
Special Article: Blood Transfusion
J Blood Disord. 2023; 10(1): 1073.
Seroprevalence of HAV and Mandatory Markers of Transfusion- Transmitted Infections in Blood Donors from Chiapas, Mexico: an Exploratory Study
Dimas-González J1,2; Jean J3; Lagunas-Martínez A2; Díaz-del Monte G1; Gómez-González SM1; Becerril-Arenas J4; Arroyo-Pérez JA1#; Trejo-Gómora JE5#*
1Centro Nacional de la Transfusión Sanguínea, Dirección Técnica y de Investigación. Ciudad de México, México
2Instituto Nacional de Salud Pública, Centro de Investigación Sobre Enfermedades Infecciosas. Cuernavaca Morelos, México
3Instituto Nacional de Salud Pública, Centro de Investigación en Nutrición y Salud. Cuernavaca Morelos, México
4Grupo Ruvel, S.A. de C.V. Ciudad de México, México
5Centro Nacional de la Transfusión Sanguínea, Dirección General. Ciudad de México, México
*Corresponding author: Jorge Enrique Trejo-Gómor Centro Nacional de la Transfusión Sanguínea, Dirección General. Av. Othón de Mendizábal Oriente 555, Nueva Industrial Vallejo, Gustavo A. Madero, C.P. 07700, Ciudad de México, México. Tel: +52 55 6392 2250 Email: jorge.trejo@salud.gob.mx
#These authors contributed equally to this article.
Received: April 26, 2023 Accepted: May 26, 2023 Published: June 02, 2023
Abstract
Introduction: Blood transfusion saves millions of lives annually. However, blood transfusion is one of the leading risk factors for Transfusion-Transmitted Infections (TTIs). Therefore, the objective of this study was to explore the seroprevalence of both HAV and mandatory markers of TTIs in blood donors from Chiapas, Mexico.
Materials and Methods: The study included 3,067 blood donors from the Dr. Domingo Chanona Rodríguez Blood Bank located in Tuxtla Gutiérrez, Chiapas, and from 9 collection centers located in different regions of Chiapas. Screening was performed using Enzyme immunoassay (anti-HAV IgM) or Chemiluminescent microparticle immunoassay (HBV HBsAg, anti-HCV IgG/IgM, HIV Ag/Ab, anti-T. pallidum IgG/IgM, and anti-T. cruzi IgG).
Results: The seroprevalence of T. pallidum was the highest at 1.3%, followed by the seroprevalence of HAV and T. cruzi, both with 0.7%. Regions X and XV of the state of Chiapas presented the six markers of the analyzed TTIs. Blood donors at risk of being TTI reactive donated in regions IX and X. However, donors with qualified and unqualified employment and those who have donated more than 2 times are considered protective factors against TTIs.
Conclusion: We observed a high seroprevalence of HAV in this exploratory study. It is indispensable to increase the sample size of blood donors from the state of Chiapas because HAV is endemic to Mexico and it is probably transmitted through blood transfusion. Also, it is necessary to augment voluntary non-remunerated blood donations in the state of Chiapas, mainly in the Southern border regions, to decrease the risk of TTIs.
Keywords: Seroprevalence; HAV; Blood donors; Transfusion-transmitted infections
Abbreviations TTIs: Transfusion-Transmitted Infections; USA: United States of America; HAV: Hepatitis A Virus; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; HIV: Human Immunodeficiency Virus; T. pallidum: Treponema pallidum; T. cruzi: Trypanosoma cruzi; WHO: World Health Organization; CNTS: Centro Nacional de la Transfusión Sanguínea; CI: Confidence intervals
Introduction
Blood transfusion is essential for patient care and sometimes the only alternative for survival. When used correctly, it helps save millions of lives every year. Nevertheless, there is a risk of Transfusion-Transmitted Infections (TTIs) [1]. The risk of TTIs depends on several factors, such as local epidemiology, migration, and endemicity [2].
