinical trial [50], 121 patients received As2O3 (0.25 mg/kg/d) on 5 consecutive days per week for 2 weeks, followed by 2 weeks' rest (one cycle). The overall major hematological remission rates were 20% in Lower-Risk (LR) and 22% in Higher-Risk (HR) patients. One higher-risk patient achieved a complete remission (3%). Transfusion independence or reduction more than 50% occurred in 33% of patients. The overall median duration of hematological remission was 6.8 months (range, 2 to 40 months). Hematological adverse events included neutropenia, thrombocytopenia, and febrile neutropenia. Most common grade 3/4 non-hematological events were pneumonia, fatigue, hemorrhage, pain, and dyspnea and many of which may have no direct relationship with the treatment itself. Thus, it suggests that As2O3 mono-therapy has moderate activity against MDS, with a manageable adverse effect profile. A latest Phase II multi-center study confirmed the similar results [47].
Given the manageable toxicity, combination trials were attempted to further improve the clinical results in comparison to with As2O3 mono-therapy. In a study of As2O3 plus thalidomide [51], there are 7 of 14 available patients (25%) experienced hematological improvement, including one CR, confirming that full dose As2O3 can be safely administered in combination with other agents. Besides the combination of As2O3, retinoic acid and thalidomide therapy were also evaluated in higher risk MDS [52]. Twenty-one patients were administered 10mg/day As2O3 intravenously for 10 days, 40mg/day retinoic acid orally for 2 weeks and 100mg/day thalidomide orally for 4 weeks per cycle. After at least 2 treatment cycles, 10 patients showed hematological responses. One achieved complete response, one achieved partial response, and three patients achieved major hematological improvements. The efficacy rate was 24% (5/21), and the response rate was 48% (10/21). The schedule was well tolerated by all patients and toxicities were moderate and reversible.
Our recent case controlled clinical study of 22 patients with Myelodysplastic Syndromes (MDS) suggested that the combination of thalidomide and arsenic trioxide is effective and well tolerated [53]. Four patients (18.2%) with thalidomide/As2O3 treatment achieved remission, comparing to none in the control group (p<0.05). Fifteen of 22 patients in the treatment group got hematologic improvement (68.2% vs. 27.3% in the control, p<0.05). Both PFS and OS were significantly prolonged (26 vs. 10 months, 36 vs. 16 months, p<0.05), while no severe adverse reactions were observed. These preliminary findings suggest that thalidomide/As2O3 combination treatment could be effective and safe option for MDS.
In summary, As2O3 can achieve hematological improvement in 22% to 26% of patients as a single therapy or in combination with other agents. Most treatment-related adverse events were mild to moderate. Preliminary data suggested that MDS patients whose cells express the EVI1 mutation may benefit more from As2O3 therapy [54]. Further studies of As2O3 on MDS with or without other agents will help to verify its role in this refractory disease. The initial experiences are encouraging, demonstrating that the potential for tri-lineage hematological improvement with mono-therapy of As2O3 may be sustained for prolonged periods even after treatment cessation.
Non-APL acute myeloid leukemia (AML): In a phase II trial of As2O3 treatment for relapsed/refractory/secondary AML [55], eleven patients with a median age of 77 years (56-90yrs) were given a daily dose of 0.25 mg/kg. Median survival following the first dose of As2O3 was 2.25 months (0.4-19mos). All subjects had progressive disease and there was no direct treatment-related mortality. Based on these results, single agent As2O3 is not recommended to be used as a treatment option for non-APLAML.
Yan et al [56] reported that of bortezomib at 32 nM and As2O3 at 1 mM synergistically induced apoptosis in some forms of acute leukemia cell lines and primary cells. These two drugs synergistically induced proteolytic activation of protein kinase C delta (PKCd) with enhanced activation of two mitogen-activated protein kinases phospho-c-Jun NH(2)-terminal kinase and p38. The specific PKC inhibitor rottlerin markedly decreased bortezomib plus As2O3- induced apoptosis, suggesting that PKC plays an important role in bortezomib plus As2O3-induced apoptosis. The combined regimen of bortezomib and As2O3 might be a potential therapeutic remedy for the treatment of leukemia.
