
Review Article
J Blood Disord. 2024; 11(1): 1087.
High Intensity Lipid Lowering Drugs Further Than Statins
Vyas A; Hajela K*
School of Life Sciences, Devi Ahilya Vishwavidyalya, Takshila Campus, Khandwa Road, Indore-452001 (M.P.), India
*Corresponding author: Hajela K School of Life Sciences, Devi Ahilya Vishwavidyalya, Indore, India. Tel: +919589860366 Email: hajelak@gmail.com
Received: April 09, 2024 Accepted: May 17, 2024 Published: May 24, 2024
Abstract
Lipid-lowering therapies are crucial in reducing the risk of Atherosclerotic Cardiovascular Disease (ASCVD), and statins are the primary drugs used for this purpose. However, statins come with some side effects such as muscle symptoms, liver dysfunction, renal insufficiency, eye conditions, and an increased risk of Type 2 diabetes mellitus. The risk of developing Type 2 diabetes mellitus is dependent on the dose and duration of statin use. Long-term use of statins (= 5 years) has been associated with a significant increase in the risk of diabetes. In this review, we will discuss several new therapies for lipid-lowering, including Ezetimibe, Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) inhibitors, Bempedoic acid, Lomitapide, Pemafibrate, and angiopoietin-like 3 inhibitor Evinacumab. In addition to durgs, there are several plant-based agents and dietary strategies such as plant sterols, soluble and dietary fiber, nuts, red yeast rice, and a plant-based diet that have shown promise in reducing cholesterol levels. Furthermore, supplementation with Bergamot has also shown benefits in managing cardio-metabolic risk in dyslipidemic subjects. These natural compounds and dietary approaches offer a complementary and natural way to manage cholesterol levels. It’s important to note that while these agents can contribute to lowering cholesterol levels, they are not meant to replace prescribed medications for individuals with high cholesterol.
Keywords: Dyslipidemia; Non-statin; PCSK9 inhibitors
Abbreviation: ACL: Adenosine triphosphate-Citrate Lyase; AMPK: Monophosphate-Activated Protein Kinase; ANGPTL3: Angiopoietin-Like 3; ApoB: Apolipoprotein B; ASCVD: Atherosclerotic Cardiovascular Disease; CV: Cardiovascular; DASH: Dietary Approaches to Stop Hypertension; EAS: The European Atherosclerosis Society; eGFR: Estimated Glomerular Filtration Rate; ELIPSE HoFH: Evinacumab Lipid Studies in Patients with Homozygous Familial Hypercholesterolemia; EMA: European Medicines Agency; ESC: European Society of Cardiology; FDA: Food and Drug Administration; GalNAc: N-acetylgalactosamine; HDL-C: High-Density Lipoprotein Cholesterol; HeFH: Heterozygous Familial Hypercholesterolemia; HMG-CoA: 3-Hydroxy-3-Methylglutaryl-Coenzyme A; HoFH: Homozygous Familial Hypercholesterolemia; hsCRP: High-Sensitivity C-Reactive Protein; IDL: Intermediate-Density Lipoproteins; I-ROSETTE: Ildong ROSuvastatin & ezETimibe for hypercholesTElolemia; LDL: Low Density Lipoprotein; LDL-C: Low Density Lipoprotein Cholesterol; LDLR: Low density lipoprotein receptor; MED: Mediterranean; MTP: microsomal triglyceride transfer protein; NPC1L1:Niemann-Pick C1-Like 1 protein; OBS: observational setting; PCSK9: Proprotein Convertase Subtilisin/kexin Type 9; PPARa: Peroxisome Proliferator-Activated Receptor Alpha; RCT: Randomized Controlled Trials; RISC : RNA-Induced Silencing Complex; siRNA: Small Interfering RNA; TG: Triglycerides; TRL: TG-Rich Lipoproteins; VLDL: Very Low Density Lipoprotein
Introduction
A metabolic disorder known as dyslipidemia causes blood Triglycerides (TG) and cholesterol levels to increase in the bloodstream. It is characterized by elevated Low-Density Lipoprotein Cholesterol (LDL-C), also known as hypercholesterolemia, and combined with low levels of high-density lipoprotein cholesterol (HDLC) and raised Triglycerides (TG), mainly in the form of TG-Rich Lipoproteins (TRL) like chylomicrons and Very-Low-Density Lipoprotein (VLDL). Cardiovascular diseases, including peripheral vascular disease, coronary heart disease, and cerebrovascular disease (stroke), are prevalent non-communicable diseases worldwide and are responsible for 31% of all deaths [1]. Elevated LDL-C is a significant risk factor for these diseases [2]. Also, the obesity epidemic and sedentary lifestyle that have exacerbated other lipid-related diseases, require aggressive lipid