
Case Report
J Blood Disord. 2015;2(1): 1021.
Essential Thrombocytopenia/JAK Mutation Causing Thrombosis: A Case Report and Review of Literature
Paolo Costa1, Michele Frigerio2, Elisabetta Del Zotto1, Alessia Giossi3, Irene Volonghi1, Loris Poli1, Andrea Morotti1, Valeria De Giuli1, Roberto Gasparotti2, Alessandro Padovani1 and Alessandro Pezzini1*
1Department of Clinical and Experimental Sciences, Clinical Neurology, University of Brescia, Italy
2Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Italy
3Neurology Unit, Hospital of Cremona, Cremona, Italy
*Corresponding author: Alessandro Pezzini, Department of Clinical and Experimental Sciences, Clinical Neurology, University of Brescia, P.le Spedali Civili, 1, 25123 Brescia, Italy.
Received: December 10, 2014; Accepted: January 27, 2015; Published: February 10, 2015
Abstract
Classic Myeloproliferative Neoplasms (MPNs) include Polycythemia Vera (PV), Essential Thrombocythemia (ET), Chronic Myeloid Leukemia (CML) and Primary Myelofibrosis (PMF). Among these Chronic Myeloproliferative Disorders (CMPDs), ET can be diagnosed by the presence of a chronic nonreactive thrombocythemic state and by the exclusion of the other CMPDs. Similar to other CMPDs; ET can lead to vascular events through quantitative and qualitative changes of the affected hematologic cell. The disease can present with any subtype of stroke including Cerebral Vein Thrombosis (CVT), which although rarely, may complicate, proceed, and occur at the moment of this CMPD. We present the case of a 65-year-old female affected by CVT complicated by ischemic stroke with haemorrhagic transformation. After detection of JAK2 V617F mutation, the patient was diagnosed with Essential Trombocythemia (ET). We discuss the relationship between MPNs, focusing on ET, and cerebrovascular disease, the possible mechanisms linking the two conditions, and the different clinical features regarding stroke subtypes (both of arterial and venous origin). We also describe the clinical relevance of JAK2 V617F mutation and its role in the pathogenesis of thrombosis, ischemic stroke and CVT.
Keywords: Essential thrombocythemia (ET); Myeloproliferative neoplasms (MPNs); Stroke; Cerebral venous thrombosis (CVT); JAK2 V617F
Abbreviations
BCSH: British Committee for Standards in Haematology; CVT: Cerebral Venous Thrombosis; SVT: Splanchnic vein thrombosis; ET: Essential Thrombocythemia; MPNs: Myeloproliferative Neoplasms; PLTS: Platelets; PV: Policythemia Vera; PMF: Primary Myelofibrosis; RBCS: Red Blood Cells
Case Presentation
A 65-year-old female was admitted to the emergency department for acute headache and delirium. She had no previous history of stroke or chronic diseases and was not assuming any medication. On admission, she showed a decreased level of consciousness and no other neurological signs. General examination was unremarkable. Routine laboratory exams were negative. Brain CT scan showed a temporo-parietal lesion consistent with an ischemic stroke complicated by haemorrhagic transformation with associated subarachnoid haemorrhage and mass effect (Figure 1, A-B). Digital subtraction cerebral angiography revealed enlargement of the cortical veins and acute right transverse sinus thrombosis (Figure 1, C-D). A diagnosis of Cerebral Vein Thrombosis (CVT) was done and the patient was treated with unfractionated heparin followed by oral anticoagulation. Before oral anticoagulation therapy was initiated, we performed an extensive screening for thrombophilic abnormalities including protein C deficiency, protein S deficiency, antithrombin III deficiency, the G20210A mutation in factor II gene, the G1691A mutation in factor V gene, anti-cardiolipin antibodies, anti-β2 glycoprotein I antibodies and lupus anticoagulant which was unremarkable. After improvement of the conscious state the neurological examination revealed sensory impairment in the left lower limb, left homonymous hemianopia and tactile neglect. The patient was discharged on oral anticoagulant therapy on day 27. One month after discharge, the patient experienced an acute pain in the left knee. Ultrasonographic examination revealed a thrombosis of the left popliteal vein, although the patient was still on oral anticoagulant therapy and within the therapeutic range. One year after the patient complained acute onset of sensory loss on the right side of the face associated with paresthesias, which resolved spontaneously in 2 hours. Symptoms were consistent with the clinical diagnosis of transient ischemic attack. Laboratory exams showed thrombocytosis (platelet count, 519,000/mm3). Blood count was otherwise normal. No iron deficiency was found. Molecular analysis of JAK2 gene was performed which revealed the JAK2 V617F mutation. Although the patient refused to undergo a bone marrow study, we diagnosed Essential Thrombocythemia (ET) according to the British Committee for Standards in Haematology (BCSH) diagnostic criteria (Table 1) [1,2], and started a long-term therapy with hydroxyurea, at an initial dose 15 mg/kg per day, then adjusted depending upon the balance between the desired effect on the PLT count. On subsequent evaluations we observed a regular course and follow up neuroimaging revealed no further complications. Laboratory, clinical and imaging findings are summarized in Table 2.

Figure 1: A, B: Brain CT scan showing temporo-parietal lesion interpreted as ischemic stroke complicated by haemorrhagic transformation (arrowhead) with associated subarachnoid haemorrhage (asterisk), mass effect and midline shift (arrow). C, D: Digital subtraction cerebral angiography showing acute right transverse sinus thrombosis (C) and enlargement of the cortical veins (D).