The World Health Organization (WHO) established mandatory screening of all donated blood for the following agents: HIV, HBV, HCV, and T. pallidum. Additionally, endemic agents of each area must be included [3]. According to regulations established in Mexico, screening tests must be carried out for HIV, HBV, HCV, T. pallidum, and T. cruzi [4]. HAV screening is not a mandatory marker in blood banks; nevertheless, it is the most common viral hepatitis worldwide. In Mexico, the prevalence is 69.3 to 79% in the general population [5-7]. In blood donors from France, the United States of America (USA), and China, the HAV prevalence is low (IgM 0.02%-0.079%) [8-11]. However, in Mexico, there is no evidence in this regard.
The state of Chiapas has high levels of extreme poverty that favor limited conditions in alimentation and health [12-14]. Additionally, the Southern region of Mexico where the state of Chiapas is located has a higher HAV seroprevalence (79.8%) in the general population and low socioeconomic status (82.2%) [7]. Therefore, the objective of this study was to explore the seroprevalence of HAV, as well as to determine mandatory markers of TTIs in blood donors from Chiapas, Mexico.
Material and Methods
Blood Donors
This study included 3,067 blood donors from the Dr. Domingo Chanona Rodríguez Blood bank located in Tuxtla Gutiérrez, Chiapas, and from 9 collection centers located in different socioeconomic regions of the state of Chiapas [12] (Figure 1B), during the period from January 2021 to April 2021. Blood donors with hemolyzed samples, insufficient volume, or incomplete information were excluded.
Screening for Transfusion-Transmissible Infections (TTIs)
The screening tests were performed in the Architect i1000RS System with the Architect HBsAg Qualitative (1P97), Architect Anti-HCV (6C37), Architect HIV Ag/Ab Combo (4J27), Architect Syphilis TP (8D06), and Architect Chagas (2P25) using serum samples. HAV screening was performed with HAV IgM Plus (72491) in the EVOLIS Twin Plus System (Bio-Rad, Marnes-la- Coquette France). The screening assays calculate the results based on sample ratio (Optical density sample/Cut-off value) for HAV and S/CO (Sample relative light units/Cut-off relative light units) for the other markers of TTIs. Samples with sample ratios or S/CO values<1.00 were considered non- reactive, and those with sample ratios or S/CO values ≥1.00 were considered reactive. All TTIs- reactive blood donors were repeatedly reactive, according to the established standards by the Official Mexican Norm NOM-253-SSA1-2012 [4], including HVA-reactive donors. All markers of TTIs were analyzed at the Centro Nacional de la Transfusión Sanguínea (CNTS) in Mexico City.
Statical Analysis
Statistical analysis was performed using Stata v. software 13 (Stata Corporation LLC, College Station, USA). The percentage of donor characteristics was calculated; seroprevalence of markers of TTIs with 95% Confidence Intervals (CI); mean, median, 25th and 75th percentile of sample ratio and S/CO of the markers of TTIs were obtained in duplicate. Additionally, a univariate logistic regression analysis was performed with characteristics of donors and donors reactive to TTIs. P values <0.05 are considered statistically significant.
Results
Characteristics of Blood Donors
The characteristics of the blood donors were: 32 years old on average, male (80%), with middle school and high school education (50.6%), qualified employment (50.6%), blood group O (80.7%), family blood donation/replacement (92.1%), and first-time donor (19.3%). In addition, region XV collected more units of blood (24.9%) (Table 1).
