
Research Article
J Blood Disord. 2024; 11(1): 1086.
Effect of pH Modulation and Anaerobic Conditions on the Prolonged Storage of Red Blood Cells
Cristina González-Fernández1,2#; Linh Nguyen T Tran1#; Mitchell Weigand3; Stefano Ciannella1; Xian Wu3; Jacob Strayer3; Hyeon Choe3; Jeffrey J Chalmers3; Jenifer Gomez-Pastora1*
1Department of Chemical Engineering, Texas Tech University, USA
2Departamento de Ingenierías Química y Biomolecular, Universidad de Cantabria, Spain
3William G. Lowrie Department of Chemical and Biomolecular Engineering, The Ohio State University, USA
*Corresponding author: Jenifer Gomez-Pastora Department of Chemical Engineering, Texas Tech University, Lubbock 79409, TX, USA. Tel: 806 742 3553 Email: jenifer.gomez@ttu.edu
#These authors have equally contributed to this article
Received: April 29, 2024 Accepted: May 16, 2024 Published: May 23, 2024
Abstract
The cold storage of Red Blood Cells (RBCs) in preservative solutions during prolonged periods causes the deterioration of the RBC’s quality and function. Such storage lesion has crucial implications for the further use of stored RBC units, thus, developing storage solutions that enhance RBC preservation is of utmost importance. Progress in that field requires elucidating how the storage conditions impact the maintenance of RBC quality. In this work, we investigate the effect of inducing anaerobic conditions and modifying the pH, both individually and simultaneously, on the storage of RBCs. The Additive Solution-3 (AS-3) was selected for this analysis and the commercial enzyme EC-Oxyrase® was used for the first time to deoxygenate the storage buffer. RBC quality was assessed in terms of hemolysis (via RBC counts, Mean Corpuscular Hemoglobin (MCH), and intracellular and free hemoglobin concentration), Mean Corpuscular Volume (MCV), average diameter, size distribution (including potential microvesiculation), and cell morphology, which were analyzed biweekly for 42 days. Our results reveal that RBC lesion is alleviated when the cells are stored at higher pH values, whereas the presence of EC-Oxyrase® seems to have a significant effect on the stored RBC units only at low pH conditions. The insights gained from this preliminary study may serve as a basis for significant advancements in developing novel preservative solutions for RBC storage.
Keywords: Red Blood Cells (RBCs); Storage lesion; Storage solution; AS-3; pH; Anaerobic storage
Introduction
Transfusion of Red Blood cells (RBCs) has become a lifesaving procedure for treating multiple medical conditions [1–3]. RBCs to be used in transfusion therapy are cold stored in a preservative solution, which improves their shelf life [2–4]. Several preservative solutions have been formulated for enhancing RBC storage [5,6]; in the United States, the Food and Drug Administration (FDA) has approved the Additive Solutions (AS) AS-1, AS-3, and AS-5 [7–11]. Despite the availability of several preservative solutions, it has been reported that none of them exhibits outstanding advantages over the others regarding the maintenance of RBC quality during storage [5,12]. Prolonged RBC storage leads to the progressive accumulation of detrimental changes in the cells, which have been collectively termed “RBC storage lesion” and are mainly caused by oxidative stress and defective Adenosine Triphosphate (ATP) [2,3,5,13–15]. From all these alterations, hemolysis, which involves the release of Hemoglobin (Hb) and microvesicles into the suspending fluid, is understood as a distinct marker of RBC storage lesion [16–19]. The abovementioned age-related changes may have negative implications for transfusion recipients, thus, the FDA limits the storage period of RBC units to 42 days [3,13,20]. Extending the shelf-life of stored RBCs is of paramount importance for the logistics of blood banks and to maintain a reliable supply of RBC units at hospitals, since it makes possible the planning for seasonal shortages and reducing outdates [21,22]. Additionally, maintaining the quality of RBCs during long-term storage is key to guarantee their successful application for transfusion medicine [12,23]. Thus, novel storage strategies may result in a more effective use of the finite available supply of RBCs units. Storage under anaerobic conditions and at high pH values have been proposed as an attractive strategy [13,21,24–26]. In this work, the effects of pH and the presence of the commercial enzyme EC-Oxyrase® (Oxyrase from now on) to induce anaerobic conditions on the storage of RBCs are investigated for the first time. With addition of a proton donor, the enzyme Oxyrase consumes Dissolved Oxygen (DO) and converts it to water in media and deoxygenates the media without airtight containers or flushing the solution with inert gases [27]. This enzyme has been used before for deoxygenating the Hb contained in healthy and sickle RBCs and has demonstrated to be as effective as conventional deoxygenation methods [27]. AS-3 was selected as the preservative solution for testing the different storage conditions. Several components of the stored RBCs were assessed biweekly for 42 days: intracellular and cell-free Hb concentration as well as RBC counts and Mean Corpuscular Hemoglobin (MCH) as indicative of hemolysis, RBC size distribution to study the Mean Corpuscular Volume (MCV), average RBC size and potential microvesicle production, and RBC morphology via microscopy. The insights derived from this preliminary research may provide a basis for moving forward in developing preservative solutions for RBC storage with improved performance.