BSCH 2010
WHO 2008
requires A1-A3 or A1 + A3-A5
requires A1-A4
A1: Sustained platelet count >450x10^9/l
A1: Sustained platelet count >450x10^9/l
A2: Presence of an acquired pathogenetic mutation (e.g. in the JAK2 or MPL genes)
A2: bone marrow showing ncreased number of enlarged, mature magakariocytes; no significant increase of left-shift of granulopoiesis or erytrhopoieis
A3: no other myeloid malignancy especially PV, PMF, CML or MDS.
A3: no meeting of WHO criteria for PV, PMF, CML, MDS or other myeloid neoplasms
A4: No reactive cause for thrombocytosis and normal iron stores
A4: acquired mutation or clonal marker or no reactive cause for thrombocytosis
A5: Bone marrow aspirate and trephine biopsy showing increased megakaryocyte numbers displaying a spectrum of morphology with predominant large megakaryocytes with hyperlobated nuclei and abundant cytoplasm. Reticulin is generally not increased (grades 0–2/4 or grade 0/3)
PV, polycythaemia vera; PMF, primary myelofibrosis; CML, chronic myeloid leukaemia; MDS, myelodysplastic syndrome.
Table 1: The British Committee for Standards in Haematology Criteria (BSCH) for ET.
normal range
CVT
VT
TIA
Follow UP
Laboratory findings
WBC
x10^3/uL
4-10,8
9.93
8.92
9.98
9.86
RBC
x10^6/uL
4-5,20
4.38
4.46
5.12
4.38
Hb
g/dL
12.0 - 16.0
13
12.2
13.3
13.5
HCT
%
37.0 - 47.0
39.2
37.6
42.8
40.2
MCV
fl
82-99
89.5
88.9
79.4
90.2
PLT
x10^3/uL
130-400
330
380
519
512
Prothrombin time
sec
12.8
24.9
33.2
23.9
%
80-120
86
32
23
34
INR
1.1
2.1
2.8
2.1
activated partial thromboplastin time
sec
24 - 38
30
-
44
-
ratio
0,70 - 1,28
1.01
-
1.44
-
JAK2V617F
positive
Thrombophilia screening (prior to OAC)
negative
Risk factors for CVT/VT
Oral contraceptives
-
-
Hormone replacement therapy
-
-
Infection
-
-
Pregnancy/puerperium
-
-
Trauma
-
-
Solid cancer
-
-
Surgery
-
-
Autoimmune disease
-
-
Fracture, prolonged immobilization
-
-
Liver disease
-
-
Clinical Findings
General: Acute headache, delirium, decreased level of consciousness.
General: acute pain in the left knee
no further clinical complication
Neurological examination: sensory impairment in the left lower limb, left homonymous hemianopia and tactile neglect
Neurological examination: acute onset of sensory loss on the right side of the face associated with paresthesias, which resolved spontaneously in 2 hours
Splenomegaly
-
-
-
-
Imaging Findings
CT scan: temporo-parietal ischemic stroke complicated by haemorrhagic transformation with associated subarachnoid haemorrhage and mass effect
Doppler Ultrasound: thrombosis of the left popliteal vein
CT scan: no new lesions
Follow Up Brain MRI: persistent right transverse sinus thrombosis with no further complication
Digital subtraction cerebral angiography: enlargement of the cortical veins and acute right transverse sinus thrombosis
Therapy
OAC
OAC
OAC + hydroxyurea
OAC + hydroxyurea
Table 2: Summary of laboratory, clinical and imaging findings.
The incidence of ET is about 1.0-2.5 cases per 100,000 people per year and increases with age, with a peak between 50-70 years [3,4], and a female preponderance, according to most of the studies (female to male ratio, ~ 2:1) [5-7]. Although a bone marrow study is always advisable to better understand the underlying pathology, it is not considered mandatory in order to make a diagnosis. Actually, for a definitive diagnosis, it is necessary to exclude other causes of thrombocytosis such as other Myeloproliferative Neoplasms (MPNs) or inflammatory causes. As most patients are asymptomatic at presentation, the diagnostic workup for ET sometimes begins after incidental laboratory findings. Symptomatic patients usually report headache, visual disturbance, and dizziness. Besides the risk of myelofibrosis progression or acute myeloid leukemia, thrombotic and haemorrhagic events are the most important complications of ET, with an incidence ranging between 11% and 25% of the cases [8]. Vascular complications encompass a broad spectrum of clinical features including easy bleeding and bruising, erythromelalgia, migraine and brain ischemia [4]. In particular, the frequency of arterial thrombosis is higher than that of venous thrombosis. The proportion is roughly two thirds on the arterial side vs one third for the venous side for Policythemia Vera (PV), and the difference is even greater for ET. Landolfi and colleagues [9] found a prevalence of major thromboses ranging between 9.7% and 29.4% among ET patients in different studies. The prevalence of arterial thrombotic events ranged from 68.2% to 96.7%, while that of venous thrombotic events from 3.3 to 31.8 %. The prevalence of major thromboses was 22% to 38.6% among PV patients (among these, the prevalence of arterial thrombotic events was 64% to 75%, that of venous thrombotic events 33% to 42%) [19].
In the multicenter ECLAP study [10], 143 episodes of arterial thrombosis (35 TIA, 36 strokes, 72 myocardial infarctions and peripheral arterial thrombosis) were recorded during a mean followup of 2.7 years in among 1638 PV patients, for an incidence rate of 32 events per 1000 patient-years. Cerebrovascular events showed an overall incidence rate of 16 per 1000 patient-years [10,11].
Two studies of 306 [12] and 532 [13] ET patients followed for a median period of 96 months and 7.6 years, described an incidence of cerebrovascular events of 8.3 per 1000 patient-years, 13 TIA (4 %) and 7 strokes (2 %) and of 7.7 per 1000 patient-years, 31 TIA or strokes (6 %), respectively [11].
Hepatic vein thrombosis is the hallmark of ET, occurring in 5-10% of patients with overt MPNs, and can be the first clinical manifestation of still undiagnosed MPNs in 25-65% of the cases [14-16].