Various other strategies [57-60] have been explored to induce synergy apoptosis aimed at enhancing the anti-tumor effect of As2O3 on AML. Examples of which include an MEK1 inhibitor that activates the p73-p53AIP1 apoptotic pathway, and 17-Allylamino- 17-demethoxygeldanamycin (17-AAG)43 which can abrogate the function of heat-shock protein Hsp90 and modulate cellular sensitivity to anticancer agents. This preliminary data provides us with a basis for studying the role of combined use of As2O3with other agents in the treatment of acute myeloid leukemia.
Chronic myeloid leukemia (CML): In the 1930's, As2O3 was reported to be effective in patients with Chronic Myelogenous Leukemia (CML) [1]. Arsenic together with irradiation was the treatment of choice for CML until busulfan was introduced in 1953.
As2O3 reduces bcr-abl levels and induces apoptosis in human myeloid leukemia cells that express bcr-abl. These effects are independent of bcr-abl kinase activity, since they occur when cells are pretreated with Imatinib, a tyrosine kinase inhibitor specific for the bcr-abl tyrosine kinase. Furthermore, As2O3 decreases proliferation of CML blasts but does not affect growth of peripheral CD34 progenitor cells. Concurrent treatment of As2O3 with Imatinib causes a greater apoptotic response compared with using either agent alone [61-63].
Lymphoma/Lymphoblastic leukemia: It was previously reported that As2O3 with or without interferon-α (IFN-α), can lead to induction of apoptosis and growth inhibition on Human T-cell Leukemia Virus type 1 (HTLV-1)-associated adult T-cell leukemia/ lymphoma (T-ALL/NHL) cells [64,65]. For non-HTLV-1 T-ALL, a French phase II trial of As2O3 combined with IFN-α or bortezomib therapy in seven patients with relapsed/refractory T-ALL showed Complete Remission (CR) in 1 patient and Partial Remission (PR) in 3 patients [64]. Though the case number was limited, this study did provide the important information that moderately-aggressive relapsed/refractory T-ALL patients are promising candidates, while those who are heavily-treated or presenting very aggressive T-ALL do not appear to benefit. Incorporation of apoptosis-inducing agents into current therapeutic regimens is an attractive strategy to improve treatment for drug-resistant or aggressive lymphoma/lymphoblastic leukemia. Further studies are needed to determine the efficacy of As2O3 treatment alone or in combination with IFN-αfor the treatment of T-ALL [66].
A phase II trial of As2O3 for the treatment of relapsed and refractory Acute Lymphoblastic Leukemia (ALL) reported the use of high dose As2O3 of 0.25 mg/kg/day intravenously for 5-7 days per week for up to 60 days. Of 11 patients eligible, eight had B-ALL, three T-ALL and two were Philadelphia chromosome-positive [67]. The median duration of therapy was 21 days (range 7-28). There were no responses noted. One patient died from infection and in the remaining patients the disease further progressed leading to death. The median survival was only 3.2 months (range 1.2-4.1). This study indicated that As2O3 is not effective as a single agent in the treatment of ALL, even with high dose. Novel combination strategy with other agents to improve the cytotoxic effect is therefore needed.
The combination of As2O3 and dexamethasone was tested in Glucocorticoid (GC)-resistant Acute Lymphoblastic Leukemia (ALL) [68]. Low-dose As2O3 markedly increased in vitro GC sensitivity of ALL cells derived from T-cell and precursor B-cell ALL patients with poor in vivo response to prednisone. In GC-resistant cell lines, this effect was mediated, at least in part, by inhibition of Akt and affecting downstream Akt targets such as Bad and the X-Linked Inhibitor of Apoptosis Protein (XIAP). Combination of As2O3 and dexamethasone resulted in increased bad and rapid down-regulation of XIAP, while levels of the anti-apoptotic regulator Mcl-1 remained unchanged. Expression of dominant-active Akt, reduction of Bad expression by RNA interference, or over-expression of XIAP abrogated the sensitizing effect of As2O3. The inhibitory effect of XIAP over-expression was reduced when the Akt phosphorylation site was mutated (XIAP-S87A). These data suggested that the combination of As2O3 and glucocorticoids can be advantageous in GC-resistant ALL.