% (n=3067)
95% CI
Age (years)
32.0
31.7
-
32.4
Sex
Male
80.0
78.6
-
81.4
Female
20.0
18.6
-
21.4
Blood collection region
I
9.6
8.6
-
10.6
V
16.0
14.7
-
17.3
VI
10.2
9.2
-
11.3
VIII
4.0
3.3
-
4.7
IX
3.8
3.2
-
4.6
X
16.3
15.0
-
17.7
XII
4.8
4.1
-
5.6
XIII
6.2
5.4
-
7.1
XIV
4.3
3.6
-
5.0
XV
24.9
23.4
-
26.5
Level of education
Illiterate
2.6
2.1
-
3.28
Primary level
23.4
22
-
24.9
Middle and high school level
50.6
49
-
52.4
Undergraduate and
postgraduate
23.4
22
-
24.9
Occupation
Unemployed
2.3
1.9
-
2.9
Qualified employment
50.6
48.9
-
52.4
Unqualified employment
39.5
37.7
-
41.2
Housekeeper
7.6
6.7
-
8.6
ITTs
VIH
0.2
0.1
-
0.4
HAV
0.7
0.5
-
1.1
HBV
0.3
0.2
-
0.6
HCV
0.5
0.3
-
0.9
T. pallidum
1.3
0.9
-
1.7
T. cruzi
0.7
0.5
-
1.1
Blood type
A
13.1
11.9
-
14.3
B
0.6
0.4
-
0.9
AB
5.6
4.9
-
6.5
O
80.7
79.3
-
82.1
Blood donor type
Voluntary non-remunerated
7.9
7.0
-
8.9
Family/replacement
92.1
88.3
-
96.0
Number of donations
First-time
19.3
17.9
-
20.7
Repeat
80.7
79.3
-
82.1
CI: Confidence Interval. Qualified employment: Employee with more 12 years than of schooling. Unqualified employment: Employee with less than 12 years of schooling.
Table 1: Characteristics of blood donors.
Seroprevalence of TTIs
The highest seroprevalence was T. pallidum (1.3%), followed by T. cruzi and HAV (both with 0.7%), and the lowest was HIV (0.2%) (Figure 1A). In regions X and XV from the state of Chiapas, the six markers of TTIs were identified. Five and four markers were found in regions V and IX, and regions I and VIII, respectively (Figure 1B). HAV and HIV were detected in younger donors, and T. pallidum in donors with an average of 40.9 years. Men presented the highest seroprevalences of HBV, HCV, T. pallidum, and T. cruzi; women presented the highest seroprevalence of HAV; and first-time donors had the highest seroprevalence of all markers of TTIs (Table 2).
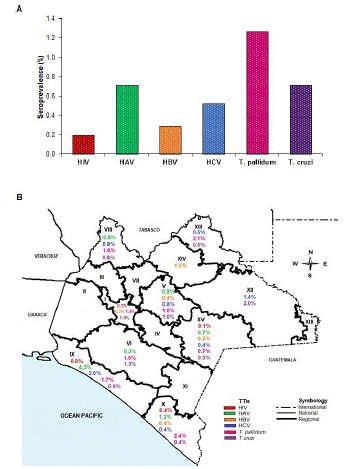
Figure 1: Seroprevalence of TTIs in blood donors. A) Seroprevalence of TTIs, B) Seroprevalence of TTIs in map of the state of Chiapas by regions.
VIH
(n=5)HAV
(n=22)HBV
(n=9)HCV
(n=16)T. pallidum
(n=39)T. cruzi
(n=22)
%
95% CI
%
95% CI
%
95% CI
%
95% CI
%
95% CI
%
95% CI
Age (years,
media)27.0
19.7-34.2
26.9
22.8-30.9
32.0
31.7-32.4
32.1
31.7-32.4
40.9
37.3-44.4
38.7
34.4-43.0
Sex
Male
0.2
0.1-0.4
0.6
0.4-1.0
0.6
0.3-1.0
0.6
0.3-1.0
1.3
1.0-1.9
0.8
0.5-1.3
Female
0.2
0.0-1.2
1.1
0.5-2.4
0.3
0.1-1.3
0.3
0.1-1.3
1.0
0.4-2.2
0.3
0.1-1.3
Blood collection region
I
0.3
0.0-2.4
0.0
0.3
0.0-2.4
0
1.4
0.5-3.6
1.0
0.3-3.1
V
0
0.8
0.3-2.2
0.4
0.1-1.6
0.8
0.3-2.2
1.0
0.4-2.4
1.0
0.4-2.4
VI
0
0.0-2.4
0.3
0.0-2.2
0
0
1.6
0.7-3.8
1.3
0.5-3.4
VIII
0
0.8
0.1-5.6
0
0.8
0.1-5.6
1.6
0.4-6.4
0.8
0.1-5.6
IX
0.9
0.1-5.9
4.3
