
Special Article: Blood Transfusion
J Blood Disord. 2024; 11(1): 1085.
Analysis of Storage Lesions in Refrigerated Red Blood Cells in Different Storage Solutions
Linh Nguyen T Tran1#; Cristina González-Fernández1,2#; Mitchell Weigand3; Jeffrey Chalmers3; Jenifer Gomez-Pastora1*
1Department of Chemical Engineering, Texas Tech University, Lubbock 79409, TX, USA
2Departamento de Ingenierías Química y Biomolecular, Universidad de Cantabria, Avda. Los Castros, s/n, 39005 Santander, Spain
3William G. Lowrie Department of Chemical and Biomolecular Engineering, The Ohio State University, Columbus 43210, OH, USA
*Corresponding author: Jenifer Gomez-Pastora Department of Chemical Engineering, Texas Tech University, Lubbock 79409, TX, USA. Tel: +1 806-742-3553 Email: jenifer.gomez@ttu.edu
#These authors have contributed equally to this article.
Received: March 18, 2024 Accepted: April 22, 2024 Published: April 29, 2024
Abstract
Background: Red Blood Cells (RBCs) undergo detrimental biochemical and morphological changes during refrigerated storage, termed “storage lesion”. Additive Solutions (ASs) aim to mitigate these effects and preserve RBC integrity and functionality during long storage. This study evaluates the performance of different storage solutions, i.e. AS-3, AS-7, and SAGM solutions and a non-nutrient Phosphate-Buffered Saline (PBS), in maintaining RBC characteristics over a 42-day storage.
Methods: RBCs from 5 healthy donors were isolated, processed, and suspended in different storage solutions at 4oC for 42 days. Samples were analyzed every 2 weeks for multiple parameters: hemoglobin levels (intracellular hemoglobin), percentage of hemolysis, RBC indices, RBC count, size distribution, average cell size, microvesicle generation, cell morphology, etc. One-way Analysis of Variance (ANOVA) and post-hoc Tukey’s tests evaluated statistical differences among the effects of the various storage solutions.
Results: Our results suggest that SAGM demonstrated the best performance in maintaining RBC quality in terms of hemolysis, RBC count, and morphology. AS-based solutions showed improved performance compared to PBS in different RBC indices and parameters, especially with regard to microvesiculation, swelling, and substantial intracellular hemoglobin decline. Still, our findings suggest that even the most enhanced formulations need optimization in order to avoid adverse effects in transfused patients.
Keywords: Red blood cell (RBC); Storage lesion; Cell preservation; Additive solutions; Blood banking
Introduction
Blood transfusion is a critical therapeutic procedure performed for treating different conditions, with approximately 85 million Red Blood Cell (RBC) units collected globally each year [1]. However, before transfused, RBC units are generally stored in blood banks under refrigerated conditions and undergo significant biochemical and biomechanical changes collectively termed “storage lesion” that can compromise post-transfusion viability and function [2-6]. For instance, refrigerated storage promotes glucose metabolic alterations, Adenosine Triphosphate (ATP) depletion, oxidative damage, membrane perturbations, and cell morphology shifts that compromise cell integrity [2,3,6-8]. Biochemically, ATP and 2,3-diphosphoglycerate (2,3-DPG) reserves become depleted while redox imbalance leads to membrane lipid peroxidation and the accumulation of oxidized hemoglobin (Hb) forms [9,10]. Concurrently, membrane changes like phospholipid scrambling, vesiculation, and progressive loss of surface area reflect cytoskeletal protein alterations and oxidative damage [11-13]. These biochemical shifts also influence biomechanics, as dehydration and swollen morphology reduce deformability while membrane defects promote increased fragility [8,10,14,15]. Ultimately, biochemical and biomechanical storage lesions are manifested as reduced oxygen delivery capacity, impaired rheological properties in the microcirculation, and shortened post-transfusion survival.
