
Review Article
J Blood Disord. 2015;2(1): 1022.
Mesenchymal Stromal Cells: Regulators of Immune Response in Hematological Malignancies
Poggi A¹* and Zocchi MR²
1Molecular Oncology and Angiogenesis Unit, National Institute for Cancer Research, Italy
2Division of Immunology, Transplants and Infectious Diseases, Scientific Institute San Raffaele Milan, Italy
*Corresponding author: Poggi A, Molecular Oncology and Angiogenesis Unit, Largo R. Benzi 10, CBA-ISTNord Tower A1 and C4, IRCCS AOU San Martino IST National Institute for Cancer Research-Genoa, 16132-Genoa, Italy.
Received: December 02, 2014; Accepted: February 11, 2015; Published: February 27, 2015
Abstract
The Bone Marrow (BM) microenvironment plays a key role in regulating the maturation of precursors of myeloid and B lymphoid cells. This microenvironment is composed of several types of cells among which Mesenchymal Stromal Cells (MSC) can be considered as a major component. Indeed, these elements produce several extracellular matrix proteins involved in triggering signals to precursors cells; furthermore, MSC posses the high plasticity to differentiate into other cell components present within BM as osteocytes and adipocytes which regulate the composition of the microenvironment. MSC can display an immunoregulatory activity leading to the impairment of recognition of leukemic transformed cells. There are preclinical and clinical evidences that treatment with Immunomodulatory Drugs (IMiDs) in multiple myeloma and aminobisphosphonates in different hematological malignancies can trigger an efficient immune response; this response can hit both tumor and stromal cell component of the BM. Herein, we briefly summarize the more recent advances on how and what MSC can regulate anti-leukemic immune response and which drugs would be employed to render MSC immunostimulatory rather than immunosuppressive.
Keywords: MSC; NK; γδT cells; NKG2D; NKG2DL; Immunosuppression
Abbreviations
APC: Antigen Presenting Cells; BM: Bone Marrow; CLL: Chronic Lymphocytic Leukemia; COX2: Cyclooxigenase 2; CTLs: Cytolytic T Lymphocytes; DC: Dendritic Cells; EGF: Epidermal Growth Factor; EGFR: Epidermal Growth Factor Receptor; EMC: Extracellular Matrix Component; EMT: Epithelial Mesenchymal Transition; HLA: Human class I Leukocyte Antigen; HL: Hodgkin Lymphoma; HSC: Hemopoietic Stem Cells; IDO: Indoleamine 2,3, Deoxigenase; IL-: Inteleukin-; IFNγ: Interferon γ; IMiDs: Immunomodulatory Drugs; LCA: Leukocyte Common Antigen; LNMSC: Lymph Node MSC; LSC: Leukemic Stem Cells; MDSC: Myeloid-Derived Suppressor Cells; MHC: Major Histocompatibility Complex; MICA/B: MHC Class I polypeptide related sequence A/B; MM: Multiple Myeloma; MSC: Mesenchymal Stromal Cells; N-BP: aminobisphosphonates; NK cell: Natural Killer cell; NHL: Non-Hodgkin Lymphoma; NKG2D: Natural-Killer Group 2 member D; NKG2DL: NKG2D Ligand; NKT: Natural Killer-like T cells; NOS2: Nitric Oxidase Synthase 2; PB: Peripheral Blood; PGE2: Prostaglandin E2; P4H: Prolyl-4- Hydroxilase; SCF: Stem Cell Factor; SDF1: Stromal Derived Factor 1; Th: T helper; TNFa: Tumor Necrosis Factor a; TGFβ: Transforming Growth Factor β; TPO: Trombopoietin; Treg; regulatory T cells; ULBP1-6: UL16 Binding Protein 1-6; VEGF: Vascular Endothelial Growth Factor
Introduction
Within the BM, leukemic cells interact with the microenvironment composed of different kind of cells, soluble factors and Extracellular Matrix Components (EMC) [1,2]. Mesenchymal Stromal Cells (MSC) can influence their surroundings producing EMC and soluble factors playing a role in maturation of hematopoietic cell precursors. Furthermore, MSC can regulate both innate and adaptive immune cell response [3]. It is becoming evident that MSC plays a key role in the development of the leukemic disease [1,2]. Herein, we will point out on the use of drugs to regulate MSC-mediated activities and we will analyze more recent findings regarding the immunosuppressive role of MSC. Indeed, we believe that influencing MSC behavior one can also affect the development and the fate of the leukemic diseases.