1.8-9.9
0
2.6
0.8-7.7
1.7
0.4-6.6
0.9
0.1-5.9
X
0.4
0.1-1.6
1.2
0.5-2.6
0.4
0.1-1.6
0.4
0.1-1.6
2.4
1.4-4.2
0.4
0.1-1.6
XII
0
0
0
1.4
0.3-5.3
0
2.0
0.7-6.2
XIII
0
0
0
0.5
0.1-3.6
2.1
0.8-5.5
0.5
0.1-3.6
XIV
0
0
1.5
0.4-5.9
0
0
0
XV
0.1
0.0-0.9
0.7
0.3-1.6
0.3
0.1-1.0
0.4
0.1-1.2
0.7
1.6-1.2
0.3
0.1-1.0
Level of
education
Illiterate
0
0
0
1.2
0.2-8.3
2.5
0.6-9.4
1.2
0.2-8.3
Primary level
0.1
0.0-1.0
0.3
0.1-1.1
0.6
0.2-1.5
0.4
0.1-1.3
1.7
1.0-2.9
0.7
0.3-1.7
Middle and high
school level0.1
0.0-0.5
0.9
0.5-1.5
0.3
0.1-0.8
0.6
0.3-1.1
0.9
0.5-1.5
0.6
0.3-1.1
Undergraduate and postgraduate
0.3
0.1-1.1
0.8
0.4-1.9
0
0.4
0.1-1.3
1.5
0.9-2.8
1.0
0.5-2.0
Occupation
Unemployed
0
2.8
0.7-10.5
1.4
0.2-9.3
1.4
0.2-9.3
1.4
0.2-9.3
2.8
0.7-10.5
Qualified
employment0.3
0.1-0.7
0.7
0.4-1.3
0.1
0.0-0.5
0.5
0.2-0.9
1.4
0.9-2.1
0.7
0.4-1.3
Unqualified employment
0
0.5
0.2-1.1
0.6
0.3-1.2
0.6
0.3-1.2
1.1
0.6-1.8
0.7
0.4-1.4
Housekeeper
0.4
0.1-3.0
1.3
0.4-3.9
0
0.4
0.1-3.0
1.7
0.6-4.5
0
Number of
donations
First-time
0.7
0.0-1.3
2.9
1.5-4.2
0.7
0.0-1.3
2.0
0.9-3.2
3.1
1.7-4.4
2.2
1.0-3.4
Repeat
0
0.0-0.1
0.2
0.0-0.4
0.2
0.0-0.4
0.2
0.0-0.3
0.8
0.5-1.2
0.4
0.1-0.6
CI: Confidence Interval. Qualified employment: Employee with more 12 years than of schooling. Unqualified employment: Employee with less than 12 years of schooling.
Table 2: Seroprevalence of TTIs according to the characteristics of the blood donors.
Association of Reactive Blood Donors and their Characteristics
Blood donors from regions IX and X are at increased risk of being reactive to TTIs markers. However, qualified and unqualified employed donors and those who have donated multiple times have a protective factor against TTIs (Table 3).
OR
95% CI
p value
Sex
Male
ref
Female
0.8
0.46 - 1.30
0.33
Blood collection region
XV
ref
I
1.3
0.582.96
0.51
V
1.7
0.87 - 3.22
0.12
VI
1.2
0.55 - 2.77
0.62
VIII
1.8
0.65 - 4.86
0.27
IX
4.7
2.22 - 10.1
<0.010
X
2.2
1.18 - 4.04
0.01
XII
1.5
0.53 - 3.99
0.46
XIII
1.3
0.53 - 3.43
0.54
XIV
0.6
0.15 - 2.8
0.56
Level of education
Illiterate
ref
Primary level
0.7
0.25 - 2.14
0.56
Middle and high school level
0.7
0.24 - 1.93
0.47
Undergraduate and
postgraduate
0.8
0.26 - 2.22
0.61
Occupation
Unemployed
ref
Qualified employee
0.3
0.14 - 0.74
0.01
Unqualified employee
0.3
0.14 - 0.77
0.01
Housekeeper
0.4
0.13 - 1.05
0.06
Blood donor type
Voluntary non-remunerated
ref
Family/replacement
0.6
0.33 - 1.06
0.07
Blood donation
First time
ref
Repeat
0.2
0.15 - 0.33
<0.001
OR: Odds Ratio. ref: Reference. CI: Confidence Interval. Qualified employment: Employee with more 12 years than of schooling. Unqualified employment: Employee with less than 12 years of schooling. In bold statistically significant differences.