Current information revealed that Primary Effusion Lymphoma (PEL) cells utilize activated NF-kB pathway for their survival [69]. In a subsequent study, the same group indicated that a dramatic inhibition of cell proliferation and induction of apoptosis was observed in cells treated by Velcade (PS-341), a proteosome inhibitor, and As2O3 plus IFN-a treated cells. This was associated with of the mitochondrial membrane potential, cytosolic release of cytochrome c, caspase activation and the effects could be reversed by the pan-caspase inhibitor, z-VAD. Velcade and As2O3 plusIFN-α treatment abrogated NF-kB translocation to the nucleus and decreased the levels of the anti-apoptotic protein Bcl-X (L). Altogether, these results provide a rational basis for a future therapeutic use of Velcade in combination with As2O3 plusIFN-α in a specific group of lymphoma patients.
Childhood non-APL hematological malignancies: Childhood APL with relapse responded equally as effective as the adult with the 0.15mg/m2 daily dose of As2O3 intravenously and the toxicity profile also showed similar pattern. But the data on the use of As2O3 on other non-APL hematological malignancies is very limited. It can be due to small number of patients that can be recruited because of the low incidence of childhood cancer compared to adults and the excellent upfront chemotherapy results for childhood leukemia/lymphoma.
A phase I study by Fox et al [70] applied As2O3 to children (median age 13 yrs, range, 2-19yrs) with refractory leukemia. As2O3 was administered intravenously over 2 hours, 5 d/wk for 20 doses/ cycle. Patients with APL (n=13) received 0.15 mg/kg per day, and patients with other types of leukemia received 0.15 mg/kg per day (n=2) or 0.2 mg/kg per day (n=4). Nineteen of the 24 enrolled patients were fully evaluable for toxicity. Morphologic complete response was achieved in 85% of patients with APL, and disappointingly, no response was observed in all non-APL patients. Arsenic trioxide has also been tested in children with infiltrating astrocytic brain tumors which showed an acceptable safety profile but the efficacy could not be evaluated due to other confounding treatments were added [73].
In our in-vitro study, non-APL leukemic cells often required higher concentration of As2O3 to achieve satisfactory cytotoxic effects. The serum concentration should be in the range of 1 to 2μM/ ml. However, in an pharmacokinetic study done on 13 children with refractory solid tumors by 0.15mg/m2 daily intravenously, most children could only achieved a peak drug concentration of <1μM/ ml (range 0.6 - 1μM/ml) (GCF Chan, unpublished data). That partly explained why the clinical effects were suboptimal in previous trials.
Conclusion and Perspectives
This review describes As2O3 has potential therapeutic benefits in a variety of other hematological malignancies other than the known advantages for patients with APL. As2O3 acts through several signal transduction pathways in a range of target cells involved. The underlying mechanisms include apoptosis induction, antiproliferation, angiogenesis inhibition and immuno-modulation. However, different types of hematological malignancies may have differences in the response pattern to As2O3.
As2O3 generally does not induce myelosuppression which is a good complement to conventional cytotoxic agents. This review describes phase I/II studies using combination of As2O3 with other anti-cancer therapeutic agents delivering promising results for MM, MDS and CML Whether such combination strategy may result in enhanced antitumor activity without significant additive side effects has yet to be confirmed.
For non-APL myeloid leukemia, T-ALL and lymphoma, the results of using As2O3 as a single agent are disappointing. Again, the combination of As2O3 with selected targeted therapeutic agents may enhance the sensitivity of resistant blast cells to As2O3. What accounts for the relative poor cytotoxic response to As2O3 in these cells and how to reverse the underlying resistant mechanisms will help to develop new therapeutic targets.