Materials and Methods
Sample Collection and Preparation
Whole blood (35 ± 5 mL) from a total of 8 healthy volunteer donors was collected in Ethylenediaminetetraacetic Acid (EDTA) tubes upon informed consent according to the Institutional Review Board (IRB)-approved protocols of the Texas Tech University Health Science Center and The Ohio State University (Protocol numbers IRB: L22-L274 and 2018H0268, respectively). RBCs were stored in AS-3 at different pH (standard, pH=5.8; acidic, pH=4.5; and basic, pH=8.5) conditions in the presence or absence of oxygen. Oxyrase was used to deoxygenate the storage buffer at two concentrations (2.5% v/v and 5% v/v). To change the pH of the media, we employed hydrochloric acid and sodium hydroxide, and the pH was measured on several samples during storage. Sample deoxygenation was proved by monitoring the evolution of the DO in some samples over the course of the storage period.
Blood samples were first washed via centrifugation (2,000 x g for 5 min) with the appropriate AS-3 storage solution (standard, acidic, or basic, without Oxyrase), to obtain the RBCs from the other blood components. The samples were stored in the AS-3 solutions without or with 2.5% v/v or 5% v/v Oxyrase in 2 mL tubes at 4°C. Analyses were performed for 5 donor samples on a biweekly basis (at 0, 14, 28, and 42 days from the donation date). Additionally, the samples from 3 donors were used to monitor pH and DO changes during storage.
Sample Analysis
Intracellular and free Hb levels were calculated by measuring the absorbance of the samples using the Agilent Cary 60 UV-Vis spectrophotometer (Agilent Technologies Inc.). Intracellular Hb was measured by washing the cells with Phosphate Buffered Saline (PBS) and lysing the cells with Deionized water (DI) to release the Hb, whereas the free Hb was measured directly from the media. All samples were diluted further in DI when required so that the absorbance around the Q bands (560 nm and 577 nm) lied in the 0.1-1 range. Each sample was tested twice or thrice to calculate the concentration of different Hb species (i.e., Oxyhemoglobin (oxy-Hb) and Methemoglobin (met-Hb)) according to the equations reported by Winterbourn [28]. To measure deoxyhemoglobin (deoxy-Hb), we converted the deoxygenated Hb into met-Hb with sodium nitrite for determining the free Hb of Oxyrase-containing samples, whereas oxy-Hb was measured in the rest of the samples [27]. The presence of oxy-Hb and met-Hb in the samples was verified by assessing the shape of their absorption spectra [27]. We also measured the Hb concentration of the original blood samples (day 0), which was on average around 14 g/dL for all donors. The sample’s cell volume, concentration, and size distribution, as well as the potential formation of microvesicles, were determined in an automated cell counter, B43905 Multisizer 4e Coulter Counter (CC, Beckman Coulter, CA). Finally, to assess the cell morphology and shape during storage, we took pictures of individual cells using a portable microscope camera (AM73515MT8A, Dino-Lite, Torrance, CA).
The statistical significance of the effects of pH and Oxyrase content on different RBC parameters (Hb levels, RBC average diameter, RBC count, MCH, etc.) was assessed through analysis of variance (ANOVA) in JMP® Pro 17.0.0 statistical software (Accessed on 11/01/2023, https://www.jmp.com/en_us/software/predictive-analytics-software.html). The variables pH and Oxyrase were treated as categorical factors at levels acidic, basic, and standard, and 0% v/v, 2.5% v/v, and 5% v/v, respectively, while the response variables were treated as numerical continuous. The dependent variables were tested for normality using Shapiro-Wilk’s W test for normal distribution goodness-of-fit and Levene’s test for equality of variances to ensure that ANOVA assumptions were satisfied. Post-hoc comparisons between groups were conducted using Tukey’s test to identify which combinations of pH and Oxyrase significantly differ from each other at a confidence level a=0.05. The probability values (p-values) for each factor and interactions terms were reported for several RBC parameters.
Results and Discussion
The impact of pH and/or aerobic/anaerobic states on the RBC storage lesion is comprehensively analyzed in this section, first individually, and subsequently, simultaneously. It should be noted that, for all the conditions presented in the following, the impact of storage time on all RBC parameters was statistically significant (p-values ranging from 0.01 to 0.04), indicating a consistent progression of the storage lesion over the 6-week storage period.