suppression medication and clinical follow-up to prevent plaque buildup and cardiovascular events. First-line treatment for hypercholesterolemia is using 3-Hydroxy-3-Methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitors, such as statins, for primary and secondary prevention. In the middle of the 1970s, statins were first developed as cholesterol-lowering medications. After being used for over four decades, statins are now among the drugs that are mostly prescribed all over the world, particularly for cardiovascular diseases. Statins decrease cholesterol synthesis in the liver by increasing LDLR expression. This leads to increased LDL uptake and decreased plasma levels of other ApoB-containing lipoproteins [3]. While statins are effective in lowering cholesterol and preventing cardiovascular events, they come with a risk of side effects, including muscle symptoms, liver dysfunction, renal insufficiency, eye conditions, and an increased risk of Type 2 diabetes mellitus [4]. The risk of new-onset Type 2 diabetes mellitus is time-varying and dose-dependent, with long-term statin use (= 5 years) realated with a statistically significant raise in the risk of diabetes. The diabetogenic effect was not statistically significant for pitavastatin, but atorvastatin and rosuvastatin showed the largest risks [5]. Statins have been associated with an increased risk of self-reported muscle symptoms, such as pain and weakness, which can affect patient compliance and quality of life [6]. Additionally, Patients with severely impaired liver function are also at risk due to the importance of hepatic excretion of all statins. Blinded placebo-controlled trials have not confirmed the existence of chronic myalgias or other pain disorders. A significant and reproducible rise in liver enzymes (alanine and aspartate aminotransferases) is observed in 1 to 3% of patients but actual liver damage may not occur [7]. Recent developments in lipid-lowering treatments have introduced promising options for managing dyslipidemia and reducing the risk of cardiovascular disease. These therapies beyond statins encompass a wide range of options, each with its unique mechanism of action and potential benefits. Evidence-based medications like angiopoietin-like 3 inhibitors, ATP-citrate lyase inhibitors, PCSK9 modulators, and microsomal triglyceride transfer protein inhibitors can effectively lower down lipids as mentioned in Table no. 1. This evidence-based review focuses on the clinical and benefit-risk evaluation of these non-statin drugs and also some plant-based compound for lowering lipids.
Features
Ezetimibe**
Bempedoic acid*
Evolocumab*
Alirocumab*
Inclisiran*
Lomitapidea*
Pemafibrate***
Evinacumab*
Class
Small molecule
Small molecule
Fully human mAb
Fully human mAb
SiRNA
Small molecule
Small molecule
Fully Human
mAbMarketing approval
2002(FDA)
2003 (EMA)2020
(FDA,
EMA)2015 (FDA,EMA)
2015 (FDA,EMA)
2020 (EMA)
2013 (EMA) 2012(FDA)
Not approved by FDA & EMA
2021 (FDA,EMA)
Target
NPC1L1
ACL
PCSK9
PCSK9
PCSK9
MTP
PPARa
ANGPTL3
Dosage
10 mg/ day
180mg/ day
Subcutaneous injection (140 mg every 2 weeks or 420 mg once monthly)
Subcutane-ous injection (75-150 mg once every 2weeks or 300 mg once every 4 weeks)
Subcutaneous injection (284 mg every3–6 months)
5mg/day (starting dose)
With titration to max 60mg/day0.1-0.4 mg twice / day
Intravenous infusion over 60 minutes (15mg/kg every 4 weeks)
LDL-C reduction from baseline
15-20%
15–23.5%
57–72%
47–61%
40–51%
45% (RCT)–60%(OBS)
7-10% LDL-C and 45% triglycerides
47%
Cardio vascular benefit
Yes
Yes
Yes
Yes
Not determined
Yes
Yes
Not determined
Table 1: Comparative pharmacological aspects of non-statin drugs [28,52,53].
Ezetimibe
Ezetimibe is a newer agent approved for the treatment of hyperlipidemia. Its mode of action has been found to be complementary to that of statins. Ezetimibe is a medication that helps to lower cholesterol by blocking a protein called Niemann-Pick C1-Like 1 (NPC1L1), which in turn prevents the absorption of dietary cholesterol. A meta-analysis of eight Randomized Controlled Trials (RCTs) showed that taking ezetimibe alone (10 mg/day) for 12 weeks can reduce LDL-C levels by an average of 18.58%. The study also found significant reductions in total cholesterol (13.49%) and triglycerides (8.6%) compared to those who received a placebo [8].