To mitigate storage lesion effects, RBCs are suspended in nutrient-enriched Additive Solutions (ASs) designed to better maintain RBC integrity during refrigerated preservation [16]. The Food and Drug Administration (FDA) allows RBC storage for up to 42 days in several US-approved solutions [2,10]. These solutions contain varying formulations of salts, sugars, buffers, antioxidants, and nutrients, with the aim of optimizing RBC viability, function and hemostatic activity during storage and following transfusion [17]. First-generation solutions such as AS-1 (Adsol) primarily focused on maintaining ATP levels, but they fell short in preserving other crucial factors like 2,3-DPG and antioxidant capacity [18,19]. In this regard, more advanced solutions, such as AS-3 (Nutricel), AS-5 (Optisol), AS-7 (Optisol-7) and SAGM (saline-adenine-glucose-mannitol) address these limitations by offering better preservation of 2,3-DPG and antioxidant defense [7,20-22]. Though ASs aim to counter storage lesion issues, all formulations have trade-offs between cost, and efficacy. On the other hand, Phosphate-Buffered Saline (PBS) is also a readily available and cost-effective solution but with the only applicability to RBC short storage. Investigating how RBCs fare in PBS, AS-3, AS-7, and SAGM can offer valuable insights into the extent of their protective effects and potential trade-offs. Several studies have analyzed the effect of various storage solutions in the metabolic profile of RBCs during hypothermic storage [3,7,17,23]. However, the majority of the studies have focused on the analysis of different metabolites and biomolecules (ATP, DPG, glucose, lactate, pH, etc.) or in the cell integrity (rheology, deformability, membrane protein profile, etc.). To the best of our knowledge, we are the first group comparing the effect of PBS, AS-3, AS-7 and SAGM in various RBC parameters that are of paramount importance to asses RBC storage during 42 days, such as RBC indices, e.g., Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Volume (MCV), RBC size distribution, potential microvesicles formation, along with traditionally reported parameters like hemolysis, Hb levels, RBC counts and cell morphology. This study therefore aims to evaluate storage-related changes in RBC viability, morphology, and function among AS-3, AS-7, SAGM and PBS. By evaluating the strengths and differences between PBS and existing ASs, we can contribute to optimizing current storage strategies. By thoroughly tracking critical indicators of RBC function, integrity, and survival, this study intends to elucidate differences among the storage media formulations. Identifying an optimal solution would guide creation of evidence-based RBC storage protocols to minimize storage lesions, improve shelf life, and enhance clinical effectiveness when transfused.
Materials and Methods
AS-3, AS-7, and SAGM were prepared according to the compositions outlined by D’Amici et al. and Lagerberg et al. [7,17], presented in Table 1; PBS was purchased from Thermo Fisher Scientific. The pH of each solution was measured with a VWR (MU 6100L) pH meter and our measured pH values agreed with the values reported in the literature [7,17].
Constituents (mmol/L)
AS-3
AS-7
SAGM
NaCl
70
—
150
NaHCO3
—
26
—
Na2HPO4
—
12
—
NaH2PO4
23
—
—
Citrate
2
—
—
Na - Citrate
23
—
—
Adenine
2
2
1.25
Glucose
55
80
45
Mannitol
—
55
30
pH
5.8
8.5
5.7
Table 1: Composition of the prepared solutions for RBC storage.
Blood Sample Collection
Whole blood samples (35±5 mL) were collected from five healthy volunteer donors using Ethylenediaminetetraacetic Acid (EDTA) tubes upon informed consent according to the Institutional Review Board (IRB)-approved protocols of the Texas Tech University Health Science Center (Protocol number IRB: L22-L274).
RBC Isolation and Storage Conditions
After collection, whole blood samples were divided into multiple vials and RBCs were mixed with either PBS, AS-3, AS-7, or SAGM. To isolate RBCs, each aliquot underwent 3 standardized wash cycles. In each cycle, the samples were centrifuged at 2,000 x g for 5 minutes, then supernatant was removed and replaced with fresh storage media. This washing process removed other blood components, while the solutions stabilized the RBCs under proper storage conditions prior to refrigeration. The resulting washed RBC suspensions were stored in 2 mL microcentrifuge tubes at 4°C in their respective solutions throughout the 42-day storage period.