Similarly, overt MPN can be identified in about one-third of patients with Splanchnic Vein Thrombosis (SVT), another frequent complication of ET, at the time of the vascular presentation [17,18]. Although splenomegaly is the most common sign on physical examination, occurring in approximately 50% of the cases at the time of diagnosis [19], it is usually relatively mild in ET, as compared to what observed in other MPNSs [4].
Essential Thrombocytemia
Pathophysiology of the thrombotic process in MPNs
Susceptibility to thrombotic diseases in patients affected by MPNs is the consequence of an underlying prothrombotic state due to the procoagulant effect of circulating malignant cells as well as of the raise of the inflammatory stimuli as a host response to the tumour, with subsequent activation of physiologic cells including Platelets (PLTs), leukocytes and endothelial cells. The increase in blood viscosity due to the raise of haematocrit, with subsequent slowing of blood flow and blood stasis are well-known microcirculatory disturbances predisposing to thrombosis. At low-shear conditions like in the venous circulation, a high haematocrit has a major influence on blood viscosity and subsequent impairment of the blood flow. Under high shear rates, the rise of Red Blood Cells (RBCs) mass may displace PLTs toward the vessel wall, facilitating the PLT-PLT and PLTleukocyte interaction, and the subsequent prothrombotic activation. Finally, RBCs and PLTs are not only increased in absolute number but they also present non-physiologic, procoagulant properties. Biochemical changes of the RBCs membrane may facilitate the formation of RBC aggregates and impair blood flow, especially in the small vessels. This mechanism may play an important role in the cerebral circulation. PLTs functional abnormalities may involve a large number of morphological, functional and biochemical abnormalities, particularly in the glycoprotein IIb/IIIa (gp IIb-IIIa), Adenosine Diphosphate (ADP) and Thromboxane (TXA) pathways. Furthermore, PLT-leukocytes interactions may also be impaired. Of note, the prevalence of inherited thrombophilic disorders, may be also higher in MPNs than in the general population [9]. Noteworthy, among ET patients, a reduction of circulating levels of the naturally occurring anticoagulant protein S has been described, mimicking the resistance to activated protein C [11,20-25]. Moreover the presence of the gain-of-function mutation factor V Leiden was associated with a 4-fold increased risk of thrombosis in a cohort of 191 PV or ET patients [11,26]. Finally, to further increase the prothrombotic tendency, several studies demonstrated elevated plasma homocysteine levels among MPN patients [9,27].
Essential trombocythemia and stroke subtypes
ET may present with CVT and virtually with any kind of ischemic and haemorrhagic stroke [28-32]. TIA may also be the first manifestation of such a disease [33]. The susceptibility to hemorrhagic events in a disease with a thrombotic tendency may be caused by the development of a Von Willenbrand syndrome due to very high PLTs count (i.e. >1,500x109/L), with consequent increased clearance of the large circulating Von Willenbrand factor multimers by PLTs themselves [34].
Cerebral venous thrombosis: CVT is an infrequent vascular disorder sometimes complicated by stroke [35-38]. The exact incidence of CVT is difficult to determine. A recent report by Coutinho and colleagues showed an overall incidence of 1.32 per 100,000 person-years [39], which is higher than previously thought. Although local susceptibility factors for CVT are well known (i.e. brain tumors, cerebral infections and traumas), the commonest predisposing conditions are oral contraceptive use, pregnancy and puerperium and prothrombotic disorders (Table 3) [40]. The diagnosis of CVT may be challenging, and is typically based on a high level of clinical suspicion and imaging confirmation (Figure 2). In patients with MPNs the prevalence of CVT has been reported to be about 1% while, among patients with CVT, a MPN is diagnosed in 3-7% of the cases [41]. Thrombotic events may occur before or after or may be concomitant to the diagnosis of MPNs. In a recent study by Martinelli and colleagues [41], conducted on 48 MPNs patients with CVT [ET, 30 (63 %); PV, 11 (23%); Primary Myelofibrosis, 6 (12%); post-ET or post-PV myelofibrosis, 1 (2%)], the diagnosis of thrombosis was concomitant to that of MPNs in 22 patients (46%), it was made within one year before the diagnosis of MPN in 8 (17%), and after this diagnosis in 18 (38%) patients. Of note, MPNs-CVT patients showed a higher probability to develop recurrent thrombosis compared to patients affected by thrombosis in sites other than the cerebral district. Noteworthy, in the same study, they compared to 178 MPN patients (ET patients, 105) with no thrombosis, 135 MPN thrombotic patients, 48 MPNs-CVT (ET patients, 30) and 87 MPNvenous thrombosis (ET patients, 53) respectively. Among those with ET, there was a higher prevalence of JAK2 mutation: 76% (19/30) and 78 % (32/53) vs 55% (53/105).
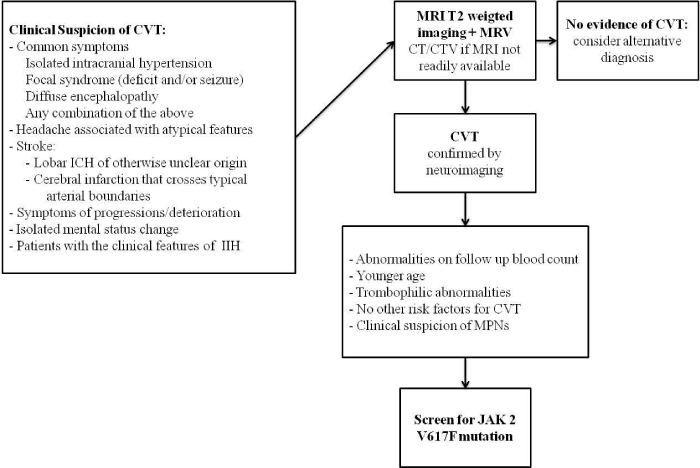
Figure 2: Proposed algorithm for the diagnosis of cerebral venous thrombosis and the screening for JAK2 V617F mutation [41,73,74]. Cerebral Venous Thrombosis (CVT), Intracranial Pressure (ICP), Intracerebral Hemorrhage (ICH), MR Venogram (MRV), CT Venogram (CTV), Idiopathic Intracranial Hypertension (IIH).