Effect of pH
Figure 1a illustrates the evolution of the intracellular Hb concentration (normalized, using the total Hb value of the sample at time 0) for the three pH values over time. The intracellular Hb concentration was decreased, on average for all donor samples, by 22% and 12% in six weeks when RBCs were stored in the standard and basic AS-3, respectively. Our measurements on the free Hb concentration, presented in Figure 1b, revealed that small free Hb concentration values were obtained for the standard and basic solutions. These values agree well with the outcomes reported by Chalmers et al. [29], that revealed that the ex vivo storage of human RBCs leads to, on average, a loss of 17% of their Hb after 42 days. When comparing the free Hb concentration at week 6 for the standard and basic solutions, we observed that the free Hb content was lower (approximately 50% lower) for higher pH values of the storage solution. Nevertheless, the maximum concentration of free Hb for both pH conditions (standard and basic) was below 0.1 g/dL during the whole storage period (< 10% of the total Hb present in the sample in week 0). In contrast to these solutions, when acidic AS-3 was used for storing RBCs, free Hb was observed in week 0 and it sharply increased in the first two storage weeks, as seen in Figure 1b. The highest free Hb concentration reported for the acidic AS-3 (0.26 g/dL on average for all donors) was one order of magnitude higher than that detected for the standard and basic AS-3. The observed trend for the free Hb concentration was consistent with the steeply decrease to zero of the intracellular Hb content for acidic AS-3 in the second storage week as seen in Figure 1a. Furthermore, the extracellular oxy-Hb concentration of the samples in the acidic solution after week 2 declined, even though the intracellular Hb concentration was zero; this suggests the potential transformation of oxy-Hb to other species (oxidized Hb) that were not measured spectrophotometrically.
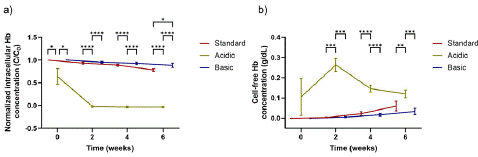
Figure 1: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
Additionally, our statistical analysis provided further insight into the impact of pH on the Hb loss over the storage duration. Our results revealed that the three pH conditions presented a significant effect on all response variables (intracellular Hb, free Hb concentration). Tukey’s post-hoc test further clarified the effect and differences between these conditions. For the intracellular Hb concentration, the acidic AS-3 resulted in significantly lower levels than the basic or standard conditions. Moreover, the acidic condition generated significantly higher levels of free Hb concentration than the basic or standard AS-3, as presented in Figure 1.
The analysis of MCH sheds light on how the pH of the storage solutions affects single RBC Hb levels. Figure 2a, which depicts the MCH distribution of the samples, reveals that standard and basic solutions maintained considerably stable MCH values. Particularly, the MCH of RBCs stored in standard and basic AS-3 was around 24.6 pg and 25.7 pg, respectively, throughout the six-week storage period. Conversely, the MCH of RBCs preserved in acidic AS-3 steeply decreased to zero in the second week of storage, which confirms the low Hb content accumulated per cell when using acidic solutions as demonstrated above. On the other hand, the effect of the pH of the preservative solution on the volume and size of RBCs was assessed using our cell counter. The average MCV, presented in Figure 2b, remained almost stable during the six weeks (variation of less than 15%) for all solutions and around the normal values reported for healthy donors (70-100 fL) when measured in our cell counter [30-32]. However, we observed higher MCV values for the acidic conditions, exhibiting approximately 20% higher MCV for all donors, even at day 0.
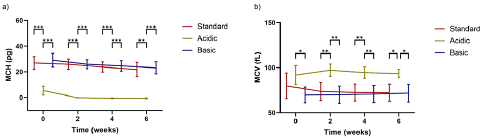
Figure 2: a) Mean MCH and b) mean MCV of RBCs when stored in acidic, standard, and basic AS-3 solutions. Significant differences were calculated using ANOVA followed by a Tukey’s post hoc test: *p<0.05; **p<0.01; ***p<0.001. Error bars indicate standard deviation.