Sample Analysis
Several RBC parameters were analyzed every 2 weeks (days 0, 14, 28, and 42) for all the samples from all donors in each storage condition. All measurements were performed in triplicate.
Spectrophotometric Analysis
Intracellular and cell free Hb levels were assessed spectrophotometrically through absorbance measurements using the Agilent Cary 60 UV-Vis spectrophotometer (Agilent Technologies Inc.). To comply with Beer-Lambert’s law, supernatant samples underwent dilution in deionized water, ensuring that the absorbance around the Q bands (560 nm and 577 nm) fell within the 0.1-1 range. Each sample underwent triplicate testing to calculate the concentrations of oxyhemoglobin (oxy-Hb) using the equations developed by Winterbourne [24].
A conversion factor of 1.611 transformed Hb concentration from millimolar to grams per deciliter. The oxy-Hb presence was verified by examining absorption spectra shapes within the 474-670 nm range.
Intracellular Hb concentration measurements involved washing the samples with PBS through centrifugation (2,000 x g for 5 min, repeated three times). A subsequent 1:4 dilution in deionized water (to promote cell lysis) and a 30-minute incubation at 4°C ensured complete intracellular Hb release. Supernatant Hb concentration was spectrophotometrically measured post-centrifugation. Free Hb levels were measured in the solution (supernatant) after separating the cells from the solution via centrifugation and before lysis.
RBC Size and Concentration Analysis
RBC sample size and concentration distributions were carefully measured using an automated cell counter, B43905 Multisizer 4e Coulter Counter (CC, Beckman Coulter, CA). To ensure an appropriate measurement, samples were diluted 1:100 in PBS. Several parameters were measured including potential microvesicle formation, average RBC size and distribution, average cell volume and distribution as well as RBC count.
Microscope Analysis
For morphology evaluation, a small quantity (< 10μL) of RBC samples was diluted 1:100 in PBS and added to a Bright-Line hemocytometer (Hausser Scientific). The sample was analyzed using a portable microscope camera (AM73515MT88A, Dino-Lite, Torrance, CA) to examine the shape of the RBCs. At least five images were captured for every sample biweekly, ensuring an entire and realistic inspection of the RBC morphology.
Statistical Analysis
The effects of the four preservative solutions (PBS, AS-3, AS-7, and SAGM) on measured RBC parameters over the 42-day storage period were analyzed using one-way Analysis of Variance (ANOVA) tests in JMP® Pro 17.0.0 statistical software. The parameters compared between groups were hemolysis percentage, Hb levels, RBC size, and RBC count. Briefly, the four storage solutions were treated as categorical factors. The dependent variables of interest, including hemolysis percentage, Hb levels, cell size, and RBC count, were analyzed as continuous numerical outcomes. The statistical significance of storage duration over the 42 days was also assessed. Prior to analysis, Shapiro-Wilk’s W test evaluated normal distributions, and Levene’s test validated equal variance assumptions for applying ANOVA. After significant overall F-tests, Tukey’s Honestly Significant Difference (HSD) post-hoc tests elucidated which storage solution combinations exhibited significant differences at a 95% confidence level for the RBC properties. ANOVA tests determined that there were significant differences between groups for all measured variables (p<0.05). For post-hoc comparisons between individual storage solutions, Tukey HSD tests were utilized to correct for multiple testing.
Results and Discussion
In this section, the effect of storage time and/or the four solutions – PBS, AS-3, AS-7, and SAGM - was analyzed on various parameters including hemolysis, swelling, and concentration. The solutions were first analyzed individually to assess their separate effects over storage time, and subsequently analyzed simultaneously. The following analyses have been presented for all five donors across all storage time points (0, 2, 4, and 6 weeks) in first.
However, our later analysis. focuses specifically on the collected data at the six-week storage time point for RBC indices and Hb concentrations.