Systemic risk factors (%)
Cerebral sinus and vein thrombosis
Inherited
Antithrombin deficiency
1-7
Protein C deficiency
3-6
Protein S deficiency
3-8
Factor V Leiden
3-12
Prothrombin G20210A
11-21
Acquired
Cancer
3-10
Myeloproliferative neoplasms
1-3
Antiphospholipid antibodies
4-17
Behcet disease
1
Autoimmune diseases
8
Paroxysmal nocturnal hemoglobinuria
1
Hyperhomocysteinemia
4-29
Inflammatory bowel diseases
2-3
L-asparaginase and other drugs
1-2
Circumstantial *
Oral contraceptives
10-77
Hormone replacement therapy
4-7
Pregnancy or puerperium
2-3
Local risk factors (%)
Acquired
Brain tumors
2
Central nervous system infections
2-5
Ear, mouth, face, and neck infections
8-14
Circumstantial
Head trauma, neurosurgery,
lumbar puncture, jugular catheter1-2
Table 3: Risk factors for cerebral sinus and vein thrombosis in adults.
Another recent study assessed the frequency of MPNs in a series of 706 patients with CVT and that of CVT in a cohort of 2,143 MPNs patients. Among CVT patients, 27 (3.8%) were diagnosed with MPNs: 4 concomitantly (0.6%), 8 before (1.1%), and 15 after CVT (2.1%). Among the MPN patients, CVT was diagnosed in 9 cases (0.4%), with a higher frequency in patients affected by PV (5 out of 735; 0.7%) as compared to those affected by ET (3 out of 964; 0.3%) or primary myelofibrosis (1 out of 444; 0.2%) [42] (Table 4). These findings suggest, therefore, a weak association between CVT and MPNs and may discourage a thorough investigation for underlying MPNs in CVT patients without overt myeloproliferative features. However, despite extensive work-up, the etiology of CVT may remain unexplained in up to 15% of patients [43]. In this regard, it is noteworthy that among those CVT patients who do not undergo a JAK2 V617F mutation testing, a considerable number of MPNs may remain undiagnosed. Finally, as highlighted by the Europen Leukemia Net Study, patients with CVT and MPN have a significant risk, compared to patients with MPNs alone, of carrying an abnormal thrombophilia testing, suggesting the importance of further evaluation in patients affected CVT even when the diagnosis of ET is already known [41].
CVT [46] (n=706)
MPNs [46] (n=2, 143)
MPNs [45] (n = 5,500)
n (%)
n (%)
n (%)
MPNs diagnosis
total
27 (3.8)
before
8 (1.1)
concomitantly
4 (0.6)
after
15 (2.1)
CVT
total
9 (0.4)
before
3 (0,14)
concomitantly
3 (0,14)
After
3 (0,14)
CVT-MPNs subgroup
ET
3/964 (0.3)
PV
5/735 (0.7)
PMF
1/ 444 (0.2)
MPNs and no thrombosis
178
ET
105 (59)
PV
51 (28)
PMF
21 (12)
post-ET or post-PV myelofibrosis
1 (1)
JAK2V617F,in ET
53 (55)
MPNs and venous thrombosis (other than CVT)
87
ET
53 (61)
PV
24 (28)
PMF
7 (8)
post-ET or post-PV myelofibrosis
3 (3)
JAK2V617F, in ET
32 (78)
MPNs and cerebral vein thrombosis
48
ET
30 (63 )
PV
11 (23)
PMF
6 (12)
post-ET or post-PV myelofibrosis
1 (2)
JAK2V617F, in ET
19 (76)
CVT diagnosis
before
8 (17)
concomitantly
22 (46)
after
18 (38)
Table 4: Cerebral venous thrombosis and myeloproliferative neoplasms relationship.
Janus kinase 2 (JAK2) V617F mutation
In 2005, the somatic gain-of-function mutation (due to a homozygous G ? T transversion) in the Janus Kinase 2 (JAK2) gene, determining the replacement of valine by phenylalanine at codon 617 (V617F), and contributing to the clonal expansion of hematopoietic cells in MPNs was identified [44]. Since then, the role of JAK2 mutation, both in better understanding the pathophysiology and contributing to the diagnosis of MPNs has increased.
A direct effect of JAK2 V617F mutation on PLTs function has long been suspected, although attempts to find a clear relationship have been elusive. A recent study by Hobbs and colleagues [45] based on the application of the JAK2 V617F knock-in mouse model (in which hematopoietic cells are heterozygous for human JAK2 V617F) revealed some important information about the relationship between JAK2 V617F, megakaryocyte and PLTs biology. The presence of JAK2 V617F mutation in megakaryocytes resulted in a hypersensitive signalling through the Thrombopoietin (TPO)/ Myeloproliferative Leukemia virus oncogene (MPL) pathway leading to increased activation of signal transducer, activator of transcription and extracellular signal-regulated kinase. JAK2 V617F-positive megakaryocytes exhibited increased ploidy and mobility as compared to wild-type JAK2 megakaryocytes. Finally, JAK2 V617F-expressing megakaryocytes exhibited an increased pro-platelet formation. Authors [45] concluded that JAK2 V617F-positive megakaryocytes are characterized by increased differentiation, greater migratory ability and pro-platelet formation.