The reduction in the number of cells for the acidic pH samples was verified by comparing the cell count data presented in Figure 3 for a representative donor. For the acidic AS-3, there was a 64.28% reduction in the cell concentration during the storage period (on average for all donors). Also, the subtle right shift of the histogram suggests an increase in cell diameter, and hence, volume, for low pH values. Indeed, the mean diameter rose 43% to 7.76 μm by week 6, compared to the initial 5.77 μm value for this specific acidic sample. Additionally, based on the shape of the histograms, we could not detect or determine the potential formation of microvesicles in our samples. Nevertheless, the slight increase in RBC count during the storage period for the standard and basic solutions suggests that a small amount of microvesicles might be forming during storage in these solutions, small enough to not significantly affect the average size of the cells. Moreover, the statistical analysis showed significant effects of pH on both RBC size and count, with p-values<0.05 for both. Specifically, Tukey’s test showed that acidic conditions induced considerably greater declines in RBC count coupled with larger increases in the cell’s mean diameter compared to basic or standard AS-3 throughout the 6-week storage duration.
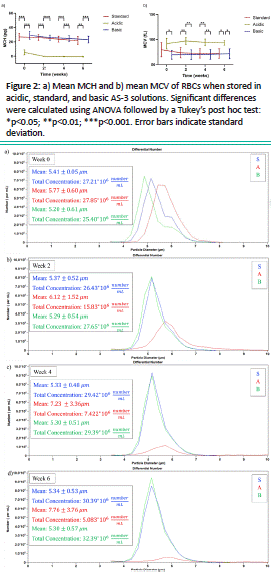
Figure 3: RBC size and concentration distributions of a representative sample when stored in standard, acidic, and basic solutions at: a) week 0, b) week 2, c) week 4, d) week 6. The sample was diluted 1/10 before analysis.
Finally, the morphology of the RBCs was inspected via microscopy (Figure 4), and we could detect substantial cell aggregates for the acidic conditions at week 6, highlighting the damaging effects of the acidic solution on RBC morphology over the 6-week timeframe. On the other hand, no notable differences were observed in the RBC morphology for RBCs stored in basic or standard conditions. Thus, it can be determined that the storage lesion was significantly affected by the pH of the solution. Specifically, the cell concentration dramatically dropped nearly 60% in just the first 2 weeks of storage under acidic conditions. We hypothesize that when the RBCs are contacted with the acidic AS-3, they capture water from the solution and swell; increasing the MCV and leading to the rupture of the cell membrane, releasing Hb to the media. Conversely, the standard and basic AS-3 solutions better enabled the preservation of RBCs, where the cell concentration and size/volume were maintained through the 6-week storage period, the free-Hb concentration did not raise much and the intracellular Hb concentration did not decrease more than 15% of its initial value, especially for the basic AS-3.
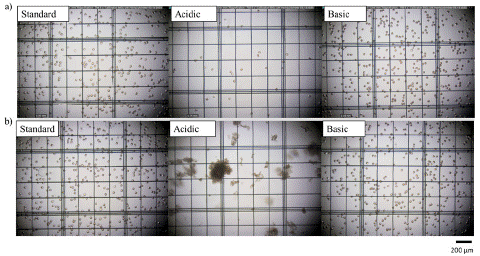
Figure 4: Microscope pictures of RBCs stored in standard, acidic and basic AS-3 in a) week 0 and b) week 6, respectively. The scale bar at the bottom of the figure is 200 μm.
Effect of the Anaerobic Conditions using Oxyrase
The effect of inducing anaerobic conditions enzymatically using different Oxyrase concentrations (2.5% v/v and 5% v/v in standard AS-3) on the intracellular and free Hb is explored in Figure 5. The presence of the enzyme Oxyrase appeared to be detrimental for the RBC storage; the RBC samples stored in anaerobic conditions reported higher cell-free Hb concentrations and lower intracellular Hb concentrations than the aerobic samples. Additionally, and unlike what was observed for the aerobic sample, free Hb levels (Figure 5b) were present in the anaerobic samples from week 0, and the final value of the free Hb concentration for 2.5% and 5% v/v Oxyrase samples was around twice the value observed for 0% Oxyrase samples. Normalized intracellular Hb concentration, presented in Figure 5a, was reduced during storage, on average for all donors, by 22%, 25%, and 19% for the 0% v/v Oxyrase, 2.5% v/v Oxyrase and 5% v/v Oxyrase, respectively. Interestingly, the solution with a higher content of Oxyrase (5% v/v) reported more similar intracellular Hb concentrations to the aerobic solution (no Oxyrase) than the storage solutions with lower concentrations of Oxyrase (2.5% v/v). The ANOVA analysis found that Oxyrase levels had a significant effect on both free Hb and intracellular Hb concentrations, with p-values < 0.0001. Further analysis performed using Tukey’s test showed that free Hb concentration increased significantly with each Oxyrase level addition from 0% v/v to 2.5% v/v to 5% v/v. For intracellular Hb concentrations, storage solutions with 0% Oxyrase had slightly higher levels than the solution containing a 2.5% v/v of Oxyrase, but this was not significantly different from the solution with a concentration of 5% v/v. Thus, while the presence of Oxyrase led to an increased Hb release from the cells over the storage duration, intracellular Hb preservation effects were more variable.