Effect of Storage Time
Figure 1 demonstrates the impact of storage time on the percentage of hemolysis when RBCs were stored in the four studied storage solutions. The percentage of hemolysis was calculated using the cell count at weeks 2, 4 and 6, in comparison to week 0. It was observed that the percentage of hemolysis progressively increased from week 0 to week 2 of all studied storage solutions, as also reported by other studies [25]. After week 2, average hemolysis levels decreased or kept constant for all the solutions but AS-7. This decrease in the hemolysis levels for AS-3, PBS and SAGM can be attributed to the fact that we employed cell counts to calculate hemolysis, and the potential formation of microvesicles can increase cell counts over storage time and decrease the hemolysis level. The average hemolysis levels for the samples at week 2 of storage were 8.34%, 10.54% and 6.04% for PBS, AS-3 and AS-7, respectively. On the contrary, SAGM showed the lowest hemolysis levels of all the solutions, with levels below 6% during the entire storage period. These results agree well with previous studies reported in the literature [25-27].
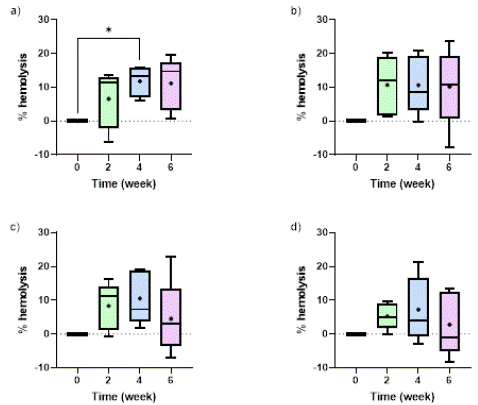
Figure 1: Comparison of time-dependent changes in hemolysis when RBCs were stored in (a) AS-7, (b) AS-3, (c) PBS, and (d) SAGM. “+” mark represents the mean value for each timepoint. Significant differences were calculated using ANOVA followed by a Tukey’s post hoc test: * p < 0.05.
RBC Indices and Hb Levels
The RBC indices (MCV, MCH), RBC counts, and Hb concentration levels (normalized intracellular Hb and cell-free Hb concentration) for the four storage solutions at week 6 are shown in this section.
The analysis of MCH and MCV provides valuable insights into the effects of different storage solutions on single RBC Hb levels and volume during storage. Regarding MCV, both AS-7 and AS-3 led to a slight decrease in MCV (by around 3%) by week 6 in comparison to week 0 (Figure 2a), likely due to the cells losing water and shrinking. Interestingly, PBS exhibited a unique pattern where its MCV initially decreased by 6% at week 4 (data not shown) but then increased, resulting in an overall increase of 10.18% by week 6, indicating significant RBC swelling. This swelling could be due to the influx of water into the cells or changes in the cell membrane's permeability, which may be influenced by the composition of the PBS solution. In contrast, SAGM had the smallest MCV change during storage, with a modest 2% increase, which suggests that SAGM is the most effective solution at preserving the volume of RBCs for 6 weeks. By comparing all the solutions, and excluding PBS, SAGM and AS-7 reported a statistically significant different MCV values.
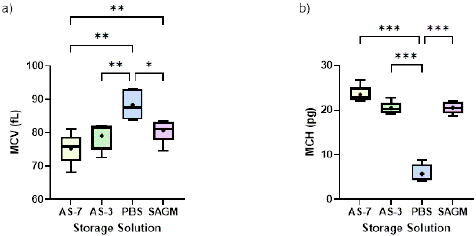
Figure 2: Effect of storage solutions on: (a) MCV (fL), (b) MCH (pg) at the end of the 42-day storage period. “+” mark represents the mean value for each solution. Significant differences were calculated using ANOVA followed by a Tukey’s post hoc test: * p < 0.05; ** p < 0.01; *** p < 0.001.
With regards to MCH, we observed that AS-7 maintained relatively stable MCH values after an initial slight decrease in week 2, while AS-3 and SAGM exhibited a gradual decrease in MCH throughout the storage period. MCH decreased 12.21% and 17.35% in 6 weeks for RBCs stored in AS-3 and SAGM, respectively. Interestingly, PBS demonstrated the most prominent decrease in MCH, surpassing all other solutions by week 4 and maintaining the lowest values until the end of the storage (as low as 5.71 pg of Hb by week 6). Figure 2b further illustrates that at the end of storage, PBS exhibited significantly lower MCH values compared to other solutions. This finding suggests that the RBCs stored in PBS accumulates a lower Hb content per cell compared to the others. AS-7, AS-3 and SAGM showed comparable MCH values at the end of the storage period, with no statistically significant differences among them, indicating a relatively stable Hb content per cell across these conditions.