The biological effects of JAK2 V617F were not limited to megakaryocytes but also affected PLTs. JAK2 V617F-PLTs exhibited increased thrombus formation, increased reactivity in response to collagen-related peptide and thrombin, and increased aggregation. Key experiments, performed by Hobbs and colleagues, allowed to exclude the possibility that the altered biological activity of JAK2 V617F-PLTs was simply due to a higher concentration of PLTs. Even considering equivalent PLTs numbers, the increased biological activity of JAK2 V617F-PLTs was still evident. PLTs reactivity to agonists was enhanced, with an increase in PLTs aggregation in vitro together with a reduced duration of bleeding in vivo. The Authors concluded that the JAK2 V617F mutation leads to intrinsic changes in both megakaryocyte and PLTs biology that extend beyond a simple increase in cell number [46]. Finally, the effect of JAK2 mutation may be not only confined to hematopoietic cells. The detection of JAK2 V617F mutant liver endothelial cells in patients with Budd- Chiari syndrome further extends the possible influence of JAK2 V617F mutation on thrombosis, although the underlying pathogenic mechanism is complex and not yet completely uncovered [46,47]. JAK2 V617F characterizes the majority of MPN patients, i.e. nearly 100% of patients with PV and about 50% of ET and PMF patients present the mutation. Its prevalence and clinical significance in the general population has been also evaluated [48]: among 10,507 participants from the Copenhagen City Heart Study with up to 17.6 years of follow-up who were screened for JAK2 mutation, the prevalence of the mutation was 0.2% and its presence was associated with increased morbidity and mortality. A subsequent study [49] by the same Authors showed a prevalence of 0,1% (n=68) among 49,488 individuals from the general population. Interestingly, an association of the JAK2 V617F mutation with age was observed, which implies that the status may change over time [49].
JAK2 mutation, venous and arterial thrombosis
The prevalence of JAK2 V617F mutation varies according to the site of thrombosis. Shetty and co-workers performed molecular screening for JAK2 mutation in a cohort of 321 patients affected by venous thrombosis in different sites. Overall, 19 cases (5.9%) carried the V617F variant of JAK2. The prevalence of the mutation were 3% (1/36) in patients with deep venous thrombosis, 8.8% (12/137) in those with Budd-Chiari syndrome, 5% in those with portal venous thrombosis (4/78), and 3% in those with CVT (2/70) [50]. In other studies the prevalence of JAK2 mutation in CVT patients was reported to range between 0% and 6.2%, with the highest frequency observed in a recent study by Passamonti and colleagues [16], in which 10 out of 152 patients (6.6%) affected by CVT turned out to be mutationcarriers (excluding the 6 patients with an overt MPNs at the time of CVT, the prevalence of the JAK2 V617F mutation was 2.7%: 4 out of 146). In particular, 6 patients met the diagnostic criteria for MPNs at the time of CVT, and 3 additional patients developed the disease during the follow-up (median duration, 7.8 years; range, 6 months to 21.3 years), giving an annual incidence of 0.26% patient-years [16], while one patient had no evidence of MPNs after 3 years of follow-up despite the JAK2 mutation. Among these patients, ET was the most frequent diagnosis (6 patients), with normal o slightly elevated PLTs count in most of the cases. It is important to consider that the JAK2 mutation was found in the absence of myeloproliferative features in 2.7% of CVT patients in the Italian cohort reported by Passamonti and colleagues [16] and in 5.9% in an Indian cohort [42,51]. In patients affected by ET, the JAK2 V617F mutation was the risk factor more frequently detected in patients with CVT [41].
The JAK2 V617F mutation is also common in patients with SVT (between 71% and 100% of the affected subjects) [15]. Up to 25% of these patients may have latent MPNs and present with normal blood counts. In this setting the presence of the JAK2 mutation was reported to be a strong predictor for the subsequent diagnosis of MPNs during follow-up [15,52-54]. These findings differ among different populations. In a recent study on thrombotic risk factors for Budd- Chiari syndrome (BCS) in a Chinese population, the JAK2 V617F mutation was found in 4 of the 169 patients tested. Overt MPNs were diagnosed in 5 patients (PV, 3; ET, 1; PMF, 1). In a previous study by Yoo and colleagues [55] on a Korean cohort, among the 26 patients with SVT, two patients (7.7%) had overt ET at the time of thrombosis and the JAK2 V617F mutation was detected in 3 (11.5%, including the two patients with ET). JAK-2 V617F mutation is an independent risk factor for thrombosis. Systematic reviews and meta-analyses on patients affected by PV, ET, or PMF show that JAK-2 V617F carriers have a 2-3 fold increased risk of thrombosis compared non carriers [11,56-58].
Finally, a thrombotic risk score (International Prognostic Score of thrombosis in Essential Thrombocythemia - IPSET thrombosis) was developed and validated in a large group of ET patients, who were divided into 3 risk groups of low (1.03 % patient-years), intermediate (2.35 % patient-years) and high (3.56 % patient-years) thrombosis risk on the basis of age above 60 years, thrombosis history, cardiovascular risk factors, and the presence of JAK-2 V617F mutation [11,59].
MPL and calreticulin (CALR) mutations: A gain-of-function mutation in the Myeloproliferative Leukemia virus (MPL) gene, which encodes the TPO receptor, has been identified in patients with ET and MPF in multiple sites within exon 10 and most of these result in the activation of MPL, even in the absence of TPO [60].MPLW515L/K has been shown to promote tumorigenesis in vivo [61], and a higher mutant allele frequency is associated with progression to myelofibrosis [60,62].
In 2013, Klampfl and colleagues [63] and Nangalia and colleagues. [64] described heterozygous Calreticulin (CALR) mutations as the second most prevalent acquired nucleotide changes in ET and PMF.