Since most previous trials are single arm studies with small sample size, well designed randomized prospective clinical trials with good sample size are needed to verify these pilot study results and find out the best combination strategy of As2O3 for different diseases types in the future.
There is an emerging trend of using other arsenicals such as tetraarsenic tetra-sulfide or realgar (As4S4) for the treatment of APL. For instance, a randomized, multicenter, phase III non-inferiority clinical trial further tested the efficacy and safety of an oral As4S4-containing formula named the Realgar-Indigo naturalis formula, comparing with intravenous As2O3 as both induction and maintenance therapies [74]. In this study, 242 newly diagnosed APL patients were randomly assigned (1:1) to oral RIF (60 mg/kg) or As2O3 (0.16 mg/kg) combined with all-trans retinoic acid (ATRA; 25 mg/m2) during induction therapy. After achieving CR, all patients received three courses of consolidation chemotherapy and maintenance treatment with sequential ATRA followed by either RIF or As2O3 for 2years. After median follow-up time of 39 months, DFS at 2 years was 98.1% in the RIF group and 95.5% in the As2O3 group. There were no significant differences in CR rate between the RIF and ATO groups (99.1% v 97.2%; P = 0.62) or the overall survival at 3 years (99.1% v 96.6%; P = 0.18). Also the rates of adverse events were similar. These results indicated that oral RIF plus ATRA is not inferior to intravenous As2O3 plus ATRA as first-line treatment of APL and may be considered as a routine treatment option for appropriate patients [74].
Mechanistic study has shown that As4S induced apoptosis in NB4- R1cells by increasing PP2A and reducing PML-RAR α expressions through the down regulation of SET protein expression [75]. Realgar is also reported to induce apoptosis and differentiation in ATRAsensitive NB4 and ATRA-resistant MR2 cell lines simultaneously. Gene expression profiles indicated that genes influenced by realgar treatment were involved in the modulation of signal transduction, translation, transcription, metabolism and the immune response. Given its low toxicity, realgar is also believed to be a promising alternative reagent for the treatment of APL [76].
Acknowledgement
We like to thank Mr. Joel By field of the University of Southampton United Kingdom for editing an initial version of this manuscript.
References
- Sears DA. History of the treatment of chronic myelocytic leukemia. Am J Med Sci. 1988; 296: 85-86.
- Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996; 88: 1052-1061.
- Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999; 94: 3315-3324.
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998; 339: 1341-1348.
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008; 111: 2505-2515.
- Hayashi T, Hideshima T, Akiyama M, Richardson P, Schlossman RL, Chauhan D, et al. Arsenic trioxide inhibits growth of human multiple myeloma cells in the bone marrow microenvironment. Mol Cancer Ther. 2002; 1: 851-860.
- Lam HK, Li K, Chik KW, Yang M, Liu VC, Li CK, et al. Arsenic trioxide mediates intrinsic and extrinsic pathways of apoptosis and cell cycle arrest in acute megakaryocytic leukemia. Int J Oncol. 2005; 27: 537-545.
- McCafferty-Grad J, Bahlis NJ, Krett N, Aguilar TM, Reis I, Lee KP, et al. Arsenic trioxide uses caspase-dependent and caspase-independent death pathways in myeloma cells. Mol Cancer Ther. 2003; 2: 1155-1164.
- Liu Q, Hilsenbeck S, Gazitt Y. Arsenic trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and synergy with APO2/TRAIL. Blood. 2003; 101: 4078-4087.
- Kajiguchi T, Yamamoto K, Hossain K, Akhand AA, Nakashima I, Naoe T, et al. Sustained activation of c-jun-terminal kinase (JNK) is closely related to arsenic trioxide-induced apoptosis in an acute myeloid leukemia (M2)-derived cell line, NKM-1. Leukemia. 2003; 17: 2189-2195.
- Morales AA, Gutman D, Lee KP, Boise LH. BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood. 2008; 111: 5152-5162.