Additionally, ezetimibe is a safe and effective drug that lowers LDL-C, particularly in high-risk individuals, such as those with post-acute coronary syndrome [9]. Moreover, it is also proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, which prevents the degradation of LDL receptors in hepatocytes [10]. Furthermore, combining ezetimibe with rosuvastatin has been studied extensively to assess its efficacy and safety in treating patients with primary hypercholesterolemia. The combination therapy has been found to significantly lower LDL-C levels compared to rosuvastatin alone. The I-ROSETTE study investigated the effect of adding ezetimibe to rosuvastatin in 396 participants. The adjusted mean LDL-C level after 8 weeks of treatment increased by 57.0% from baseline in the ezetimibe/rosuvastatin group, which was superior to the total rosuvastatin groups [11]. Additionally, the combination therapy resulted in achieving the target LDL-C levels in 90.7% of participants, which was significantly higher than the 72.9% in the rosuvastatin monotherapy group [12].
Bempedoic Acid
Bempedoic acid, also known as Nexletol, inhibits ATP-Citrate Lyase (ACL) which is an enzyme involved in the pathway for de novo cholesterol synthesis [13]. Bempedoic acid also activates Adenosine Monophosphate-Activated Protein Kinase (AMPK), which in turn downregulates glucose-6-phosphatase and phosphoenolpyruvate carboxykinase. This mechanism may lower LDL-C in the liver, with upregulation of hepatic LDLR, thus increasing LDL-C clearance from the blood and also downregulates pro-inflammatory pathways [14,15]. It is noteworthy that Bempedoic acid is metabolized only in the liver and not in muscle, which could be advantageous for patients with statin intolerance. A Phase II trial, which included statin-intolerant patients and utilized PCSK9 inhibition, demonstrated a significant reduction in LDL-C levels of up to 24% with favorable tolerability [16,17]. In randomized controlled trials conducted on patients with ASCVD disease and/or heterozygous familial hypercholesterolemia who were already on the maximum possible dose of lipid-lowering therapy, the treatment of bempedoic acid was found to be effective. Compared to the placebo, it reduced the levels of LDL-C by 18%, non-HDL-C by 13.3%, apoB by 11.9%, and hsCRP by 21.5%. This study was called CLEARHarmony and it was conducted on a large number of patients [18].
When added to ezetimibe with or without additional lipid-lowering medication (CLEARSerenity), bempedoic acid 180 mg once daily decreased LDL-C by 23.6% and hsCRP by 25.4% in patients with a history of statin intolerance in requiring additional LDL-Clearing [19]. A convincing case for their combination emerged as it was found that ezetimibe and bempedoic acid decrease LDL-C through various pathways. A combination of fixed doses of bempedoic acid and ezetimibe was found to significantly reduce LDL-C levels by 38% when compared with a placebo in a Phase 3 double-blind trial, with a highly favorable safety profile [20]. In contrast to the impact of statins on blood sugar levels, bempedoic acid was associated with a lower risk of new-onset diabetes and hyperglycemia when compared to a placebo [21]. The finding might have to do with bempedoic acid's stimulation of AMPK, which lowers gluconeogenesis. Moreover, fatty acid production is inhibited by AMPK activation, statins do not have a similar effect [13].
Strategies Targeted towards Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9)
The discovery of proprotein convertase subtilisin/kexin9 (PCSK9) and the development of its inhibitors have opened up a new and exciting era in lipid management for many patients with residual cardiovascular risk, despite intensive statin therapy [22]. The PCSK9 protein acts as a chaperone to transport the LDL receptor to the lysosome. Lowering the levels of PCSK9 promotes the recycling of LDL receptors to the cell surface, which in turn helps in clearing LDL from the bloodstream as shown in Figure 1A. The discovery that lifelong low levels of LDL-C and reduced cardiovascular risk are associated with loss of function mutations in PCSK9, has led to the development of targeted therapies that inhibit PCSK9 [23]. PCSK9 inhibitors are a new class of drugs used to treat hypercholesterolemia in patients with intolerance or inadequate response to statins. They are also used for secondary prevention or in cases of familial hypercholesterolemia. The first strategy for lowering PCSK9 was based on monoclonal antibodies (mAbs) targeting the protein. Alirocumab and evolocumab are two of the medications that have undergone clinical trial examination and are presently available for purchase. They are both fully humanized mAbs that bind specifically to human PCSK9. Monoclonal antibodies against PCSK9 neutralize it outside cells, while gene expression strategies act inside cells (Figure 1B). Strategies to suppress PCSK9 are being developed, such as inhibiting function or interfering with expression. PCSK9-specific gene silencing by siRNA with the agent inclisiran has seen rapid translation to clinical use.