CALR mutations were mutually exclusive of JAK2 and MPL mutations. CALR mutational frequencies are estimated to range between 15% and 24% in ET, and between 25% and 35% in PMF [65]. In terms of prognosis, CALR mutations were associated with better thrombosis-free survival in ET and overall survival in PMF [65]. Klampf and colleagues [63] were the first to notice a two-fold difference in cumulative incidence of thrombosis between JAK2 and CALR mutated ET (13% vs 6.3% at 5 years, 21% vs 11% at 10 years, and 27.1% and 12.8% at 15 years, respectively). In contrast, Nangalia and colleagues [64] did not find a difference in thrombosis rates between JAK2 and CALR mutated patients. A subsequent study by Rotunno and colleagues [66] which included 576 ET patients, reported a significantly longer thrombosis-free survival in CALR mutated and triple-negative patients, compared to JAK2 or MPL mutated cases. JAK2 mutated ET patients had a two-fold increased risk of thrombosis compared to those with CALR mutations, and the difference remained significant after adjusting for age in the analysis by Rumi and colleagues [67]. Finally, Gangat and colleagues [65] analyzed on 300 ET subjects describing mutational frequencies of 53% for JAK2, 32% CALR, 3% MPL, and 12% 'triple-negative'. Triple negative ET patients (who carried the wild type genotype of JAK2, calreticulin-CALR and MPL) showed better thrombosisfree survival compared to JAK2 mutated cases, and these findings remained significant after correction for age and previous thrombosis in multivariable analysis. These observations further underline the importance of JAK2 mutational status in the clinical assessment of these patients [65].
When searching for JAK2 mutations in stroke patients?: Because of its pathogenic role in the thrombotic process, screening for JAK2 mutation might be a reasonable diagnostic approach in selected clinical situations, such as stroke in young adults and cryptogenic stroke, even in the presence of normal blood count. In line with this view, Richard and colleagues [68] found PLTs count < 600 x 109/L in 5 out of 14 patients with ischemic stroke who were subsequently diagnosed as affected by ET, further emphasizing the difficulty of diagnosing the disease at stroke onset, and suggested that searching for the JAK2 V617F mutation might be considered in selected stroke patients irrespective of PLT count. In contrast, Xavier and co-workers [69] recently concluded that routine testing for JAK2 V617F is not recommended for patients with venous thrombosis (except for those with SVT) and for all patients with arterial thrombosis, because of the low prevalence of JAK2 V617F in these clinical situations. Nevertheless, they also observed that the value of testing for JAK2 V617F mutation in patients with CVT is yet to be established. Actually, while JAK2 V617F mutation is reported to range from 12% to 74% in SVT patients lacking clinical evidence of MPN, studies that evaluated the frequency of JAK2 V617F mutation in patients with venous thrombosis at other sites (including lower limb deep venous thrombosis, pulmonary embolism, and retinal vein occlusion), found absence or very low prevalence (0.1-3%) of this genetic variant [69]. Similarly, mutation frequency in patients affected by arterial thrombosis (ischemic stroke, acute myocardial infarction, and peripheral arterial thrombosis) is very low (1.1% according to a meta-analysis of 6 studies including 535 subjects) [70].
Although these data could be considered an argument against molecular screening, the role of JAK2 V617F in arterial thrombosis may still be considered. Zerjavic and co-workers detected JAK2 V617F mutation in 1.4% of patients with venous thrombosis and in 3.3% of patients with ischemic stroke from a cohort of 444 patients with venous peripheral thrombosis and/or pulmonary embolism and 60 patients with ischemic stroke of arterial origin. When compared to a control group of 2,430 subjects, the Authors found a significant association between JAK2 V617F mutation and both venous thrombosis (OR 5.53, CI 1.77 to 17.2, p = 0.0053) and ischemic stroke (OR 13.9, CI 2.75 to 70.5, p = 0.0145) [71]. In line with these findings, all the 11 patients who complicated with stroke out of the 102 affected by ET enrolled in a recent longitudinal study [8], showed the presence of JAK2 V617F mutation, suggesting a major role of this genetic variant in the pathogenesis of stroke of arterial origin. A systematic search for MPNs might be especially important for young patients with stroke of undetermined cause [72].
Conclusion
ET may present and complicate with any kind of stroke, and stroke may precede or follow the diagnosis of ET. Clinicians should keep in mind that stroke is a possible manifestation of MPNs, and this relation should be considered especially in those cases of ischemic stroke of arterial origin occurring at young age without plausible, alternative, causative mechanisms, as well as in cases of CVT in which no obvious risk factors are detectable, regardless of PLTs count. The link between JAK2 V617F mutation and stroke (including arterial and venous thrombosis) may extend beyond their connection with MPNs. Searching for JAK2 V617F mutation, although presently not recommended as a screening tool in all patients with stroke, may lead to earlier diagnosis of ET in a considerable number of patients, especially in those with normal or slightly elevated PLTs count and no obvious manifestation of the disease.
References
- Harrison CN, Bareford D, Butt N, Campbell P, Conneally E, Drummond M, et al. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol. 2010; 149: 352-375.
- Beer PA, Erber WN, Campbell PJ, Green AR. How I treat essential thrombocythemia. Blood. 2011; 117: 1472-1482.
- Beer PA, Green AR. Essential thrombocythemia. Chapter 87. 8th edn. Prchal JT, Kaushansky K, Lichtman MA, et al, editors. In: Williams’s hematology. New York: McGraw-Hill. 2010; 1237–1248.
- Meier B, Burton JH. Myeloproliferative disorders. Emerg Med Clin North Am. 2014; 32: 597-612.
- Cortelazzo S, Viero P, Finazzi G, D'Emilio A, Rodeghiero F, Barbui T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol. 1990; 8: 556-562.