- Wei LH, Lai KP, Chen CA, Cheng CH, Huang YJ, Chou CH, et al. Arsenic trioxide prevents radiation-enhanced tumor invasiveness and inhibits matrix metalloproteinase-9 through down regulation of nuclear factor kappa B. Oncogene. 2005; 24: 390-398.
- Kerbauy DM, Lesnikov V, Abbasi N, Seal S, Scott B, Deeg HJ. NF-kappaB and FLIP in arsenic trioxide (ATO)-induced apoptosis in myelodysplastic syndromes (MDSs). Blood. 2005; 106: 3917-3925.
- Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol. 2004; 24: 2627-2636.
- Baysan A, Yel L, Gollapudi S, Su H, Gupta S. Arsenic trioxide induces apoptosis via the mitochondrial pathway by upregulating the expression of Bax and Bim in human B cells. Int J Oncol. 2007; 30: 313-318.
- Gupta S, Yel L, Kim D, Kim C, Chiplunkar S, Gollapudi S. Arsenic trioxide induces apoptosis in peripheral blood T lymphocyte subsets by inducing oxidative stress: a role of Bcl-2. Mol Cancer Ther. 2003; 2: 711-719.
- Lam HK, Li K, Chik KW, Yang M, Liu VC, Li CK, et al. Arsenic trioxide mediates intrinsic and extrinsic pathways of apoptosis and cell cycle arrest in acute megakaryocytic leukemia. Int J Oncol. 2005; 27: 537-545.
- Shackelford D, Kenific C, Blusztajn A, Waxman S, Ren R. Targeted degradation of the AML1/MDS1/EVI1 oncoprotein by arsenic trioxide. Cancer Res. 2006; 66: 11360-11369.
- Seo T, Urasaki Y, Takemura H, Ueda T. Arsenic trioxide circumvents multidrug resistance based on different mechanisms in human leukemia cell lines. Anticancer Res. 2005; 25: 991-998.
- List A, Beran M, DiPersio J, Slack J, Vey N, Rosenfeld CS, et al. Opportunities for Trisenox (arsenic trioxide) in the treatment of myelodysplastic syndromes. Leukemia. 2003; 17: 1499-1507.
- Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, et al. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000; 60: 3065-3071.
- Chen YB, Fu WJ, Hou J, Ding SQ, Wang DX, Yuan ZG, et al. [Effects of arsenic trioxide on cell cycle and expression of cyclin dependent kinase inhibitors of multiple myeloma cells]. Zhonghua Xue Ye Xue Za Zhi. 2003; 24: 193-196.
- Ling YH, Jiang JD, Holland JF, Perez-Soler R. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol Pharmacol. 2002; 62: 529-538.
- Roboz GJ, Dias S, Lam G, Lane WJ, Soignet SL, Warrell RP, et al. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000; 96: 1525-1530.
- Bardelli M, Leucci E, Schürfeld K, Bellan C, Passiatore G, Rocchigiani M, et al. VEGF-D is expressed in activated lymphoid cells and in tumors of hematopoietic and lymphoid tissues. Leuk Lymphoma. 2007; 48: 2014-2021.
- Li L, Zhang R, Zhu ZL. [Down-regulation of expression of vascular endothelial growth factor induced by arsenic trioxide in bone marrow cells of chronic myeloid leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003; 11: 263-265.
- Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-kappa B activation in cultured aortic endothelial cells. Free Radic Biol Med. 1996; 21: 783-790.
- Barchowsky A, Roussel RR, Klei LR, James PE, Ganju N, Smith KR, et al. Low levels of arsenic trioxide stimulate proliferative signals in primary vascular cells without activating stress effector pathways. Toxicol Appl Pharmacol. 1999; 159: 65-75.
- Di Gioacchino M, Verna N, Di Giampaolo L, Di Claudio F, Turi MC, Perrone A, et al. Immunotoxicity and sensitizing capacity of metal compounds depend on speciation. Int J Immunopathol Pharmacol. 2007; 20: 15-22.
- Nayak AS, Lage CR, Kim CH. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio). Toxicol Sci. 2007; 98: 118-124.