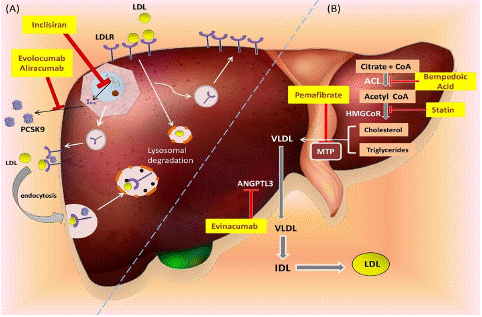
Figure 1: Targets and mechanisms of action of non-statin therapies for lipid lowering. A) The regulation of LDL particle clearance involves the binding of secreted Proprotein convertase subtilisin/kexin type 9 (PCSK9) to LDLR on the liver surface, leading to the degradation of LDL-LDLR-PCSK9 complex. This process reduces the cell surface LDLR. However, the presence of PCSK9 inhibitors such as monoclonal antibodies evolocumab and alirocumab, as well as siRNA agent Inclisiran, can increase the uptake of LDL cholesterol by the LDLR and recycle more LDLRs at the cell surface. B) New cholesterol-lowering treatments are being developed that focus on different aspects of lipid metabolism. Statins target the enzyme hydroxymethylglutaryl coenzyme A reductase (HMGCoR). The latest medicines aim at various facets of lipid metabolism, as shown in figure.
Evolocumab
Evolocumab is a monoclonal Immunoglobulin G2 (IgG2). It can be administered subcutaneously in doses of 140 mg or 420 mg. Administering this medication resulted in median peak blood concentrations within 3-5 days. The medication reduced plasma LDL-C levels by 53% to 75%. It can be used as a monotherapy or in conjunction with statin therapy. It can also be administered to patients with statin intolerance or patients with heterozygous familial hypercholesterolemia. Maximum suppression of circulating unbound PCSK9 occurred by four hours [24]. Evolocumab decreased plasma LDL-C levels by 31% in patients with homozygous familial hypercholesterolemia (HoFH) with dysfunctional LDLRs [4]. Additional PCSK9-Related Cardiovascular Outcomes Research the FOURIER study, which included patients with ASCVD and LDL-C levels =70 mg/dL or non-HDL-C levels =100 mg/dL on statin therapy, showed that evolocum ab decreased the risk of cardiovascular disease by 15% Hazard Ratio [HR], 0.85; 95% Confidence Interval [CI], 0.79 to 0.92) [25].
Alirocumab
IgG1 monoclonal antibody is known as alirocumab. The recommended initial dose is 75 mg subcutaneously once every two weeks or 300 mg monthly once every four weeks. The dosage may be changed to 150 mg administered every two weeks if the LDL-C response is inadequate [26]. When given as a monotherapy, in combination with statin therapy, or to individuals who have developed a statin intolerance, alirocumab decreased plasma LDL-C levels by 45% to 53%. Alirocumab lowered plasma LDL-C levels by 39%–58% in patients with HeFH and by 11.9%–34.3% in individuals with HoFH, depending on the patient's genotype. In the ODYSSEY OUTCOMES trial, which evaluated the effects of alirocumab on cardiovascular outcomes after acute coronary syndrome, patients with an acute coronary syndrome, plasma LDL-C =70 mg/dL, non-HDL-C =100 mg/dL, or apolipoprotein B (apo B) =80 mg/dL who had been on statin therapy observed a 15% reduction in cardiovascular risk (HR, 0.85; 95% CI, 0.78 to 0.93) [27].
