
Case Report
J Blood Disord. 2023; 10(1): 1070.
Precipitation of Insoluble Fibrin in the Brains of Alzheimer’s Disease Model Mice
Osman B1, Wang Z2, Shiraishi K3, Yokoyama M3 and Matsumura Y4,5*
1Division of Developmental Therapeutics, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, Japan
2Division of Artificial Intelligence in Medicine, Research Center for Medical Sciences, The Jikei University, School of Medicine, Japan
3Division of Medical Engineering, Research Center for Medical Sciences, The Jikei University, School of Medicine, Japan
4Department of Immune Medicine, National Cancer Center Research Institute, Japan
5RIN Institute Laboratory, National Cancer Center Research Institute, Japan
*Corresponding author: Yasuhiro Matsumura Department of Immune Medicine, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
Received: December 27, 2022; Accepted: January 24, 2023; Published: January 31, 2023
Abstract
Purpose: To clarify the location of blood coagulation and its relationship with the stage of Alzheimer’s Disease (AD), the brains of AD model mice were immunostained with an antibody that binds only to Insoluble Fibrin (IF) but not to fibrinogen or fibrin degradation products.
Material and Methods: Experiments were performed in AD model mice of three different ages, reflecting three different stages of AD. Brains of AD model mice were immunostained with anti-IF, Anti-β-amyloid (Aβ), and anti-CD31 antibodies to localize IF in the brain and analyze its accumulation and correlation with disease severity.
Results: IF deposited around and inside blood vessel walls in the brain, and its amount increased with disease progression. A significant increase in IF deposition was observed mainly in perivascular areas in the cortex and hippocampus of AD mice. IF co-localized with Aβ protein in the small capillaries and arterioles of the brain in mice with advanced stage AD. The amount of both proteins in lesions was positively correlated with disease stage and severity.
Conclusion: This study selectively detected IF in AD model mice, and clearly demonstrated that hypercoagulation occurs at the sites of Aβ deposition in Alzheimer’s disease model mice. These findings pave the way for future studies clarifying the pathology of AD.
Abbreviations: IF: Insoluble fibrin; AD: Alzheimer’s disease; Aβ: Amyloid β-protein; BBB: Blood-brain barrier
Introduction
Alzheimer’s Disease (AD) is a neurodegenerative disorder that causes the most prevalent form of dementia. The 2022 World Alzheimer Report estimated that approximately 139 million people will be living with dementia by 2050. Amyloid β-protein (Aβ) has been at the center of AD research because of its etiological role described by the amyloid cascade hypothesis [1]. A considerable body of data has confirmed that Aβ plays a critical role in the pathogenesis of AD. It is deposited in the brain parenchyma or around cerebral blood vessels [2], thereby contributing to vascular pathology in AD. In addition, Blood-Brain Barrier (BBB) dysfunction enables fibrinogen and other components of the blood to extravasate into the brain parenchyma, leading to neuronal damage [3].
Against this background, the role of blood coagulation in the progression of AD has received increasing attention. Recent results have revealed that fibrin and amyloid are deposited along cerebral vessel walls and brain parenchyma in AD patients and mouse models of AD [4,5]. Further, fibrinogen accumulates in Aβ-positive vessels in an AD mouse model and is considered an additional feature of AD [6]. Most of these previous studies used antibodies that bind to both fibrinogen and Insoluble Fibrin (IF), or used extraction procedures that remove all soluble fibrinogen and then stain with IF-specific antibodies to demonstrate increased amounts of IF in AD brains [7]. In this context, we conducted the present study to specifically identify IF and thereby provide robust evidence that blood coagulation occurred in the brain lesions of knock-in APP NL-G-F/NL-G-F AD model mice, and to determine if IF correlates with the degree of pathology [8]. We also examined the co-localization of IF with Aβ in this disease model.
We took advantage of our previously produced anti-IF monoclonal antibody, which recognizes an uncovered region that is exposed only when a fibrin clot forms. This highly specific anti-IF antibody distinguishes the IF clot from fibrinogen, soluble fibrin, and D-dimer that are present in blood flow [9-11]. Another advantage of this mAb is that the amino acid sequences of the epitope are completely conserved from fish to humans, allowing experimental results in mice to be extrapolated to humans.
Materials and Methods
Mouse Model
To confirm the persistence of IF clots in the brains of AD model mice, our mAb (clone 1101) against IF was used as the primary antibody [11]. We compared three different groups of knock-in (AppNL-G-F/NL-G-F) AD model mice, representing early, middle, and advanced stages of disease (120, 263, 396 days old) [8]. As a control, normal brains from C57Black mice were used.