Complexity of bone marrow microenvironment: relevance of MSC
Generally, the leukemic microenvironment in BM is composed of cancer cells at different stages of maturation, endothelial cells, immune cells, myeloid cells, EMC and different types of MSC [1-6]. These MSC can be fibroblasts which produce and secrete the collagen component of the extracellular matrix, osteocytes and adipocytes which are involved in the mineralization of the EMC or in the storing of fatty acids respectively. Further due to the anatomic site, also the interaction with endothelial cells or pericytes located at the vascular sinusoid component of BM can influence the destiny of leukemic cells during their egression from BM to Peripheral Blood (PB) [5,6]. Indeed, the endothelium senses the microenvironment modifications controlling the trafficking of leukemic cells and different stem cell precursors [7]; further, endothelial cells can influence the fate of Hematopoietic Stem Cell (HSC) precursors releasing VEGF and angiopoietin 1 and 2. It is to note that within the BM the amount of a component of the microenvironment is not the same at the different sites; this leads to a different influence on the growth of leukemic cell [6-8]. There are some evidences in the literature that subsets of MSC can differentiate to endothelial cells suggesting that also this BM microenvironment component derives from MSC. In addition, inside the BM, several types of monocyte-derived cells are present [5]. Macrophages, dendritic cells in different stages of differentiation, histiocytes, fibrocytes, Myeloid Derived Suppressors Cells (MDSC) can function as scavengers, professional Antigen Presenting Cells (APC), extracellular matrix producer or immunoregulatory elements together with the other components of BM [5]. Also, these cells are really difficult to be distinguished from MSC on the basis of their morphology, expression of defined molecular markers and functions [9]. In this complex scenario, MSC can regulate the proliferation and maturation of HSC precursors [7] through the Jagged 1, Delta 1, Trombopoietin (TPO) and Stem Cell Factor (SCF) and for these reasons MSC may be considered as one of the first cell which may sense the neoplastic transformation [8]. Indeed, it has been claimed that Leukemic Stem Cells (LSC) are responsible for the onset of leukemia; however, the functional MSC behavior is essential to favor or impede the LSC expansion [6-8]; for this reason MSC should be considered as a target to treat leukemia’s [9-11]. In addition, BM is a store of totipotent undifferentiated MSC, thus tumor cell precursors may affect the differentiation of MSC in the tumor niche and determine the fate of leukemia suggesting a strong relevance for the cross-talk between MSC and LSC [6-8]. It is of note that MSC can produce Transforming Growth Factor (TGF) β which is known to play a key role within the BM niche [12]; indeed, it has been shown that TGFβ can inhibit the cytokine-triggered clustering of lipid rafts and it induces HSC hibernation ex vivo. The surface downstream mediators of TGFβ signaling as Smad2 and Smad3 are specifically activated in HSCs in the hibernation state, but not in proliferating CD34+ progenitor’s cells [12]. These data would indicate that TGFβ is a candidate to control of HSC hibernation suggesting that this cytokine can model the HSC niche [12]. Further, MSC can produce IL6 that is a key cytokine for the growth and maturation of B lymphocytes and Multiple Myeloma (MM) cells [5] indicating that MSC within BM are essential for both normal and neoplastic development [5-10]. We should further note that in several reports the definition of stromal cells is not limited to MSC but also to monocyte-derived elements and endothelial cells. This leads to a confounding ground which does not aid to define precisely and unequivocally the BM scenario [1-4].
Phenotype and immunoregulatory role of BM-derived MSC
MSC isolated from the BM and expanded in vitro cell culture express CD73 CD105 CD146 and CD90 but not hematopoietic lineage markers as CD34 or the Leukocyte Common Antigen (LCA). They can produce different kinds of collagens and express the prolyl-4- hydroxylase which is the enzyme involved in the hydroxylation of prolyn residues of collagen. The MSC can be distinguished from monocytes as they do not express the CD14 marker and from professional APC by the lack of expression of B7-1 (CD80) and B7-2 (CD86) surface molecules [13]. However, MSC can share several markers and functions with other microenvironment components and this can depend on either the experimental conditions used for their in vitro culture expansion or it is related to the tissue specimen from which they are isolated [9,13,14]. For these reasons, some challenging questions have been raised regarding the use of MSC as a tool for modulating immune response [14]. Indeed, it has been proposed that MSC should be subjected to a process termed ‘licensing’ to get the ability to regulate immune response. The licensing process would consist of different steps as activation with pro-inflammatory cytokines such as IFNγ. TNFa and IL1a or IL1β, b) the prevalence of stimuli as Toll ligands which favor rather than hamper the inhibiting behavior of MSC and finally c) the moment at which MSC are involved together the activation signal delivered to immune effectors cells. It appears that the direct interaction between MSC and lymphocyte is a relevant requisite for the delivery of the inhibiting signal [13]. This inhibiting effect, although mainly contact dependent, is mediated through different