Inclisiran
Inclisiran is a synthetic small interfering RNA (siRNA) that targets PCSK9. It is conjugated to triantennary N-acetylgalactosamine carbohydrates (GalNAc), which directs siRNA to the liver [28]. It functions within hepatocytes by attaching itself to the RNA-Induced Silencing Complex (RISC) and preventing PCSK9 mRNA translation. This lowers PCSK9 synthesis and its release into the extracellular environment. The Inclisiran treatment for low-density lipoprotein cholesterol (LDL-C) in patients with heterozygous familial hypercholesterolemia (HeFH) showed significant reductions in plasma LDL-C levels. In the ORION-9 trial, patients with HeFH and plasma LDL-C =100 mg/dL were treated with a 300-mg dose of inclisiran sodium. In the ORION-10 trial, patients with ASCVD reduced their LDL-C levels by 52.3%. The ORION-11 trial showed a 49.9% reduction in LDL-C levels. The ongoing phase-3 trial will further clarify the cardiovascular benefits of inclisiran [29]. Inclisiran was recently approved by the FDA (December 22, 2021) and the EMA (December 9, 2020) for the treatment of residual hypercholesterolemia in patients based on the existing clinical evidence with heart failure or cardiovascular disease, failed to achieve the desired LDL goal with maximally tolerated statin treatment [30,31]. These novel strategies may have advantages over antibody treatments, such as increased durability, more convenient dose schedules, and potentially lower costs if they are proven to decrease CV events in outcomes trials.
Lomitapidea
Lomitapidea is an FDA approved medication used to reduce cholesterol in patients with HoFH when used with other lipid-lowering drugs and a low-fat diet. The endoplasmic reticulum lumen contains Microsomal Triglyceride transfer Protein (MTP), which is immediately bound by lipitapide and inhibited. Suppressing MTP prevents the hepatocytes and enterocytes from forming lipoproteins containing apo-B, which lowers the production of VLDL and chylomicrons and, as a result, lowers plasma levels of LDL-C [32]. With dosages ranging from 5 to 60 mg per day, lomitapide was given in addition to statins. After 26 weeks, the mean LDL-C decrease was 50%, and after 78 weeks of treatment, it was 38% for the patients. The most prevalent adverse effects were hepatic steatosis (liver fat content raised from 1.0% at baseline to 8.6%), gastrointestinal problems (27 out of 29 participants were reduced with a low-fat diet), and raised alanine transaminase levels in 4 people (which resolved upon dose reduction) [33].
Pemafibrate
Pemafibrate, marketed as Parmodia, is the first selective peroxisome proliferator-activated receptor alpha (PPARa) modulator. It shows more than 2,500 times stronger PPARa activation compared with fenofibric acid, the active form of fenofibrate [34]. Pemafibrate and fenofibrate significantly decreased plasma TG levels by 46% and 39%, respectively, in a phase 3 comparative trial comprising Japanese patients whose plasma TG levels ranged from 150 to 500 mg/dL. In comparison to fenofibrate, pemafibrate has shown less adverse effects over the years. According to clinical trials, it decreases plasma triglyceride levels in patients from Europe by 54.4% and in Japanese patients by 50% [35]. Ten thousand patients with type 2 diabetes mellitus and plasma TG levels of 200 to 500 mg/dL while receiving statin therapy participated in a phase 3 cardiovascular trial that observed Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides [36]. Notably, Pemafibrate has demonstrated to improve liver function test values and is less likely to increase serum creatinine or decrease the Estimated Glomerular Filtration Rate (eGFR) in comparison to other available fibrates. Moreover, very few drug-drug interactions were noticed even when used simultaneously with statins [34]. Additionally, the use of pemafibrate was linked to an increased occurrence of adverse renal events and venous thromboembolism, which is consistent with findings from other trials. and suggest that among statin-treated patients, fibrates, including pemafibrate, cannot be recommended for cardiovascular risk reduction [36]. Pemafibrate has also been found to have dose-dependent beneficial effects on liver enzymes and to increase splanchnic glucose uptake. However, it does not seem to improve glucose metabolism. It is important to consider the potential benefits and risks of pemafibrate, especially in the context of individual patient characteristics and medical history [37].