- Lemarie A, Morzadec C, Bourdonnay E, Fardel O, Vernhet L. Human macrophages constitute targets for immunotoxic inorganic arsenic. J Immunol. 2006; 177: 3019-3027.
- Gonzalez-Rangel Y, Portales-Perez DP, Galicia-Cruz O, Escudero-Lourdes C. Chronic exposure to arsenic sensitizes CD3+ and CD56+ human cells to sodium arsenite-mediated apoptosis. Proc West Pharmacol Soc. 2005; 48: 89-91.
- Deaglio S, Canella D, Baj G, Arnulfo A, Waxman S, Malavasi F. Evidence of an immunologic mechanism behind the therapeutical effects of arsenic trioxide (As(2)O(3)) on myeloma cells. Leuk Res. 2001; 25: 227-235.
- Kim JY, Bae JH, Lee SH, Lee EY, Chung BS, Kim SH, et al. Induction of NKG2D ligands and subsequent enhancement of NK cell-mediated lysis of cancer cells by arsenic trioxide. J Immunother. 2008; 31: 475-486.
- Berenson JR, Boccia R, Siegel D, Bozdech M, Bessudo A, Stadtmauer E, et al. Efficacy and safety of melphalan, arsenic trioxide and ascorbic acid combination therapy in patients with relapsed or refractory multiple myeloma: a prospective, multicentre, phase II, single-arm study. Br J Haematol. 2006; 135: 174-183.
- Rousselot P, Larghero J, Arnulf B, Poupon J, Royer B, Tibi A, et al. A clinical and pharmacological study of arsenic trioxide in advanced multiple myeloma patients. Leukemia. 2004; 18: 1518-1521.
- Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, Neil J, Reis I, Kharfan-Dabaja M, et al. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res. 2002; 8: 3658-3668.
- Chauhan D, Pandey P, Ogata A, Teoh G, Treon S, Urashima M, et al. Dexamethasone induces apoptosis of multiple myeloma cells in a JNK/SAP kinase independent mechanism. Oncogene. 1997; 15: 837-843.
- Abou-Jawde RM, Reed J, Kelly M, Walker E, Andresen S, Baz R, et al. Efficacy and safety results with the combination therapy of arsenic trioxide, dexamethasone, and ascorbic acid in multiple myeloma patients: a phase 2 trial. Med Oncol. 2006; 23: 263-272.
- Wu KL, Beksac M, van Droogenbroeck J, Amadori S, Zweegman S, Sonneveld P. Phase II multicenter study of arsenic trioxide, ascorbic acid and dexamethasone in patients with relapsed or refractory multiple myeloma. Haematologica. 2006; 91: 1722-1723.
- Zhou LL, Hou J, Fu WJ, Wang D, Yuan Z, Jiang H. Arsenic trioxide and 2-methoxyestradiol reduce beta-catenin accumulation after proteasome inhibition and enhance the sensitivity of myeloma cells to Bortezomib. Leuk Res. 2008; 32: 1674-1683.
- Campbell RA, Sanchez E, Steinberg JA, Baritaki S, Gordon M, Wang C, et al. Antimyeloma effects of arsenic trioxide are enhanced by melphalan, bortezomib and ascorbic acid. Br J Haematol. 2007; 138: 467-478.
- Hofmeister CC, Jansak B, Denlinger N, Kraut EH, Benson DM, Farag SS. Phase II clinical trial of arsenic trioxide with liposomal doxorubicin, vincristine, and dexamethasone in newly diagnosed multiple myeloma. Leuk Res. 2008; 32: 1295-1298.
- Berenson JR, Matous J, Swift RA, Mapes R, Morrison B, Yeh HS. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res. 2007; 13: 1762-1768.
- Qu X, Du J, Zhang C, Fu W, Xi H, Zou J, et al. Arsenic trioxide exerts antimyeloma effects by inhibiting activity in the cytoplasmic substrates of histone deacetylase 6. PLoS One. 2012; 7: e32215.