Table 3: Univariate logistic regression analysis with characteristic of blood donors.
Sample Ratio and S/CO of Reactive Blood Donors
HIV, HBV, HCV, T cruzi, and T. pallidum’s S/CO rates were primarily from family/replacement donors with wide ranges for HBV. The sample rates of HAV were from both types of blood donors (Figure 2).
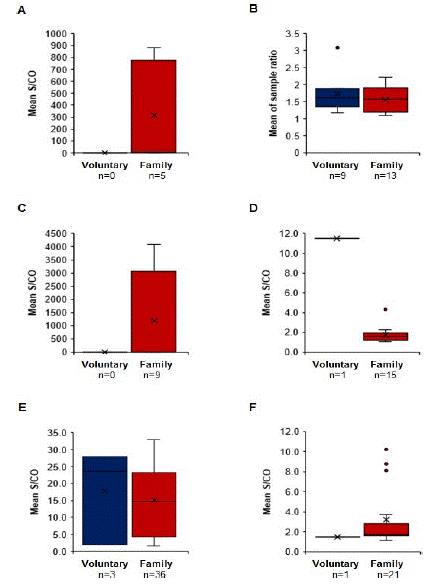
Figure 2: S/CO ratio of blood donors reactive to TTIs. A) HIV, B) HAV, C) HBV, D) HCV, E) T. pallidum, F) T. cruzi.
Discussion
The seroprevalence of anti-HAV IgM was high (0.7%) in blood donors. Additionally, we observed that the seroprevalence of T. pallidum was the highest in the study. The X and VX regions of the state of Chiapas located on the border with Guatemala presented all the markers of TTIs.
The state of Chiapas is located in Southern Mexico and it consists of 124 municipalities that are grouped into 15 regions. It is bordered by the states of Oaxaca to the west, Veracruz to the northwest, and Tabasco to the north, and shares approximately 600 kilometers of border with Guatemala which facilitates the transmission of infectious diseases by the actual migratory flow [12,13]. Moreover, Chiapas has high levels of extreme poverty that can contribute to worsening diet, educational, and health conditions [12-14].
An interesting finding of the study was the high seroprevalence of anti-HAV IgM (0.7%) in blood donors compared with blood donors from France, USA, and China (IgM 0.02-0.079%) [8-11]. It is the first exploratory study in Mexican blood donors because HAV is not a mandatory marker of TTIs. Mexico is considered an area of high/intermediate endemicity for HAV infection, and HAV is the most common cause of viral hepatitis [5-7]. The National Health and Nutrition Survey carried out in Mexico reported high seroprevalence of HAV (79.8%) in the Southern region [7], which coincides with characteristics of extreme poverty and low socioeconomic status from the state of Chiapas. It is important to mention that the seroprevalences determined in Mexico were anti-HAV IgG in the general population including age group (10-19 and 20-25 years) and other variables (gender, region, type of location, and socioeconomic status) that is suggestive of a prior infection or innate immunity [7]. In contrast, our study was carried out in blood donors (18 to 65 years) and anti-HAV IgM was determined. A positive result suggests an acute infection and it is disturbing because these reactive- blood donors can transmit HAV to recipients.
For example, in France, thirteen asymptomatic blood donors were identified as HAV-positive post- donation. Unfortunately, platelet concentrates and erythrocyte concentrates were transfused before HAV detection, and one receptor transfused with these components of blood was infected with HAV [9,11]. In the USA and China, HAV IgM seroprevalence was low in blood donors (0.02%, 0.079%) [8-11]. Therefore, it is necessary to increase the sample size of blood donors from the state of Chiapas. Furthermore, a national study can be necessary for determining the seroprevalence of anti-HAV IgM and identifying risk regions.