Evinacumab
Evinacumab, available under the trade name Evkeeza, is a monoclonal antibody that inhibits circulating Angiopoietin-like 3 (ANGPTL3). It is an effective and safe treatment for homozygous and heterozygous Familial Hypercholesterolemia, resistant hypercholesterolemia, hypertriglyceridemia and homozygous familial hypercholesterolemia (HoFH) [38]. Evinacumab binds to ANGPTL3, allowing lipoprotein lipase (LPL) and endothelial lipase (EL) to enhance the clearance of Very Low-Density Lipoprotein (VLDL) remnants via remnant receptors in the liver, resulting in the decrease of LDL cholesterol levels [39]. In the ELIPSE HoFH trial, patients diagnosed with Homozygous Familial Hypercholesterolemia and having a plasma LDL-C level of 70 mg/dL or more while undergoing statin therapy, were administered evinacumab intravenously every four weeks at a dosage of 15 mg/kg body weight. As a result, they experienced a significant decrease of 49% in their plasma LDL-C levels and 50% reduction in plasma TG levels. Evinacumab reduced plasma LDL-C levels by 43.4% in patients with LDLR null-null variants, compared to a 16.2% increase in the placebo group. Adverse events were similar, including liver fat increase. In a phase 2 trial, it reduced plasma LDL-C levels by over 50% [40].
The Specific Components Found in Plants Play a Crucial Role in Controlling Lipid Levels
There is substantial evidence to suggest that specific foods, food groups, and overall dietary practices can improve dyslipidemia and reduce the risk of cardiovascular disease. The Mediterranean (MED) diet and Dietary Approaches to Stop Hypertension (DASH) diet, Nordic diet and Portfolio diet, and other plant-based dietary patterns have been shown to protect against cardiovascular disease risk and related risk factors, such as LDL-C. These foods, including fruits, legumes, nuts, whole grains, seeds and vegetables, are rich in unsaturated fatty acids, plant proteins, dietary fiber, phytonutrients, and vitamins [41]. They are low in saturated fats and energy density compared to animal sources. The beneficial effects of these plant-based foods have different mechanisms that influence cardiovascular disease development, such as dyslipidemia. Replacing saturated fats with unsaturated fatty acids, particularly vegetable oil PUFA, has been shown to lower LDL-C and lower the risk of cardiovascular disease. Dietary fiber, particularly viscous Soluble Fibers like beta-glucan, reduces cholesterol absorption and re-absorption, and produces short-chain fatty acids in the colon, which may affect hepatic cholesterol synthesis [42].
Various studies have investigated the effect of bergamot, a nutraceutical derived from Citrus bergamia, on human lipid parameters. The data from these studies revealed that 75% of them showed a significant decrease in LDL-C, triglycerides, and total cholesterol. The reduction in LDL-C ranged from 12.3% to 31.3%, in triglycerides from 7.6% to 40.8%, and in total cholesterol from 11.5% to 39.5%. Additionally, eight trials reported an increase in HDL-C after the intervention of bergamot. These studies suggest that bergamot has a dose-dependent effect and can potentially work synergistically when combined with statins [43].
The revised ESC/EAS guidelines for the management of dyslipidemias included for the first time a recommendation for plant sterols as part of lifestyle changes to reduce serum cholesterol levels by inhibiting intestinal cholesterol absorption [44]. These plant sterols are part of plant foods, mainly in unrefined vegetable oils, grains, nuts, and olive oil. A typical Western diet contains equal amounts (approximately 400 mg) of both plant sterols and cholesterol each day. It was found that Phytosterol-enriched low-fat milk and margarine results in a 10–15% LDL-C reduction [45], whereas fortified cereals reduce LDL-C by 5.4% [46]. To date, different food products enriched with phytosterols are available: milk, soy, and yogurt products; soy and fruit drinks; cereal; sausage, etc. However, not all plant-based dietary patterns are equally effective in lowering cardiovascular disease risk. Shifting to a more plant-based dietary pattern will not only improve cardiovascular health but also be more environmentally sustainable.
Also, small amount of statin like compound monacolin K, is found in nutritional supplement obtained from red yeast rice. In several randomized trials, it was demonstrated that there was a reduction of approximately 15% in LDL levels [47]. On March 24th, 2024, The Economics Times News agency Kyodo reported that the consumption of red yeast rice supplements (beni-koji) manufactured by Kobayashi Pharmaceutical Co. has been linked to two deaths and over a hundred hospital admissions. One of the deaths resulted from kidney illness caused by long-term use of beni-koji supplements. It is important to exercise caution when using this unregulated substance with a diverse product composition, as it was found to be nephrotoxic [48]. It's important to note that while these agents can contribute to lowering cholesterol levels, they are not meant to replace prescribed medications for individuals with high cholesterol. It is suggested to consult with a healthcare professional before making any significant changes to your diet or starting any new supplements or natural remedies.