soluble factors as IL10, TGFβ, IFNγ, TNFa, IL1β, hepatocyte growth factor, heme oxygenase, indoleamine 2-3 dioxygenase, prostaglandin E2, nitric oxide and peculiar histocompatibility antigens as HLAG5 [13-15] (Figure 1). These factors, with several and still undefined mediators, can apparently function alone or in association depending on the origin and ontogenic stage of MSC. This picture is somehow too complex to be checked to use MSC in clinical setting and it would suggest that the “primum movens” of the commitment of an inhibiting MSC is not defined yet. Whatever the molecular mechanism underlined, MSC can deliver in vitro a negative signal on several subset of T and non-T lymphocytes blocking proliferation to either antigenic, polyclonal or oligoclonal stimuli such as phytohemoagglutinin A or monoclonal antibodies to CD3/ T cell receptor complex [13-16]. This inhibiting effect is evident when the ratio between T lymphocyte and MSC are similar and it progressively decreased when the amount of responding lymphocytes increase. This finding indicates that MSC cannot modulate immune response in the presence of an excess of lymphocytes, suggesting that an increment of anti-tumor effectors cells could overcome inhibitory signals mediated within the BM microenvironment. Conflicting reports have been reported regarding the effects on B lymphocytes both in vitro and in vivo experimental settings [17-19]. Indeed, it has been shown that Immunoglobulin (Ig) synthesis is either inhibited [17] or triggered if B cells are stimulated either through the engagement of B cell receptor or via Toll like Receptors (TLR) respectively [18]. Conflicting results have been also reported on the possibility that MSC may trigger rather than inhibit the generation of cytotoxic T lymphocytes and that MSC can function as stimulator in mixed lymphocyte reaction [15,20]. On the other hand, it has been claimed that also the generation of Treg cells is another relevant mechanisms by which MSC can regulate immune response [21-23]. Also in this case not all reports indicate a relevant role for these cells [24].
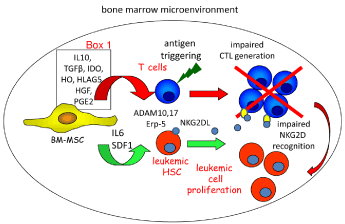
Figure 1: Scheme of the role of mesenchymal stromal cells (MSC) in favoring
leukemic cell proliferation within the bone marrow.
MSC can produce factors involved in the normal maturation of Hematopoietic
Stem Cell (HSC) precursors and they can divert the immune system by several
means. Indeed, several soluble factors (listed in box 1) can be responsible for
the inhibition of T lymphocyte proliferation and generation of specific Cytolytic
T Lymphocytes (CTL). These CTL can recognize leukemic cells bearing
antigens which are up regulated compared to healthy counterpart. MSC
releasing IL6 and SDF1 can support survival and proliferation of leukemic
Hematopoietic Cell precursors (HSC). ADAM10-17 and Erp5 present on
MSC can release NKG2D Ligands (L) into the microenvironment as soluble
molecule and also in exosomes. The generation of extracellular NKGDL
determines a competitive effect with NKG2DL expressed at the leukemic cell
surface leading to an impairment of leukemic cell recognition.
We should also acknowledge that the large part of the studies discussed herein has employed cultured MSC and that these culture conditions may be relevant in “licensing” the inhibiting effect of MSC [25]. No reports using chemically defined media have been performed, thus it is difficult to ascertain the reason why these discrepancies have been found. In addition, the media used for culturing MSC and to trigger lymphocytes are different, thus the results obtained should be analyzed carefully; the main message one can take home is that MSC are indeed plastic not only regarding their differentiation capabilities but also in regulating immune response.
BM-derived MSC as a target for therapy of leukemic disorders
It is conceivable that one can consider a cell type as a suitable target for a given therapy if there is a way to identify it clearly and selectively. As shown above, it is difficult to assign either a single or a set of molecular markers to MSC. Indeed, the MSC heterogeneity and intrinsic plasticity render their molecular definition difficult. In addition, most of data reported on MSC are referred to in vitro expanded cells; this expansion can favor the outgrowth of a less represented kind of MSC or even trigger MSC to a differentiation stage where is prevalent the proliferative capacity of these cells or a peculiar functional behavior. Taking into account these caveats, MSC can be considered a good target for therapy in BM diseases; indeed, the finding that Immunomodulatory Drugs (IMiDs) as thalidomide can affect BM stromal microenvironment, besides triggering anti- Multiple Myeloma (MM) immune response, supports this notion [26,27]. Importantly, IMiDs as single agents or in combination with corticosteroid have shown a strong clinical impact. In particular, lenalidomide has shown a better toxicity profile than thalidomide. Moreover, pomalidomide can be used to overcome resistance to lenalidomide suggesting differences in their mechanisms of action and resistance [26,27]. IMiDs appeared to act also on the generation of lipid rafts in stromal cells [26,27] and this finding is in line with the reported down regulation of MSC-mediated inhibition of T lymphocyte proliferation when MSC were exposed to inhibitors of mevalonate synthesis such as hydroximethylgluatryl Coenzyme A-reductase (HMG-CoAR) inhibitors which leads to decrement of membrane cholesterol content and consequent impairment of lipid rafts formation [16].