The dimensionless intracellular Hb level (as the ratio between the intracellular Hb level at different storage times over the total Hb levels at week 0) as well as the cell free Hb concentration at the end of storage are depicted in Figures 3a and 3b, respectively. Among the additive solutions, AS-7 maintained the highest intracellular Hb levels (around 0.9 g/dL and 0.8 when normalized, on average for all donors) during storage. This suggests that AS-7 may better preserve RBC integrity compared to SAGM, AS-3, and PBS, in agreement with the findings from Lagerberg and co-workers [17]. On the other hand, the use of PBS led to a worse maintenance of cell quality, in terms of intracellular Hb concentration and increased cell free Hb, compared to the other storage solutions, as presented in Figure 3. The detrimental effects of PBS on RBC storage lesion have been reported in the literature [26] and these results agree well with our observations. We demonstrated that RBC storage in PBS caused a significant increase of the free Hb concentration, with the highest free Hb concentration value recorded at week 6 (around 0.66 g/dL on average for all donors). The unfavorable performance of PBS is also revealed by the significant decrease of the intracellular Hb concentration. Particularly, a ~78% reduction in the intracellular Hb is reported on average for all donors during the six weeks of storage. Conversely, release of intracellular Hb to the medium for the other solutions was not high; the free Hb concentration remained below 0.1 g/dL for SAGM, AS-3, and AS-7 preservative solutions during the whole storage period, thus demonstrating that these solutions may ensure the maintenance of RBC quality. In this regard, the use of SAGM, AS-3, and AS-7 resulted in a small reduction of intracellular Hb concentration (in the range 5-29% depending on the donor) during the six weeks of storage. Further analysis using Tukey’s test revealed significantly higher levels of cell-free Hb in PBS compared to all other enhanced solutions, confirming its poorer ability to prevent storage lesion during storage. Additionally, while no significant differences were observed among AS-based and SAGM solutions themselves, PBS also demonstrated substantially lower intracellular Hb retention. Thus, this confirms PBS as an inferior solution for preserving RBC integrity compared to AS-based and SAGM additive solutions.
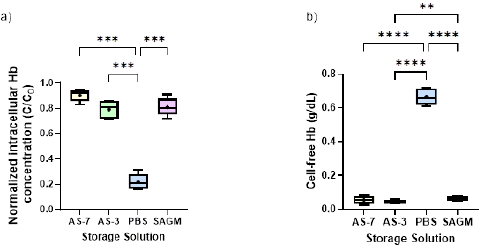
Figure 3: The impact of the four studied storage solutions on (a) intracellular Hb concentration (dimensionless), and (b) cell-free Hb concentration (g/dL) at the end of the storage period. “+” mark represents the mean value for each solution. Significant differences were calculated using ANOVA followed by a Tukey’s post hoc test: ** p < 0.01; *** p < 0.001; **** p < 0.0001.
In Figure 4, we present the final RBC counts (number of cells/dL) at the end of the storage period for the different storage solutions. Our statistical analysis suggests that the differences in RBC counts among the different storage solutions were not statistically significant. By analyzing both RBC count and hemolysis (Figure 1), we can note that SAGM emerged as the most promising solution, demonstrating the lowest average hemolysis percentage (2.17%) and the highest RBC count (3.22 billion of cells per dL), albeit no statistically significant differences were found in comparison to the other solutions. Even though PBS reported significantly free Hb concentration (especially in week 2), its RBC count at week 6 of approximately 3.17 billion of cells per dL was comparable to the other solutions, with differences within 2%. This can be attributed to the generation of microvesicles of micrometer sizes during storage, that were detected and counted as RBCs by our Cell Counter (as we demonstrate in the following analyses), but with insignificant amounts of Hb, generating high cell-free Hb concentration. AS-7 reported the lowest RBC count (3.10 billion of cells per dL), although the differences in RBC count were not statistically significant.