- Fenaux P, Simon M, Caulier MT, Lai JL, Goudemand J, Bauters F. Clinical course of essential thrombocythemia in 147 cases. Cancer. 1990; 66: 549-556.
- Bellucci S, Janvier M, Tobelem G, Flandrin G, Charpak Y, Berger R, et al. Essential thrombocythemias. Clinical evolutionary and biological data. Cancer. 1986; 58: 2440-2447.
- Posfai E, Marton I, Szoke A, Borbenyi Z, Vecsei L, Csomor A, et al. Stroke in essential thrombocythemia. J Neurol Sci. 2014; 336: 260-262.
- Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia. 2008; 22: 2020-2028.
- Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007; 109: 2446-2452.
- Artoni A, Bucciarelli P, Martinelli I. Cerebral thrombosis and myeloproliferative neoplasms. Curr Neurol Neurosci Rep. 2014; 14: 496.
- Radaelli F, Colombi M, Calori R, Zilioli VR, Bramanti S, Iurlo A, et al. Analysis of risk factors predicting thrombotic and/or haemorrhagic complications in 306 patients with essential thrombocythemia. Hematol Oncol. 2007; 25: 115-120.
- Palandri F, Polverelli N, Catani L, Ottaviani E, Baccarani M, Vianelli N. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol. 2011; 90: 933-938.
- Bazzan M, Tamponi G, Schinco P, Vaccarino A, Foli C, Gallone G, et al. Thrombosis-free survival and life expectancy in 187 consecutive patients with essential thrombocythemia. Ann Hematol. 1999; 78: 539-543.
- Austin SK, Lambert JR. The JAK2 V617F mutation and thrombosis. Br J Haematol. 2008; 143: 307-320.
- Passamonti SM, Biguzzi E, Cazzola M, Franchi F, Gianniello F, Bucciarelli P, et al. The JAK2 V617F mutation in patients with cerebral venous thrombosis. J Thromb Haemost. 2012; 10: 998-1003.
- Denninger MH, Chait Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, et al. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000; 31: 587-591.
- Briere JB. Budd-Chiari syndrome and portal vein thrombosis associated with myeloproliferative disorders: diagnosis and management. Semin Thromb Hemost. 2006; 32: 208-218.
- Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, Lee SJ, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol. 2005; 131: 208-213.
- Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC, Colomer D, Villamor N, Bellosillo B, et al. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: relationship with thrombosis occurrence and JAK2 V617F allele burden. Am J Hematol. 2009; 84: 102-108.
- Conlan MG, Haire WD. Low protein S in essential thrombocythemia with thrombosis. Am J Hematol. 1989; 32: 88-93.
- Wieczorek I, MacGregor IR, Ludlam CA. Low proteins C and S and activation of fibrinolysis in treated essential thrombocythemia. Am J Hematol. 1995; 49: 277-281.
- Bucalossi A, Marotta G, Bigazzi C, Galieni P, Dispensa E. Reduction of antithrombin III, protein C, and protein S levels and activated protein C resistance in polycythemia vera and essential thrombocythemia patients with thrombosis. Am J Hematol. 1996; 52: 14-20.
- Marchetti M, Castoldi E, Spronk HM, van Oerle R, Balducci D, Barbui T, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008; 112: 4061-4068.
- Dienava-Verdoold I, Marchetti MR, te Boome LC, Russo L, Falanga A, Koene HR, et al. Platelet-mediated proteolytic down regulation of the anticoagulant activity of protein S in individuals with haematological malignancies. Thromb Haemost. 2012; 107: 468-476.
- Trifa AP, Cucuianu A, Popp RA, Coada CA, Costache RM, Militaru MS, et al. The relationship between factor V Leiden, prothrombin G20210A, and MTHFR mutations and the first major thrombotic episode in polycythemia vera and essential thrombocythemia. Ann Hematol. 2014; 93: 203–209.
- Sun T, Zhang L. Thrombosis in myeloproliferative neoplasms with JAK2V617F mutation. Clin Appl Thromb Hemost. 2013; 19: 374-381.
- D'Ambrosio D, Della-Morte D, Gargiulo G, Rossetti M, Rundek T, Rengo F, et al. Intrapetrous internal carotid artery dissection and essential thrombocythemia: what relationship? A case report. Cases J. 2008; 1: 354.
- Verdure P, Lefaucheur R, Guegan-Massardier E, Triquenot-Bagan A, Gerardin E, Maltete D. Bilateral vertebral artery dissection and essential thrombocythemia with JAK2 mutation. Rev Neurol (Paris). 2012; 168: 543-544.
- Freilinger T, Saam T, Duering M, Dichgans M, Peters N. Internal carotid artery dissection and ischemic cerebral infarction in the setting of essential thrombocythemia. Clin Appl Thromb Hemost. 2011; 17: 138-140.
- Miller TD, Farquharson MH. Essential thrombocythaemia and its neurological complications. Pract Neurol. 2010; 10: 195-201.
- Adam R, Priglinger M, Harrington T, Gottlieb D, Krause M. An unusual cause of cerebella hemorrhage in a young patient: essential thrombocythemia. J Stroke Cerebrovasc Dis. 2014; 23: 373-374.
- Kalala F, Mamara A, Ioannou M, Speletas M. Transient ischemic attacks as the first presentation of JAK2-V617F positive chronic myeloproliferative neoplasm. Hematol Rep. 2012; 4: e12.
- Falanga A, Marchetti M. Thrombotic disease in the myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2012; 2012: 571-581.
- Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005; 352: 1791-1798.
- Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004; 35: 664-670.
- Wasay M, Bakshi R, Bobustuc G, Kojan S, Sheikh Z, Dai A, et al. Cerebral venous thrombosis: analysis of a multicenter cohort from the United States. J Stroke Cerebrovasc Dis. 2008; 17: 49-54.