- List A, Beran M, DiPersio J, Slack J, Vey N, Rosenfeld CS, et al. Opportunities for Trisenox (arsenic trioxide) in the treatment of myelodysplastic syndromes. Leukemia. 2003; 17: 1499-1507.
- Vey N, Bosly A, Guerci A, Feremans W, Dombret H, Dreyfus F, et al. Arsenic trioxide in patients with myelodysplastic syndromes: a phase II multicenter study. J Clin Oncol. 2006; 24: 2465-2471.
- Foss FM. Nucleoside analogs and antimetabolite therapies for myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004; 17: 573-584.
- List AF, Schiller GJ, Mason J, Douer D, Ellison R. Trisenox (arsenic trioxide) in patients with myelodysplastic syndromes (MDS): preliminary findings in a phase 2 clinical study [abstract]. J Clin Oncol. 2004; 22: s6512.
- Schiller GJ, Slack J, Hainsworth JD, Mason J, Saleh M, Rizzieri D, et al. Phase II multicenter study of arsenic trioxide in patients with myelodysplastic syndromes. J Clin Oncol. 2006; 24: 2456-2464.
- Raza A, Buonamici S, Lisak L, Tahir S, Li D, Imran M, et al. Arsenic trioxide and thalidomide combination produces multi-lineage hematological responses in myelodysplastic syndromes patients, particularly in those with high pre-therapy EVI1 expression. Leuk Res. 2004; 28: 791-803.
- Zheng WL, Zhang GS, Xu YX, Shen JK, Dai CW, Pei MF. Arsenic trioxide, thalidomide and retinoid acid combination therapy in higher risk myelodysplastic syndrome patients. Leuk Res. 2008; 32: 251-254.
- Wei W, Zhou F, Zhang Y, Guo L, Shi H, Hou J. A combination of thalidomide and arsenic trioxide is effective and well tolerated in patients with myelodysplastic syndromes. Leuk Res. 2012; 36: 715-719.
- Shackelford D, Kenific C, Blusztajn A, Waxman S, Ren R. Targeted degradation of the AML1/MDS1/EVI1 oncoprotein by arsenic trioxide. Cancer Res. 2006; 66: 11360-11369.
- Parmar S, Rundhaugen LM, Boehlke L, Riley M, Nabhan C, Raji A, et al. Phase II trial of arsenic trioxide in relapsed and refractory acute myeloid leukemia, secondary leukemia and/or newly diagnosed patients at least 65 years old. Leuk Res. 2004; 28: 909-919.
- Yan H, Wang YC, Li D, Wang Y, Liu W, Wu YL, et al. Arsenic trioxide and proteasome inhibitor bortezomib synergistically induce apoptosis in leukemic cells: the role of protein kinase Cdelta. Leukemia. 2007; 21: 1488-1495.
- Lunghi P1, Costanzo A, Levrero M, Bonati A. Treatment with arsenic trioxide (ATO) and MEK1 inhibitor activates the p73-p53AIP1 apoptotic pathway in leukemia cells. Blood. 2004; 104: 519-525.
- Wetzler M, Earp JC, Brady MT, Keng MK, Jusko WJ. Synergism between arsenic trioxide and heat shock protein 90 inhibitors on signal transducer and activator of transcription protein 3 activity-pharmacodynamic drug-drug interaction modeling. Clin Cancer Res. 2007; 13: 2261-2270.
- Takahashi S, Harigae H, Yokoyama H, Ishikawa I, Abe S, Imaizumi M, et al. Synergistic effect of arsenic trioxide and flt3 inhibition on cells with flt3 internal tandem duplication. Int J Hematol. 2006; 84: 256-261.
- Pelicano H, Carew JS, McQueen TJ, Andreeff M, Plunkett W, Keating MJ, et al. Targeting Hsp90 by 17-AAG in leukemia cells: mechanisms for synergistic and antagonistic drug combinations with arsenic trioxide and Ara-C. Leukemia. 2006; 20: 610-619.