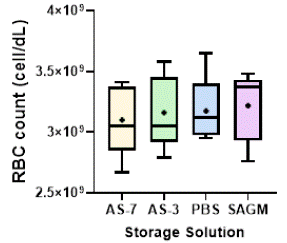
Figure 4: RBC count (cell/dL) by the end of storage. “+” mark represents the mean value for each solution. Statistical analysis using ANOVA and Tukey’s post hoc test revealed no significant differences among the groups (ns, p > 0.05). Samples were diluted 1:100 before analysis.
Morphology and Size/Concentration Distribution
In Figure 5, we present the stored RBC size distributions at week 0, 2, 4 and 6 for all the storage solutions tested in this study for a representative donor. Regarding the average RBC size, it should be noted that RBCs stored in PBS reported the highest average size for all the storage times tested. In fact, one may see how the histogram shifted to the right for the samples in PBS during storage. The storage solution significantly affected RBC size (p< 0.0001). Tukey’s HSD test further revealed that storage in PBS resulted in significantly larger RBC diameters compared to AS-3 and AS-7, while SAGM only differed significantly from AS-7 itself. Although PBS contrasted with all other solutions, fewer significant differences were observed between SAGM, AS-3, and AS-7 preservatives. When examining the histograms to evaluate the potential formation of microvesicles, we observed an increase in the number of cells detected in the size range of 3-4.5 μm for the samples stored in PBS after week 2. Indeed, vesicles smaller than 4 μm not present in weeks 0 and 2 appeared in the histograms for weeks 4 and 6.
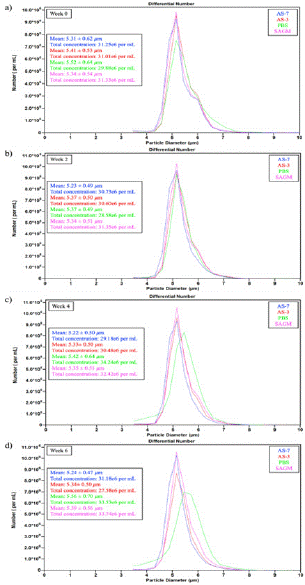
Figure 5: RBC average size and size distribution in the four storage solutions under investigation: (a) Week 0, (b) Week 2, (c) Week 4, (d) Week 6. Samples were diluted 1:100 before analysis.
The number of these microvesicles at week 6 was as high as 1.52 million cells/mL, which is 4.5% of the total count, for this representative donor. However, no observable microvesiculation was found for the other storage solutions by inspecting the histograms in Figure 5. As previously demonstrated by Bebesi et al. using infrared spectroscopy analysis, PBS causes increased vesiculation and release of RBC-derived extracellular vesicles containing higher levels of Hb, including oxidized Hb species [26], which agrees well with our size distribution analysis.
The RBC size and concentration distribution trends presented in Figure 5 closely align with the MCV data and hemolysis levels highlighted above. Analyzing data across all donors, the SAGM samples maintained the highest overall concentrations after the 6-week storage, averaging around 30-33 million cells/mL with a 0.85% reduction in cell counts compared to week 0. Meanwhile, diameters remained very consistent for all the solutions over time, fluctuating within the small range of 5.2 – 5.4 μm for all preservatives across all donors with the exception of PBS which caused diameters larger than 5.5 μm. The sustained cell populations coupled with the maintained size distributions (and MCV) for SAGM correlated to a reduced hemolysis and inhibited swelling, as presented above. In contrast, averaging the results from all donors, AS-3 and AS-7 experienced moderate concentration reductions of 13.97% and 13.45%, respectively, during the storage time. Similarly, the 5% concentration drop along with 3% diameter expansion as well as the observed microvesiculation after week 2 for PBS-stored RBCs correlates to the previously presented data and high free Hb levels in the samples. Thus, combined metrics continue to support more favorable RBC integrity and viability with SAGM, followed by AS-based storage versus PBS across multiple donor samples.