Conclusions
In conclusion, lipid-lowering therapies play a crucial role in reducing the risk of Atherosclerotic Cardiovascular Disease (ASCVD). While statins are the primary drugs used for this purpose, they come with certain side effects and an increased risk of Type 2 diabetes mellitus, particularly with long-term use. This emphasizes the importance of exploring alternative therapies that can effectively lower cholesterol levels while minimizing adverse effects.
References
- Cardiovascular diseases (CVDs). 2020.
- Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. European heart journal. 2017; 38: 2459-72.
- Morofuji Y, Nakagawa S, Ujifuku K, Fujimoto T, Otsuka K, Niwa M, et al. Beyond lipid-lowering: effects of statins on cardiovascular and cerebrovascular diseases and cancer. Pharmaceuticals. 2022; 15: 151.
- Macedo AF, Taylor FC, Casas JP, Adler A, Prieto-Merino D, Ebrahim S. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC medicine. 2014; 12: 1-3.
- Na E, Cho S, Kim DJ, Choi J, Han E. Time-varying and dose-dependent effect of long-term statin use on risk of type 2 diabetes: a retrospective cohort study. Cardiovascular diabetology. 2020; 19: 1-1.
- Cai T, Abel L, Langford O, Monaghan G, Aronson JK, Stevens RJ, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. Bmj. 2021; 374: n1537.
- Brown WV. Safety of statins. Current opinion in lipidology. 2008; 19: 558-62.
- Pandor A, Ara RM, Tumur I, Wilkinson AJ, Paisley S, Duenas A, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009; 265: 568-580.
- Jad Al Danaf, Seth Shay Martin, Roger S Blumenthal. Ezetimibe: The Lower the LDL-C, the Better (Even for Total Cardiovascular Events). 2016.
- Lee J, Lee SH. Expanding the therapeutic landscape: ezetimibe as non-statin therapy for dyslipidemia. The Korean Journal of Internal Medicine. 2023; 38: 797-809.
- Vavlukis M, Vavlukis A. Adding ezetimibe to statin therapy: latest evidence and clinical implications. Drugs in context. 2018; 7: 212534.
- Chilbert MR, VanDuyn D, Salah S, Clark CM, Ma Q. Combination therapy of ezetimibe and rosuvastatin for dyslipidemia: current insights. Drug design, development and therapy. 2022; 16: 2177-86.
- Pinkosky SL, Filippov S, Srivastava RA, Hanselman JC, Bradshaw CD, Hurley TR, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. JLipidRes. 2013; 54: 134–151.
- Morrow MR, Batchuluun B, Wu J, Ahmadi E, Leroux JM, Mohammadi-Shemirani P, et al. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metabolism. 2022; 34: 919-36.
- Verberk SGS, Kuiper KL, Lauterbach MA, Latz E, Vanden Bossche J. Themulti faceted therapeutic value of targeting ATP-citrate lyase in atherosclerosis. Trends Mol Med. 2021; 27: 1095–1105.
- Thompson PD, MacDougall DE, Newton RS, Margulies JR, Hanselman JC, Orloff DG, et al. Treatment with ETC-1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. Journal of clinical lipidology. 2016; 10: 556-67.
- Ballantyne CM, Banach M, Mancini GJ, Lepor NE, Hanselman JC, Zhao X, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018; 277: 195-203.
- Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. New England Journal of Medicine. 2019; 380: 1022-32.
- Laufs U, Banach M, Mancini GJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. Journal of the American Heart Association. 2019; 8: e011662.
- Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. European journal of preventive cardiology. 2020; 27: 593-603.
- Bays HE, Banach M, Catapano AL, Duell PB, Gotto Jr AM, Laufs U, et al. Bempedoic acid safety analysis: pooled data from four phase 3 clinical trials. Journal of clinical lipidology. 2020; 14: 649-59.
- Ganda O. Beyond statins: who and when to prescribe?. Current Diabetes Reports. 2018; 18: 1-9.
- Tokgözoglu L, Libby P. The dawn of a new era of targeted lipid-lowering therapies. European heart journal. 2022; 43: 3198-208.
- Kasichayanula S, Grover A, Emery MG, Gibbs MA, Somaratne R, Wasserman SM, et al. Clinical pharmacokinetics and pharmacodynamics of evolocumab, a PCSK9 inhibitor. Clinical Pharmacokinetics. 2018; 57: 769-79.
- Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. The Lancet. 2015; 385: 341-50.
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. New England journal of medicine. 2017; 376: 1713-22.
- Cicero AF, Bove M, Borghi C. Pharmacokinetics, pharmacodynamics and clinical efficacy of non-statin treatments for hypercholesterolemia. Expert Opinion on Drug Metabolism & Toxicology. 2018; 14: 9-15.