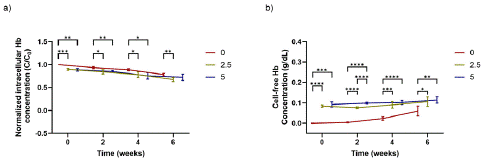
Figure 5: a) Mean intracellular Hb concentration (dimensionless), and b) mean cell-free Hb concentration (g/dL) for donor samples stored in AS-3 with different Oxyrase concentrations. C0 was considered to be the total (free and intracellular) Hb concentration in the samples in week 0. Significant differences were calculated using ANOVA followed by a Tukey’s post hoc test: *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. The legend contains a number indicating the concentration of Oxyrase in % v/v. Error bars indicate standard deviation.
According to Figure 6a, the MCH values of all the samples (with and without Oxyrase) decreased starting from week 2 throughout the storage period. At week 0, the MCH was highest for the 5% Oxyrase samples (31.40 pg) and lowest for the 0% Oxyrase samples (26.90 pg). However, by week 2, the MCH for the 2.5% and 5% Oxyrase samples had sharply decreased to an average of 23.84 pg and 24.81 pg, respectively, and continued to decline until the end of storage. On the other hand, the MCV was similar for the three preservative solutions analyzed in this subsection and these MCV values remained almost stable during the six weeks of storage, as noticed from Figure 6b.
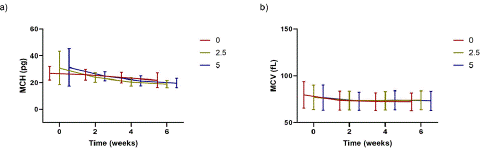
Figure 6: a) Mean MCH and b) mean MCV of RBCs when stored in standard AS-3 without and with 2.5% v/v and 5% v/v Oxyrase. Statistical analysis using ANOVA and Tukey’s post hoc test revealed no significant differences among the groups (p>0.05). The legend contains a number indicating the concentration of Oxyrase in % v/v. Error bars indicate standard deviation.
These trends are further examined in Figure 7, where the size and concentration distributions for the samples are presented for a representative donor. For the majority of the samples, the mean diameter did not significantly change during the storage period for both the aerobic and anaerobic samples. On the other hand, cell count across all samples increased by 3.56%, 16.1%, and 20.9% during storage for the samples without Oxyrase, 2.5% v/v Oxyrase, and 5% v/v Oxyrase, respectively. This increment suggests the formation of microvesicles during storage.

Figure 7: RBC size and concentration distributions of a representative sample when stored in standard AS-3 without and with 2.5%v/v and 5%v/v Oxyrase: a) week 0, b) week 2, c) week 4, d) week 6.
In contrast to the above explanation, the statistical analysis showed that Oxyrase had no significant impact on the RBC size or count over the storage duration. Tukey’s test confirmed that there was no significant difference in either the RBC count or size between the different Oxyrase levels. This finding could be due to the fact that Oxyrase presents better activity when dissolved at high pH values and temperatures (above 8 and 55°C, respectively), whereas the samples examined here had lower pH values and extremely colder temperatures [27]. Direct microscopic analysis (Figure 8) provides evidence of the absence of significant alterations when Oxyrase was added to the solution.
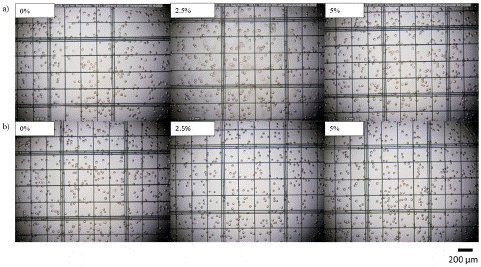
Figure 8: Microscope images of RBCs stored in standard AS-3 without and with 2.5%v/v and 5%v/v Oxyrase in a) week 0 and b) week 6, respectively.