- Zhang QY, Mao JH, Liu P, Huang QH, Lu J, Xie YY, et al. A systems biology understanding of the synergistic effects of arsenic sulfide and Imatinib in BCR/ABL-associated leukemia. Proc Natl Acad Sci U S A. 2009; 106: 3378-3383.
- Puccetti E, Guller S, Orleth A, Bruggenolte N, Hoelzer D, Ottmann OG, et al. BCR-ABL mediates arsenic trioxide-induced apoptosis independently of its aberrant kinase activity. Cancer Res. 2000; 60: 3409-3413.
- La Rosee P, Johnson K, Corbin AS, Stoffregen EP, Moseson EM, Willis S, et al. In vitro efficacy of combined treatment depends on the underlying mechanism of resistance in imatinib-resistant Bcr-Abl-positive cell lines. Blood. 2004; 103: 208-215.
- Bazarbachi A, El-Sabban ME, Nasr R, Quignon F, Awaraji C, Kersual J, et al. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood. 1999; 93: 278-283.
- Ishitsuka K, Hanada S, Uozumi K, Utsunomiya A, Arima T. Arsenic trioxide and the growth of human T-cell leukemia virus type I infected T-cell lines. Leuk Lymphoma. 2000; 37: 649-655.
- Hermine O, Dombret H, Poupon J, Arnulf B, Lefrere F, Rousselot P, et al. Phase II trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol J. 2004; 5: 130-134.
- Litzow MR, Lee S, Bennett JM, Dewald GW, Gallagher RE, Jain V, et al. A phase II trial of arsenic trioxide for relapsed and refractory acute lymphoblastic leukemia. Haematologica. 2006; 91: 1105-1108.
- Bornhauser BC, Bonapace L, Lindholm D, Martinez R, Cario G, Schrappe M, et al. Low-dose arsenic trioxide sensitizes glucocorticoid-resistant acute lymphoblastic leukemia cells to dexamethasone via an Akt-dependent pathway. Blood. 2007; 110: 2084-2091.
- Abou-Merhi R, Khoriaty R, Arnoult D, El Hajj H, Dbouk H, Munier S, et al. PS-341 or a combination of arsenic trioxide and interferon-alpha inhibit growth and induce caspase-dependent apoptosis in KSHV/HHV-8-infected primary effusion lymphoma cells. Leukemia. 2007; 21: 1792-1801.
- Fox E, Razzouk BI, Widemann BC, Xiao S, O'Brien M, Goodspeed W, et al. Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood. 2008; 111: 566-573.
- Berenson JR, Yang H, Vescio R, et al. Preliminary findings in a phase 1/2 study of Trisenox® (arsenic trioxide) dosed twice weekly in patients with advanced multiple myeloma. Hematol J. 2003; 4: S255.
- Munshi NC, Tricot G, Desikan R, Badros A, Zangari M, Toor A, et al. Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leukemia. 2002; 16: 1835-1837.
- Cohen KJ, Gibbs IC, Fisher PG, Hayashi RJ, Macy ME, Gore L. A phase I trial of arsenic trioxide chemoradiotherapy for infiltrating astrocytomas of childhood. Neuro Oncol. 2013; 15: 783-787.
- Zhu HH, Wu DP, Jin J, Li JY, Ma J, Wang JX, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013; 31: 4215-4221.
- Liu Y, He P, Liu F, Zhou N, Cheng X, Shi L, et al. Tetra-arsenic tetra-sulfide (As4S 4) promotes apoptosis in retinoid acid-resistant human acute promyelocyticleukemicNB4-R1 cells through down regulation of SET protein. Tumour Biol. 2014; 35: 3421-3430.
- Chen S, Fang Y, Ma L, Liu S, Li X. Realgar-induced apoptosis and differentiation in all-trans retinoic acid (ATRA)-sensitive NB4 and ATRA-resistant MR2 cells. Int J Oncol. 2012; 40: 1089-1096.
- Hussein MA, Saleh M, Ravandi F, Mason J, Rifkin RM, Ellison R. Phase 2 study of arsenic trioxide in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2004; 125: 470-476.