The seroprevalence of T. pallidum was the highest (1.3%) in the study. Similar results for T. pallidum were observed in blood donors from the state of Veracruz (1.4%)[15], and in blood donors from the Southern region of Mexico (~1.0%) [Unpublished results].
The seroprevalence of T. cruzi was 0.7%. T. cruzi is an endemic parasite of Central and South America including Southern Mexico[16]. Our results are similar to those results obtained in blood donors from the states of Veracruz and Yucatán located in the Southern region of Mexico with prevalence of 0.5% and 0.7%, respectively [17,18]. Furthermore, in the X and XV regions of Chiapas located on the Southern border, all the markers of TTIs were identified.
Human migration represents a risk to increase the prevalence of TTIs. For example, migrants from the Southern border of Mexico (Mexico-Guatemala) presented a seroprevalence of Chagas disease (T. cruzi) of 3.1%, syphilis (T. pallidum) 1.2% in women and 2.3% in men, and HIV 2.4% in women and 1.3% in men [19,20]. Chagas disease has also been reported in Switzerland and Italy despite that these are not endemic areas for this parasite (promoted by Latin American migrants), with a seroprevalence of 16.9% and 8.7%, respectively [21,22].
Furthermore, we found that first-time donors had the highest seroprevalence of TTIs. This had been observed in donors from other countries (China, Philippines, and Tanzania) [23-25]. It is necessary to promote voluntary non-remunerated donation that decreases the risk of TTIs given that this type of donation was <10% in the study. This percentage is consistent with reports of voluntary non- remunerated donors to level national [Unpublished results].
We found that the S/CO ratios in reactive-blood donors to TTIs were observed mainly in family/replacement donations for HIV, HBV, HCV, T. pallidum, and T. cruzi; the highest S/CO ratios correspond to HBV, and the lowest S/CO ratios were for HCV and T. cruzi. However, HAV was detected in both types of blood donation and the values of S/CO ratios were similar in both types of donations. This observation allows us to conclude that HAV does not depend on the characteristics of blood donation but whether the characteristics from the state of Chiapas (migration, extreme poverty). A limitation of this study is that confirmatory testing of reactive ITT donors was not performed; however, their units of blood were discarded (final destination).
Conclusion
We observed a high seroprevalence of HAV in blood donors in this exploratory study, therefore, it is necessary to increase the sample size of blood donors from the state of Chiapas because Mexico is considered an area of high/intermediate endemicity for HAV infection and which can be transmitted through blood transfusion. In addition, it is necessary to increase voluntary non-remunerated donations in the state of Chiapas, mainly in the Southern border regions, in order to decrease the risk of TTIs.
Author Statements
Disclosure of Interest
The authors declare that they have no competing interest.
Funding
This study was carried out with resources from the Centro Nacional de la Transfusión Sanguínea.
References
- World Health Organization. Action framework to advance universal access to safe, effective and quality-assured blood products. 2020–2023.
- Ainley LI, Hewitt PE. Haematology patients and the risk of transfusion transmitted infection. Br J Haematol. 2018; 180: 473-483.
- World Health Organization. Screening donated blood for transfusion-transmissible infections: recommendations.
- Diario Oficial de la Federación. Norma Oficial Mexicana NOM-253-SSA1-2012. Para la disposición de sangre humana y sus componentes con fines terapéuticos. 2012.
- Panduro A, Escobedo G, Fierro N, Ruiz-Madrigal B, Zepeda E, et al. Epidemiología de las hepatitis virales en México. Salud Publica Mex. 2010; 53: S37-S45.
- Trujillo-Ochoa JL, Viera-Segura O, Fierro NA. Challenges in Management of Hepatitis A Virus Epidemiological Transition in Mexico. Ann Hepatol. 2019; 18: 14-22.
- López-Gatell H, García-García L, Echániz-Avilés G, Cruz-Hervert P, Olamendi-Portugal M, et al. Hepatitis A seroprevalence in adolescents and young adults in Mexico: A 2012 National Health and Nutrition Survey analysis. Vaccine. 2018; 36: 8094-8099.