- Dentali F, Ageno W. Cerebral vein thrombosis. Intern Emerg Med. 2010; 5: 27-32.
- Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012; 43: 3375-3377.
- Martinelli I, Passamonti SM, Rossi E, De Stefano V. Cerebral sinus-venous thrombosis. Intern Emerg Med. 2012; 7 Suppl 3: S221-225.
- Martinelli I, De Stefano V, Carobbio A, Randi ML, Santarossa C, Rambaldi A, et al. Cerebral vein thrombosis in patients with Philadelphia-negative myeloproliferative neoplasms. An European Leukemia Net study. Am J Hematol. 2014; 89: E200-205.
- Dentali F, Ageno W, Rumi E, Casetti I, Poli D, Scoditti U, et al. Cerebral venous thrombosis and myeloproliferative neoplasms: results from two large databases. Thromb Res. 2014; 134: 41-43.
- Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007; 6: 162-170.
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352: 1779-1790.
- Hobbs CM, Manning H, Bennett C, Vasquez L, Severin S, Brain L, et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood. 2013; 122: 3787-3797.
- Fleischman AG, Tyner JW. Causal role for JAK2 V617F in thrombosis. Blood. 2013; 122: 3705-3706.
- Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009; 113: 5246-5249.
- Nielsen C, Birgens HS, Nordestgaard BG, Kjaer L, Bojesen SE. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011; 96: 450-453.
- Nielsen C, Birgens HS, Nordestgaard BG, Bojesen SE. Diagnostic value of JAK2 V617F somatic mutation for myeloproliferative cancer in 49 488 individuals from the general population. Br J Haematol. 2013; 160: 70-79.
- Shetty S, Kulkarni B, Pai N, Mukundan P, Kasatkar P, Ghosh K. JAK2 mutations across a spectrum of venous thrombosis cases. Am J Clin Pathol. 2010; 134: 82-85.
- De T, Prabhakar P, Nagaraja D, Christopher R. Janus kinase (JAK) 2 V617F mutation in Asian Indians with cerebral venous thrombosis and without overt myeloproliferative disorders. J Neurol Sci. 2012; 323: 178-182.
- Colaizzo D, Amitrano L, Tiscia GL, Scenna G, Grandone E, Guardascione MA, et al. The JAK2 V617F mutation frequently occurs in patients with portal and mesenteric venous thrombosis. J Thromb Haemost. 2007; 5: 55-61.
- De Stefano V, Fiorini A, Rossi E, Za T, Farina G, Chiusolo P, et al. Incidence of the JAK2 V617F mutation among patients with splanchnic or cerebral venous thrombosis and without overt chronic myeloproliferative disorders. J Thromb Haemost. 2007; 5: 708-714.
- Dentali F, Squizzato A, Brivio L, Appio L, Campiotti L, Crowther M, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood. 2009; 113: 5617-5623.
- Yoo EH, Jang JH, Park KJ, Gwak GY, Kim HJ, Kim SH, et al. Prevalence of overt myeloproliferative neoplasms and JAK2 V617F mutation in Korean patients with splanchnic vein thrombosis. Int J Lab Hematol. 2011; 33: 471-476.
- Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica. 2008; 93: 1412-1414.
- Dahabreh IJ, Zoi K, Giannouli S, Zoi C, Loukopoulos D, Voulgarelis M. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res. 2009; 33: 67-73.
- Lussana F, Caberlon S, Pagani C, Kamphuisen PW, Buller HR, Cattaneo M. Association of V617F Jak2 mutation with the risk of thrombosis among patients with essential thrombocythaemia or idiopathic myelofibrosis: a systematic review. Thromb Res. 2009; 124: 409-417.
- Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012; 120: 5128-5133.
- Takei H, Morishita S, Araki M, Edahiro Y, Sunami Y, Hironaka Y, et al. Detection of MPLW515L/K mutations and determination of allele frequencies with a single-tube PCR assay. PLoS One. 2014; 9: e104958.
- Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, Cuker A. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006; 3: e270.
- Rumi E, Pietra D, Guglielmelli P, Bordoni R, Casetti I, Milanesi C, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood. 2013; 121: 4388-4395.
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013; 369: 2379-2390.
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013; 369: 2391-2405.
- Gangat N, Wassie EA, Lasho TL, Finke C, Ketterling RP, Hanson CA, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol. 2014.
- Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014; 123: 1552-1555.
- Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014; 123: 1544-1551.
- Richard S, Perrin J, Baillot PA, Lacour JC, Ducrocq X. Ischaemic stroke and essential thrombocythemia: a series of 14 cases. Eur J Neurol. 2011; 18: 995-998.
- Xavier SG, Gadelha T, Rezende SM, Zalcberg IR, Spector N. JAK2V617F mutation in patients with thrombosis: to screen or not to screen? Int J Lab Hematol. 2011; 33: 117-124.
- Dentali F, Squizzato A, Appio L, Brivio L, Ageno W. JAK2V617F mutation in patients with arterial thrombosis in the absence of overt myeloproliferative disease. J Thromb Haemost. 2009; 7: 722-725.
- Zerjavic K, Zagradisnik B, Stangler Herodez S, Lokar L, Glaser Krasevac M, Kokalj Vokac N. Is the JAK2 V617F mutation a hallmark for different forms of thrombosis? Acta Haematol. 2010; 124: 49-56.
- Alvarez-Larran A, Cervantes F, Bellosillo B, Giralt M, Julia A, Hernandez-Boluda JC, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia. 2007; 21: 1218-1223.
- Saposnik G, Barinagarrementeria F, Brown RD, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42: 1158-1192.
- Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007; 6: 162-170.