Direct microscopic analysis provides further evidence of the effect of storage solutions on RBC preservation. As shown in Figure 6, the storage in PBS results in the severe decline in intact RBCs and careful inspection of the figure shows the prevalence of ghost cells after 6 weeks (Figure 6c). These ghost cells are RBCs with no intracellular Hb, thus they appear as transparent cells (empty) and only can be distinguished due to the presence of the cell membrane. Some of these entities have been circled in Figure 6c for easier visualization. On the contrary, the AS-based and SAGM samples seemed to maintain the cell concentration consistently during the storage period. Moreover, no notable differences were observed in the RBC morphology for RBCs stored across these solutions.
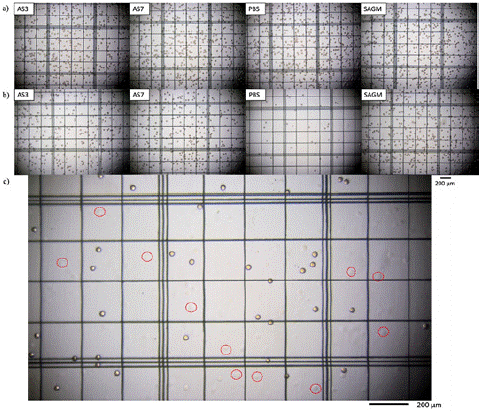
Figure 6: Microscope images of RBCs when stored in AS-3, AS-7, PBS, and SAGM in (a) Week 0 and (b) Week 6. (c) Cells stored in PBS at week 6 with red circles indicating the presence of ghost cells.
Conclusion
As the demand for lifesaving blood transfusions continues rising worldwide, enhancing storage methods to prevent the progression of harmful biochemical and mechanical lesions in donated RBCs remains imperative. This study aimed to evaluate the effect of several RBC storage solutions, namely, AS-3, AS-7, SAGM, and PBS, in mitigating deleterious storage effects over 42 days. Analysis over 6 weeks demonstrated clear differences between PBS and the nutritionally enriched AS-based and SAGM formulations.
Measurements of low intracellular Hb and high cell free Hb concentrations, evident swelling, size and morphology changes and formation of microvesicles signified rapid and visual decay advent of RBCs in PBS samples. It is worth mentioning that we observe increased microvesiculation and RBC ghosts in the samples stored in PBS at week 6, but not different/global RBC counts in comparison to the other solutions. In contrast, SAGM emerged as the most promising solution, exhibiting the lowest hemolysis, the highest RBC count, and the most effective preservation of RBC size and volume. AS-3 and AS-7 also showed improved performance compared to PBS, but surprisingly, AS-7, despite containing higher concentrations of beneficial additives, did not exhibit superior performance compared to the other solutions at least in the parameters that we are reporting in this study. By guiding clinically-impactful blood storage techniques while elucidating mechanisms underlying suboptimal PBS preservation, findings pave the way for developing enhanced, affordable, and efficient storage methods. Further efforts will build upon fundamental insights tracking temporal changes in vital parameters related to RBC metabolism, structure, and function. Advancing techniques to curb biochemical and morphological lesions remains vital for maximizing shelf life and improving patient outcomes through transfusion therapies.
Author Statements
Acknowledgement
This study was financially supported by Texas Tech University through HEF New Faculty Startup, NRUF Start Up, and Core Research Support Fund. Dr. Cristina González-Fernández thanks the Spanish Ministry of Universities for the Margarita Salas postdoctoral fellowship (grants for the requalification of the Spanish university system for 2021-2023, University of Cantabria), funded by the European Union-NextGenerationEU.
References
- Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB*. Annals of Internal Medicine. 2012; 157: 49-58.
- Sparrow RL. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus, 2010; 8: s26-30.
- D’Alessandro A, Nemkov T, Hansen KC, Szczepiorkowski ZM, Dumont LJ. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015; 55: 2955-66.
- D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010; 8: 82-88.
- D’Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, et al., An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015; 55: 205-219.
- Hess JR. Measures of stored red blood cell quality. Vox Sanguinis. 2014; 107: 1-9.