- Han T, Li C, Zhang Y, Wang Y, Wu B, et al. The prevalence of hepatitis A virus and parvovirus B19 in source-plasma donors and whole blood donors in China. Transfus Med. 2015; 25: 406-410.
- Gallian P, Barlet V, Mouna L, Gross S, Lecam S, et al. Hepatitis A: an epidemiological survey in blood donors, France 2015 to 2017. Euro Surveill. 2018; 23: 1800237.
- Tejada-Strop A, Zafrullah M, Kamili S, Stramer SL, Purdy MA. Distribution of hepatitis A antibodies in US blood donors. Transfusion. 2018; 58: 2761-2765.
- Gallian P, Barlet V, Mouna L, Gross S, Morel P, et al. Persisting higher prevalence of hepatitis A virus RNA in blood donors, France, 2018. Euro Surveill. 2019; 24: 1900695.
- Instituto Nacional de Estadística y Geografía (INEGI). México en Cifras, Chiapas.
- Alerm-González A. Health Must Be Recognized as the Human Right It Is: Héctor Javier Sánchez MD MSSenior Researcher, Department of Society, Culture and Health El Colegio de la Frontera Sur (ECOSUR), Chiapas, Mexico. MEDICC Rev. 2020; 22: 20-23.
- CONEVAL Consejo Nacional de Evaluación de la Política de Desarrollo Social. Medición de la Pobreza.
- López-Balderas N, Hernández-Romano J, Cámara-Contreras M, Bravo-Sarmiento E, Hernández- Romano PA. Trends in prevalence of HIV and syphilis in a central blood bank of Veracruz, Mexico. Transfus Apher Sci. 2019; 58: 94-99.
- Organización Panamericana de la Salud. Suministro de sangre para transfusiones en los países de América Latina y el Caribe 2016-2017. [hyperlinked with https://www.paho.org/es/documentos/suministro-sangre-para-transfusiones-paises-america-latina- caribe-2016-2017]
- García-Montalvo B. Trypanosoma cruzi antibodies in blood donors in Yucatan state, Mexico. Rev Med Inst Mex Seguro Soc. 2011; 49: 367-372.
- Hernández-Romano P, Cámara-Contreras M, Bravo-Sarmiento E, López-Balderas N. Prevalence of Trypanosoma cruzi antibodies in blood donors from Veracruz State, Mexico. Transfusion. 2015; 55: 647-656.
- Conners EE, Ordoñez TL, Cordon-Rosales C, Casanueva CF, Miranda SM, et al. Chagas Disease Infection among Migrants at the Mexico/Guatemala Border. Am J Trop Med Hyg. 2017; 97: 1134-1140.
- Conners EE, Swanson K, Morales-Miranda S, Fernández Casanueva C, Mercer VJ, et al. HIV Risk Behaviors and Correlates of Inconsistent Condom Use Among Substance Using Migrants at the Mexico/Guatemala Border. AIDS Behav. 2017; 21: 2033-2045.
- Jackson Y, Gétaz L, Wolff H, Holst M, Mauris A, et al. Prevalence, clinical staging and risk for blood-borne transmission of Chagas disease among Latin American migrants in Geneva, Switzerland. PLoS Negl Trop Dis. 2010; 4: e592.
- Pane S, Giancola ML, Piselli P, Corpolongo A, Repetto E, et al. Serological evaluation for Chagas disease in migrants from Latin American countries resident in Rome, Italy. BMC Infect Dis. 2018; 18: 212.
- Li C, Xiao X, Yin H, He M, Li J, et al. Prevalence and prevalence trends of transfusion transmissible infections among blood donors at four Chinese regional blood centers between 2000 and 2010. J Transl Med. 2012; 10: 176.
- Mohamed Z, Kim JU, Magesa A, Kasubi M, Feldman SF, et al. High prevalence and poor linkage to care of transfusion-transmitted infections among blood donors in Dar-es-Salaam, Tanzania. J Viral Hepat. 2019; 26: 750-756.
- Rivera NS, Tiongco REG, Salita CL, Kawano RL. Seroprevalence of selected transfusion transmissible infections among blood donors in Region 3, Philippines: A 5-year retrospective study. Trop Biomed. 2019; 36: 993-1002.