Coupled Effect of pH and Anaerobic Conditions
The coupled effect of Oxyrase and pH was explored first by analyzing the change in the intracellular and cell-free Hb concentration over the storage time, presented in Figure 9. When storing RBCs in basic AS-3, cell-free Hb was hardly detected during the whole storage period regardless of the Oxyrase concentration. Therefore, Hb was mainly conserved intracellularly when basic AS-3 solutions were used as noticed from Figure 9a, especially for the higher Oxyrase concentration of 5% v/v. The favorable behavior that basic 2.5% v/v Oxyrase and 5% v/v Oxyrase solutions exhibited contrasted with the high hemolysis level induced by the acidic ones. A sharp rise in the cell-free Hb concentration was observed for the acidic solutions in the first two storage weeks (Figure 9b), and the intracellular Hb concentration decreased to zero regardless of the Oxyrase concentration. The statistical analysis further confirmed the significant coupled effects of pH and Oxyrase on both cell-free and intracellular Hb concentrations. All pairings were significant at the 2-, 4-, and 6-week timepoints. Interestingly, for intracellular Hb at week 0, the basic 2.5% v/v Oxyrase pairing was significant (adjusted p = 0.0149), while the basic 5% v/v Oxyrase pairing was not significant (adjusted p = 0.2621), despite being relatively close to the significance threshold.
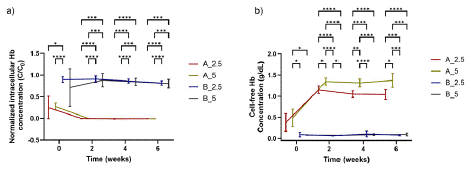
Figure 9: Effect of pH and Oxyrase concentration on a) intracellular Hb concentration (dimensionless), and b) cell-free Hb concentration (g/dL) for different storage solutions and for all donors. C0 was considered to be the total (free and intracellular) Hb concentration at week 0. Significant differences were calculated using ANOVA followed by a Tukey’s post hoc test: *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. The legend contains a letter and a number indicating the pH of the solution (A: acidic; B: basic) and the number the concentration of Oxyrase in % v/v. Error bars indicate standard deviation.
Figure 10a shows that a significant difference in the MCH was not noticed for both Oxyrase concentrations (when the pH was kept constant). However, while the MCH values for samples stored in basic solutions with 2.5% v/v and 5% v/v Oxyrase remained relatively stable throughout the six weeks of storage, at approximately 29.70 pg and 28.76 pg, respectively, the MCH values for samples stored in acidic solutions decreased significantly during the first two weeks reaching a value of 0 in 14 days. On the other hand, the MCV reported for the acidic and basic storage solutions (Figure 10b) was comparable for both levels of Oxyrase and these values hardly varied (below 5%) during the six weeks of storage. For acidic solutions, a slight but appreciable maximum in the MCV was noticed in the second week for all donors. We hypothesize that when RBCs are stored in acidic AS-3, they swell and their volume increases after 14 days of storage, which leads to the damage of the RBC membrane and ultimately to their death, which can be corroborated by looking at the RBC count data.
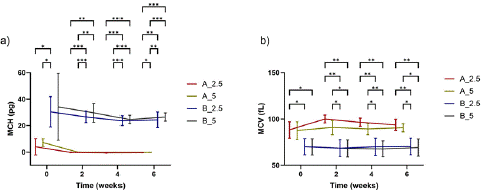
Figure 10: a) Mean MCH and b) mean MCV of RBCs when stored in acidic and basic AS-3 solutions with 2.5% v/v and 5% v/v Oxyrase. Significant differences were calculated using ANOVA followed by a Tukey’s post hoc test: *p<0.05; **p<0.01; ***p<0.001. The legend contains a letter and a number indicating the pH of the solution (A: acidic; B: basic) and the number the concentration of Oxyrase in % v/v. Error bars indicate standard deviation.
The cell size, volume, and RBC count quantified through the storage period (Figure 11) and microscopy images (Figure 12) revealed that the acidic samples exhibited an increase in the cell size and a decrease in the cell count. The cell diameter within all samples increased from 5.42 to 6.21 μm for the acidic solutions, reflecting cellular swelling and death due to acid stress. Nevertheless, for the acidic solutions, the RBC count was improved significantly when higher Oxyrase concentrations (of 5% v/v) were used, as can be perceived by inspecting the histograms reported in Figure 11. Indeed, for low pH values, RBC count throughout all donor samples decreased 22.7% and 13.4% during the storage period for 2.5% v/v and 5% v/v Oxyrase, respectively. Moreover, the average count for all donors under basic solutions followed a similar trend, as the RBC count was maintained for the 5% v/v Oxyrase solution whereas it decreased by 4.78% for the 2.5% v/v Oxyrase. For the basic solutions, the average diameter remained around 5 μm for both Oxyrase concentrations during the whole storage period. Thus, cell concentration seems to be higher after increasing the Oxyrase concentrations in both acidic and basic solutions. Finally, inspection of the size distribution histograms (Figure 11) revealed the presence of microvesicles at the end of the storage period, specifically for acidic samples, as a small amount of microparticles smaller than 4 μm were present in week 6, but not in previous time measurements. Statistical analysis confirmed that all coupled effects of pH and Oxyrase were significant for the cell size except 2.5% v/v Oxyrase at all pH conditions and basic pH at 5% v/v Oxyrase. This means that there is no sufficient evidence to assert that 2.5% v/v Oxyrase, either alone or coupled to pH, caused a significant effect on cell size. Regarding RBC count, there is sufficient evidence that leads us to conclude that only 5% v/v Oxyrase at acidic conditions caused a significant effect (p-value<0.05) on RBC concentration.