The animal experiments were approved by the Committees for Animal Experimentation of the Jikei University Medical School, Japan.
Elisa
Human and mouse IF-immobilized plates were prepared from human and mouse fibrinogen, respectively, according to the previously described method [9]. Briefly, 100 μL of 10 μg/mL fibrinogen (Sigma-Aldrich) solution was dispensed into each well of 96-well IF-immobilized plates, and the plates were incubated overnight at 4OC. One hundred microliters of 0.05 U/mL thrombin (Sigma-Aldrich), 7 mM L-cysteine, and 1 mM CaCl2 was added to each well, and the plates were incubated at 37°C for 2 hours. After blocking with N102 (Nichiyu) containing 10% sucrose, blocking was performed again with TBS-T (Tris-buffered saline with Tween-20) containing 1% BSA.
For ELISA, fibrinogen (Sigma-Aldrich), soluble fibrin (Sekisui Medical), and fibrin degradation products (FDPs; Sekisui Medical) were also immobilized on 96-well plates (1 μg/well of each compound). The plates were reacted with antibody solution (1 μg/mL) for 1 hour, washed with TBS-T, reacted with horseradish peroxidase-conjugated anti-human IgG-Fc antibody or anti-mouse IgG-Fc antibody (Bethyl) for 1 hour, and washed with TBS-T. After 15-minute incubation with 3,3’,5,5’-tetramethylbenzidine, a color-developing reagent, the reaction was stopped by addition of 2 N H2SO4, and absorbance at 450 nm was measured. The mAbs used in this experiment were clone 1101, a negative control, and the previously established mAb 59D8, which recognizes the N-terminus of fibrinogen after thrombin cleavage [12].
Immunohistochemistry
Paraffin-embedded brain sections at 6-μm thickness were deparaffinized and serially rehydrated with descending ethanol concentrations. The sections were then treated with 30% H2O2 in MetOH to inactivate endogenous peroxidases. Sections were placed in a microwave oven in Tris EDTA buffer (pH 9) to induce antigen retrieval, then blocked for 1 h with 5% skim milk. Brain sections were incubated with each of the following primary mAbs overnight at 4°C: IF humanized 1101 mAb [11], mouse monoclonal anti-β-amyloid mAb, 1-16 mAb (6E10, Covance), and anti-CD31/PECAM (platelet endothelial cell adhesion molecule) mAb (Biotech).
The following fluorescent secondary mAbs corresponding to the primary mAbs were used: Alexa Fluor 647-conjugated donkey anti-human IgG (Jackson Immunoresearch Laboratories; for 1101 mAb), Alexa Fluor 488-conjugated donkey anti-mouse IgG (Jackson Immunoresearch Laboratories; for anti-Aβ mAb), and Brilliant Violet 421-conjugated donkey anti goat IgG (Jackson Immunoresearch Laboratories; for anti-CD31 mAb). For Aβ we used 0.3% Sudan Black B in 70% ethanol to reduce lipofuscin green autofluorescence.
Sections were then covered with Vectashield (Vector Labs) and mounted on glass slides.
Results
Characterization of mAb 1101
As reported previously by our group [9-11], mAb 1101 bound only IF and not soluble fibrinogen, fibrin monomer, or FDPs (Figure 1A). In addition, mAb 1101 bound not only human IF but also mouse IF (Figure 1B). Importantly, mAb 59D8, which recognizes the N-terminus of fibrinogen cleaved by thrombin, recognized not only IF but also fibrinogen (Figure 1C).
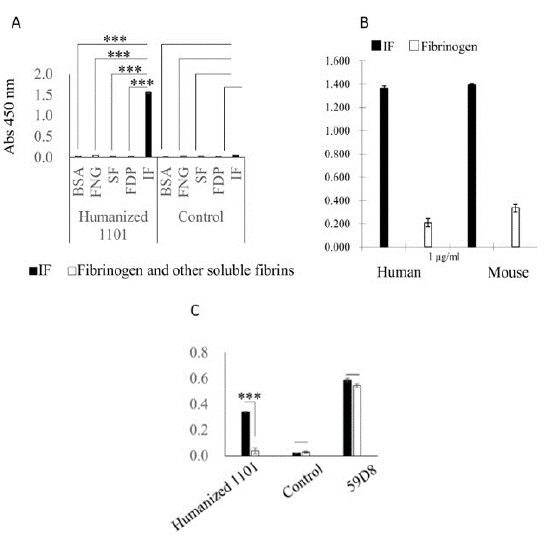
Figure 1: ELISA with anti-fibrin mAb 1101
Each ELISA experiment was performed three times.