- Raschi E, Casula M, Cicero AF, Corsini A, Borghi C, Catapano A. Beyond statins: New pharmacological targets to decrease LDL-cholesterol and cardiovascular events. Pharmacology & therapeutics. 2023; 250: 108507.
- Khvorova A. Oligonucleotide therapeutics—a new class of cholesterol-lowering drugs. n Engl J med. 2017; 376: 4-7.
- Cordero A, Santos-Gallego CG, Fácila L, Rodríguez-Mañero M, Bertomeu-González V, Castellano JM, et al. Estimation of the major cardiovascular events prevention with Inclisiran. Atherosclerosis. 2020; 313: 76-80.
- US Food and Drug Administration. Prescribing information. 2020.
- Leqvio—EPAR Product Information. 2022.
- Rayan RA, Sharma S. Lomitapide-Stat Pearls-NCBI Bookshelf. National Center for Biotechnology Information. Citované. 2022; 17: 560849.
- Larsen LE, Stoekenbroek RM, Kastelein JJ, Holleboom AG. Moving targets: recent advances in lipid-lowering therapies. Arteriosclerosis, thrombosis, and vascular biology. 2019; 39: 349-59.
- Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. European heart journal. 2014; 35: 2146-57.
- Santos RD, Raal FJ, Donovan JM, Cromwell WC. Mipomersen preferentially reduces small low-density lipoprotein particle num ber in patients with hypercholesterolemia. J Clin Lipidol. 2015; 9: 201–9.
- Yamashita S, Masuda D, Matsuzawa Y. Pemafibrate, a new se lective PPARa modulator: drug concept and its clinical appli cations for dyslipidemia and metabolic diseases. Curr Athero scler Rep. 2020; 22: 5.
- Ginsberg HN, Hounslow NJ, Senko Y, Suganami H, Bogdans ki P, Ceska R, et al. Efficacy and safety of K-877 (Pemafibrate), a selective PPARa modulator, in European patients on statin therapy. Diabetes Care. 2022; 45: 898-908.
- Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, et al. Rationale and design of the Pemafibrate to Re duce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J. 2018; 206: 80-93.
- Vishnu Priya Pulipati, Eliot A. Brinton. Pemafibrate. Clinical Lipidology (Third Edition). 2024.
- Sosnowska B, Adach W, Surma S, Rosenson RS, Banach M. Evinacumab, an ANGPTL3 inhibitor, in the treatment of dyslipidemia. Journal of Clinical Medicine. 2022; 12: 168.
- Patel N, Parmar M, Patel P. Evinacumab. 2023.
- Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. Journal of lipid research. 2020; 61: 1271-86.
- Trautwein EA, McKay S. The role of specific components of a plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients. 2020; 12: 2671.
- Anderson JW, Baird P, Davis Jr RH, Ferreri S, Knudtson M, Koraym A, et al. Health benefits of dietary fiber. Nutrition reviews. 2009; 67: 188-205.
- Lamiquiz-Moneo I, Giné-González J, Alisente S, Bea AM, Pérez-Calahorra S, Marco-Benedí V, et al. Effect of bergamot on lipid profile in humans: A systematic review. Critical reviews in food science and nutrition. 2020; 60: 3133-43.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal. 2020; 41: 111-188.
- Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med. 1995; 333: 1308–12.
- Clifton PM, Noakes M, Sullivan D, Erichsen N, Ross D, Anni son G, et al. Cholesterol-lowering effects of plant sterol esters differ in milk, yoghurt, bread and cereal. Eur J Clin Nutr. 2004; 58: 503–9.
- Dujovne CA. Red yeast rice preparations: are they suitable substitutions for statins? Am J Med. 2017; 130: 1148–50.
- Raschi E, Casula M, Cicero AF, Corsini A, Borghi C, Catapano A. Beyond statins: New pharmacological targets to decrease LDL-cholesterol and cardiovascular events. Pharmacology & therapeutics. 2023; 250: 108507.
- Lee J, Lee SH. Expanding the therapeutic landscape: ezetimibe as non-statin therapy for dyslipidemia. The Korean Journal of Internal Medicine. 2023; 38: 797-809.
- Ida S, Kaneko R, Murata K. Efficacy and safety of pemafibrate administration in patients with dyslipidemia: a systematic review and meta-analysis. Cardiovascular Diabetology. 2019; 18: 38.