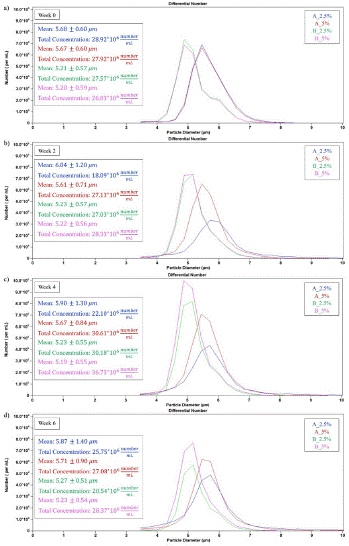
Figure 11: Impact of pH on diameter and RBC count for a sample stored in acidic and basic solutions with 2.5% v/v and 5% v/v Oxyrase: a) week 0, b) week 2, c) week 4, and d) week 6.

Figure 12: Microscope pictures of RBCs in a), b) acidic solution 2.5% v/v and 5% v/v in week 0 and week 6; c), d) basic solution with 2.5% v/v and 5% v/v in week 0 and week 6.
Conclusions
Enhancing prolonged RBC storage to achieve a successful and efficient transfusion therapy has received a great deal of interest. In this work, we have preliminarily demonstrated the effect of inducing anaerobic conditions using the enzyme Oxyrase and modifying the pH of the preservative solution, both individually and simultaneously, on several RBC parameters. Our results revealed that RBC quality is markedly affected by the pH of the storage solution. We found that the storage of RBCs in basic AS-3 resulted in low hemolysis levels, small variations in RBC diameter, and the conservation of MCV values during the storage time. Conversely, the acidic AS-3 solution reported high hemolysis levels, an increase in the RBC diameter, and a decrease in the cell count. On the other hand, the analysis of the individual effect of the anaerobic storage conditions did reveal a slightly increase in the concentration of free Hb for the samples with Oxyrase. Finally, the coupled effect of the anaerobic storage and the pH reported that high Oxyrase concentration on acidic solutions might have a beneficial effect in maintaining RBC counts and promoting preservation of RBCs even though the addition of Oxyrase did not prevent hemolysis. Hence, while alkalinizing the preservative solution AS-3 results in the maintenance of RBC quality compared with their acidification, the induction of anaerobic conditions by adding Oxyrase does not have such an important effect on the alleviation of the storage lesion. Although the current study has yielded encouraging findings that may preliminarily guide the development of preservative solutions, further research is required to obtain a comprehensive understanding of RBC lesion in different storage media.
Author Statements
Acknowledgements
This research was funded by Texas Tech University (HEF New Faculty Startup, NRUF Startup, and Core Research Support Fund) and Cristina González-Fernández thanks the Spanish Ministry of Universities for the Margarita Salas postdoctoral fellowship (grants for the requalification of the Spanish university system for 2021-2023, University of Cantabria), funded by the European Union-Next Generation EU.
References
- Franchini M, Marano G, Mengoli C, Pupella S, Vaglio S, Muñoz M, et al. Red blood cell transfusion policy: A critical literature review. Blood Transfus. 2017; 15: 307–317.
- Barshtein G, Arbell D, Livshits L, Gural A. Is it possible to reverse the storage-induced lesion of red blood cells? Front. Physiol. 2018; 9: 914.
- Sparrow RL. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus. 2010; 8: s26–s30.
- García-Roa M, Vicente-Ayuso MDC, Bobes AM, Pedraza AC, González-Fernández A, Martín MP, et al. Red blood cell storage time and transfusion: Current practice, concerns and future perspectives. Blood Transfus. 2017; 15: 222–231.
- D’Amici GM, Mirasole C, D’Alessandro A, Yoshida T, Dumont LJ, Zolla L. Red blood cell storage in SAGM and AS3: A comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus. 2012; 10: s46–s54.
- Hess JR. An update on solutions for red cell storage. Vox Sang. 2006; 91: 13–19.
- Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion. 2011; 51: 1574–1579.
- Sparrow RL, Sran A, Healey G, Veale MF, Norris PJ. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: A paired study. Transfusion. 2014; 54: 560–568.