(A) Comparison of mAb 1101 and control antibody. BSA, bovine serum albumin (blocking agent); FNG, fibrinogen; SF, soluble fibrin; FDP, fibrin degradation product; IF, insoluble fibrin.
(B) ELISA with mAb 1101 against human and mouse fibrinogen and IF. The humanized anti-IF mAb 1101 recognized both human and mouse IF.
(C) Comparison of 1101, control, and 59D8 mAbs. Only mAb 1101 bound exclusively to IF.
IF (black), fibrinogen (white). ELISA results are shown as means ± S.E. Statistical analysis was performed using Tukey’s test. (2A)
IF is Deposited in the Brains of AD Model Mice and is Associated with Disease Stage
There was no IF or Aβ deposition in the brains of C57 Black control mice (Figure 2A). In AD model mice, IF was deposited in the brain parenchyma and extravascular areas of the cortex and hippocampus. Specifically, IF was present within the vasculature and lining the vessels, and thrombi were observed in the brain (Figure 2B, C). IF deposition increased with disease stage.
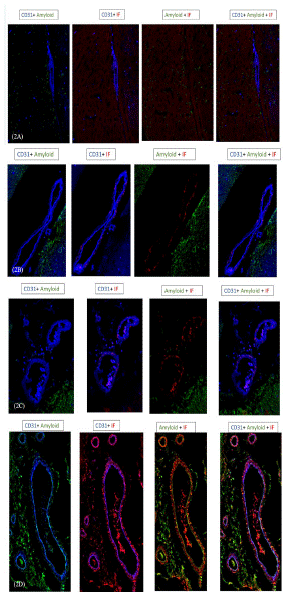
Figure 2: Immunohistochemistry in normal and AD mouse model brains.
Triple immunofluorescence staining of IF in combination with Aβ and PECAM was performed on brain sections of APP mutation mice and wild-type C57Black mice to investigate the deposition of IF (red) and Aβ (green) in the brain and their spatial relationship with cerebral blood vessels (blue).
No IF or Aβ deposition was identified in the brains of C57Black control mice (A).
IF (red) deposits are visible in the parenchyma and perivascular vessels of the cortex and hippocampus in AD model mice (blue), and its accumulation increase with age. AD brains were obtained from AD model mice aged 120 (B), 263 (C), and 396 (D) days.
Merging of the three colors shows that IF co-localizes with Aβ inside the small capillaries and arterioles of the brain in advanced AD (D).
Aβ, amyloid β-protein; IF, insoluble fibrin; PECAM, platelet endothelial cell adhesion molecule.
IF Co-localizes with Aβ
An immunofluorescence assay showed that in AD model mice of advanced age, IF co-localized with Aβ in the small capillaries and arterioles of the brain, especially in the cortex and hippocampus (Figure 2D). This may have affected blood flow.
Discussion
Fibrinogen is a major blood component that is excluded from the brain parenchyma by the BBB under normal conditions, but enters the parenchyma upon BBB breakdown and forms a pathological lesion [3]. Soluble fibrinogen is converted into an IF network that forms a blood clot via a cascade of enzymatic reactions that converts soluble fibrinogen to IF. Once IF is formed, plasminogen activator is specifically activated on IF to convert plasminogen to active plasmin, which then selectively degrades IF to produce FDPs that dissolve in the blood. This study used an APP mutation mouse model of AD to explore the existence of brain IF deposits and the contribution of these deposits to disease progression.
Using our recently developed, highly specific anti-IF mAb 1101, which binds exclusively to IF clots, we succeeded in exclusively detecting the deposition of IF without interference from soluble fibrinogen, fibrin monomers, or FDP, unlike previous studies. The anti-IF mAb 1101 recognizes the pit structure that appears only during IF formation, unlike antibodies established in the past. This pit structure disappears with soluble fibrinogen and fibrin degradation products. Therefore, positive staining with mAb 1101 means that blood coagulation has definitely occurred in the lesion, which may contribute to classifying AD and determining appropriate treatment [11,13].