- D’Amici GM, Mirasole C, D’Alessandro A, Yoshida T, Dumont LJ, Zolla L. Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood transfusion = Trasfusione del sangue. 2012; 10: s46-54.
- Tsoi WC. Advances in blood storage bags and preservative solutions. ISBT Science Series. 2016; 11: 49-54.
- Jana S, Kassa T, Wood F, Hicks W, Alayash AI. Changes in hemoglobin oxidation and band 3 during blood storage impact oxygen sensing and mitochondrial bioenergetic pathways in the human pulmonary arterial endothelial cell model. Frontiers in Physiology. 2023; 14: 1278763.
- Sut C, Tariket S, Chou ML, Garraud O, Laradi S, Hamzeh-Cognasse H, et al. Duration of red blood cell storage and inflammatory marker generation. Blood Transfus, 2017; 15: 145-152.
- Alaarg A, Schiffelers RM, van Solinge WW, van Wijk R. Red blood cell vesiculation in hereditary hemolytic anemia. Frontiers in Physiology. 2013; 4: 365.
- D’Alessandro A, D’Amici GM, Vagilo S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012; 97: 107-115.
- Roussel C, Buffet PA, Amireault P. Measuring Post-transfusion Recovery and Survival of Red Blood Cells: Strengths and Weaknesses of Chromium-51 Labeling and Alternative Methods. Frontiers in Medicine. 2018; 5: 130.
- Barshtein G, Gural A, Arbell D, Barkan R, Livshits L, Pajic-Lijakovic I, et al. Red Blood Cell Deformability Is Expressed by a Set of Interrelated Membrane Proteins. International Journal of Molecular Sciences. 2023; 24: 12755.
- Almac E, Ince C. The impact of storage on red cell function in blood transfusion. Best Practice & Research Clinical Anaesthesiology. 2007; 21: 195-208.
- Wagner SJ, Glynn SA, Welniak LA, NHLBI Working Group on Strategies to Optimize Red Blood Cell Products. Research opportunities in optimizing storage of red blood cell products. Transfusion, 2014; 54: 483-494.
- Lagerberg JW, KorstenH, Van Der Meer PF, de Korte D. Prevention of red cell storage lesion: a comparison of five different additive solutions. Blood Transfus. 2017; 15: 456-462.
- Heaton A, Miripol J, Aster R, Hartman P, Dehart D, Rzad L, et al. Use of Adsol® preservation solution for prolonged storage of low viscosity AS-1 red blood cells. British Journal of Haematology. 1984; 57: 467-478.
- Roback JD, Josephson CD, Waller EK, Newman JL, Karatela S, Uppal K, et al. Metabolomics of ADSOL (AS-1) Red Blood Cell Storage. Transfusion Medicine Reviews. 2014; 28: 41-55.
- García-Roa M, Vicente-Ayuso MDC, Bobes AM, Pedraza AC, Gonzalez-Fernandez A, Martin MP, et al. Red blood cell storage time and transfusion: current practice, concerns and future perspectives. Blood Transfus. 2017; 15: 222-231.
- Sparrow RL, Sran A, Healey G, Veale MF, Norris PJ. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: a paired study. Transfusion. 2014; 54: 560-568.
- Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion. 2011; 51: 1574-1579.
- Bebesi T, Kitka D, Gaal A, Szigyarto IC, Deak R, Beke-SomfaiT, et al. Storage conditions determine the characteristics of red blood cell derived extracellular vesicles. Scientific Reports. 2022; 12: 977-977.
- Winterbourn CC. Oxidative reactions of hemoglobin. Methods Enzymol. 1990; 186: 265-272.
- Müller-Steinhardt M, et al. Impact of various red cell concentrate preparation methods on the efficiency of prestorage white cell filtration and on red cells during storage for 42 days. Transfusion. 1997; 37: 1137-1142.
- Gammon RR, Strayer SA, Avery NL, Mintz PD. Hemolysis during leukocyte-reduction filtration of stored red blood cells. Ann Clin Lab Sci. 2000; 30: 195-199.
- Sawant RB, et al. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007; 1: 47-51.