- Valeri CR, Ragno G. The effects of preserved red blood cells on the severe adverse events observed in patients infused with hemoglobin based oxygen carriers. Artif Cells, Blood Substitutes, Biotechnol. 2008; 36: 3–18.
- Bohoněk M, Petráš M, Turek I, Urbanová J, Hrádek T, Chmátal P, et al. Quality evaluation of frozen apheresis red blood cell storage with 21-day postthaw storage in additive solution 3 and saline-adenine-glucose-mannitol: Biochemical and chromium-51 recovery measures. Transfusion. 2010; 50: 1007–1013.
- Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, et al. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012; 116: 637–647.
- Adams F, Bellairs G, Bird AR, Oguntibeju OO. Biochemical storage lesions occurring in nonirradiated and irradiated red blood cells: A brief review. Biomed Res Int. 2015; 2015: 968302.
- Chang AL, Kim Y, Seitz AP, Schuster RM, Pritts TA. pH modulation ameliorates the red blood cell storage lesion in a murine model of transfusion. J Surg Res. 2017; 212: 54–59.
- D’Alessandro A, Nemkov T, Hansen KC, Szczepiorkowski ZM, Dumont LJ. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: A metabolomics approach. Transfusion. 2015; 55: 2955–2966.
- Tsoi WC. Advances in blood storage bags and preservative solutions. ISBT Sci Ser. 2016; 11: 49–54.
- Sawant RB, Jathar SK, Rajadhyaksha SB, Kadam PT. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007; 1: 47–51.
- Hess JR, Sparrow RL, Van Der Meer PF, Acker JP, Cardigan RA, Devine DV. Red blood cell hemolysis during blood bank storage: Using national quality management data to answer basic scientific questions. Transfusion. 2009; 49: 2599–2603.
- Shin DA, Lee JC, Shin H, Cho YJ, Kim HC. Point-of-care testing of plasma free hemoglobin and hematocrit for mechanical circulatory support. Sci Rep. 2021; 11: 3788.
- Merle NS, Grunenwald A, Rajaratnam H, Gnemmi V, Frimat M, Figueres ML, et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI insight. 2018; 3: e96910.
- Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: Role of red blood cell breakdown. Transfusion. 2011; 51: 844–851.
- Yoshida T, AuBuchon JP, Dumont LJ, Gorham JD, Gifford SC, Foster KY, et al. The effects of additive solution pH and metabolic rejuvenation on anaerobic storage of red cells. Transfusion. 2008; 48: 2096–2105.
- Weigand M, Gomez-Pastora J, Palmer A, Zborowski M, Desai P, Chalmers J. Continuous-flow magnetic fractionation of red blood cells based on hemoglobin content and oxygen saturation— Clinical blood supply implications and sickle Cell anemia treatment. Processes. 2022; 10: 927.
- Almoshary M, Al Mussaed E, Arab-Din M. Biochemical profile changes in stored donor blood for transfusion. Pakistan J. Med. Sci. 2019, 35: 1697–1700.
- Yoshida T, AuBuchon JP, Tryzelaar L, Foster KY, Bitensky MW. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007; 92: 22–31.
- Dumont LJ, Yoshida T, AuBuchon JP. Anaerobic storage of red blood cells in a novel additive solution improves in vivo recovery. Transfusion. 2009; 49: 458–464.
- D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol. Biosyst. 2013; 9: 1196–1209.
- Weigand MRH, Gómez-Pastora J, Kim J, Kurek MT, Hickey RJ, Irwin DC, et al. Magnetophoretic and spectral characterization of oxyhemoglobin and deoxyhemoglobin: Chemical versus enzymatic processes. PLoS ONE. 2021; 16: e0257061.
- Winterbourn CC. Oxidative reactions of hemoglobin. Methods Enzymol. 1990; 186: 265–272.
- Chalmers JJ, Jin X, Palmer AF, Yazer MH, Moore L, Amaya P, et al. Femtogram resolution of iron content on a per cell basis: Ex vivo storage of human red blood cells leads to loss of hemoglobin. Anal Chem. 2017; 89: 3702–3709.
- Gómez-Pastora J, Weigand M, Kim J, Palmer AF, Yazer M, Desai PC, et al. Potential of cell tracking velocimetry as an economical and portable hematology analyzer. Sci Rep. 2022; 12: 1692.
- Kim J, Gómez-Pastora J, Gilbert CJ, Weigand M, Walters NA, Reátegui E, et al. Quantification of the mean and distribution of hemoglobin content in normal human blood using cell tracking velocimetry. Anal. Chem. 2020; 92: 1956–1962.
- Kim J, Gómez-Pastora J, Weigand M, Potgieter M, Walters NA, Reátegui E, et al. A subpopulation of monocytes in normal hu