We found that IF accumulated in the brains of AD model mice and co-localized with Aβ around and inside brain blood vessels in the advanced stage of the disease. As mentioned above, the persistence of IF is not a normal condition and could obstruct blood flow, thus potentially causing neuronal damage. IF deposition was correlated with disease stage. Its presence could result in abnormal blood coagulation that contributes to disease progression through vascular dysfunction that hinders the distribution of oxygen, nutrients, and other factors to the brain parenchyma. Although the thrombi observed in this study were microthrombi, they would cause serious brain damage if they occurred throughout the brain.
Vascular Aβ accumulated around blood vessel walls in the brains of AD model mice, but only in those of advanced age (396 days), corresponding to late-stage disease. This may help explain the previous findings of a Aβ fibrin complex that is structurally altered and resistant to fibrinolysis [4].
Our study is the first to examine the accumulation of IF in relation to Aβ throughout the disease course. We concluded that IF accumulation is progressive, and in late-stage AD, Aβ co-localizes with IF around blood vessels, suggesting a downstream effect. The gradual accumulation of IF as the disease progresses may worsen Aβ pathology in AD by inhibiting its clearance from blood vessels.
These results may pave the way for new AD treatment strategies that target IF to decrease its accumulation in the brain and block its interaction with Aβ. Further studies are needed to determine if anti-IF mAbs can be used as a marker for brain pathology in AD, and if so, they may significantly improve the diagnostic accuracy and sensitivity of Alzheimer’s disease.
Funding
This study was supported in part by JSPS KAKENHI Grant Number JP20H04536 (K.S. & M.Y.), the National Cancer Center Research and Development Fund (29-A-9 to Y.M.) from the National Cancer Center, Japan; the 13th Kobayashi Foundation Cancer Science Award (to Y.M.); the Takeda Science Foundation (to Y.M.); and Research Grants from Bristol Myers Squibb K.K. (to Y.M.)
Conflicts of Interest
Y.M. is co-founder, shareholder, and Board Member of RIN Institute, Inc., a venture company spun out from the National Cancer Center.
Acknowledgments
We thank Dr. Saijou S. for his technical advice and Ms. Shindo H. for her secretarial support.
References
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991; 12: 383-8.
- Yamada M. Cerebral amyloid angiopathy: Emerging concepts. J Stroke. 2015; 17: 17–30.
- Petersen M, Ryu J, Akassoglou K. Fibrinogen in Neurological Diseases: Mechanisms, Imaging and Therapeutics. Nat. Rev. Neurosci. 2018; 19: 283–301.
- Cortes-Canteli M, Zamolodchikov D, Ahn HJ, Strickland S, Norris E.H. Fibrinogen and altered hemostasis in Alzheimer’s disease. J Alzheimers Dis. 2012; 32: 599–608.
- Cortes-Canteli M, Paul J, Norris E.H, Bronstein R, Ahn H J, Zamolodchikov D, et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: A possible contributing factor to Alzheimer’s disease. Neuron. 2010; 66: 695-709.
- Cajamarca SA, Norris EH, van der Weerd L, Strickland S, Ahn HJ. Cerebral amyloid angiopathy-linked β-amyloid mutations promote cerebral fibrin deposits via increased binding affinity for fibrinogen. Proc Natl Acad Sci. 2020; 117: 14482-14492.
- Cortes-Canteli M, Mattei L, Richards AT, Norris EH, Strickland S. Fibrin deposited in the Alzheimer’s disease brain promotes neuronal degeneration. Neurobiol Aging. 2015; 36: 608-617.
- Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, et al. Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci. 2014; 17: 661–663.
- Hisada Y, Yasunaga M, Hanaoka S, Saijou S, Sugino T, Tsuji A, et al. Discovery of an uncovered region in fibrin clots and its clinical significance. Sci Rep. 2013; 3: 2604.
- Matsumura Y. Cancer and blood coagulation. Matsumura Y and Tarin D, editors. In: Cancer Drug delivery systems Based on the Tumor Microenvironment, Tokyo: Springer Japan KK, part of Springer Nature 2019; 23-40.
- Fuchigami H, Matsumura Y. Characterization of antibody clones that bind exclusively to insoluble fibrin. Blood Coagulation and Fibrinolysis. 2022; 34: 20-27.
- Hui KY, Haber E, Matsueda GR. Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science. 1983; 222: 1129-1132.
- Strickland S. Blood will out: vascular contributions to Alzheimer’s disease. J Clin Invest. 2018; 128: 556-563.