Taken together, these findings would suggest that these drugs can affect MSC behavior mainly blocking their mediated inhibiting effects. Furthermore, it has been reported that MSC can prolong survival of B lymphocytes and interfere with the pro-apoptotic effect of corticosteroids [16] indicating that MSC can counteract drugs employed in the treatment of several lymphoid malignancy as MM, Hodgkin and Non-Hodgkin Lymphomas (HL and NHL).
More recently, we have found that MSC isolated and expanded from lymph node of patients suffering from NHL can indeed impair cytokine dependent up regulation of NKG2D surface receptor on antilymphoma Vδ2+ γδ effector T cells [28,29]. NKG2D is an activating receptor expressed on the large majority of anti-tumor effector cells such as CD8+ aβ+ T cells, γδ T cells and Natural Killer (NK) cells [30-32] (Figure 1). The interaction between NKG2D and NKG2D ligands, such as MICA/B and ULBPs, evokes a triggering signal which leads to killing of leukemic target cells [32] and production of proinflammatory cytokines as IFNγ and TNFa. These latter cytokines are relevant in order to elicit an anti-tumor immune response and induce the shift from a regulatory/inhibiting tumor microenvironment to a stimulating one [29]. Perhaps more importantly, the MSCmediated inhibiting behavior is abolished when MSC are primed with the aminobisphosphonate (N-BP) zoledronic acid. This priming is indeed able to favor in MSC the increment of transcription and release of IL15 accompanied with the decrement of transcription and secretion of TGFβ. This imbalance in relative production of stimulating versus inhibiting cytokine renders the MSC able to stimulate rather than inhibit immune response. Furthermore, in this modified microenvironment Vδ2+ γδ effector T cells shift from the production of the immunoregulatory cytokine IL10 to the anti-tumor cytokines IFNγ and TNFa, favoring a Th1 switch relevant for an efficient anti-tumor response [29]. The relevance of these findings is further supported by the possibility to administer zoledronic acid to patients as this drug is already used in the treatment of osteoporosis and osteolytic lesions that follow to MM cell growth within BM. The ADAM10, ADAM17 and Erp5 enzymes involved in the process and release of the NKG2DL from leukemic cells can be considered as another target of therapy [33,34] (Figure 1). Indeed, these enzymes are expressed in MSC expanded from lymph nodes and it is evident that MSC can release NKG2DL that affect the NKG2D-NKG2DL recognition. Notably, both neoplastic cells and MSC can release NKG2DL, thus the use of inhibitors of ADAMs can reduce the immune escaping. In this case, it is important to define well how and where ADAMs and Erp5 play their enzymatic activity (Figure 2). Indeed, the definition of whether these enzymes are soluble in the extracellular microenvironment or expressed in exosomes can have a relevance in built up more specific and efficient inhibitors [35]. Indeed, exosomes have been claimed to be relevant in generating an immunosuppressive milieu and it is still to define whether the same inhibitor can influence ADAM enzyme of MSC-derived exosome as well as that expressed on MSC.
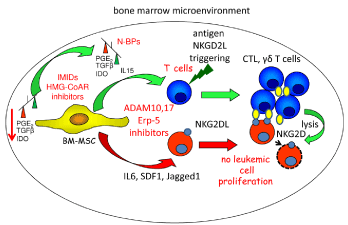
Figure 2: Anti-leukemic therapy with drug able to influence the MSC
functional behavior.
Potential target to divert the MSC-mediated effects is the therapeutic use
of drugs which interfere with the molecular mechanisms responsible for the
immune escape elicited by MSC. Two main mechanisms can be exploited
to down regulate the immune suppressive properties of MSC: a) down
regulation of MSC-mediated inhibiting signal and b) switching the functional
behavior of MSC from inhibition to stimulation of the immune system.
Evidences are reported claiming that the alteration of lipid rafts formation
in MSC can down regulate their inhibiting signal on T lymphocytes. Drugs
able to influence the lipid raft formation such as IMIDs and HMG-CoAR
inhibitors may relieve the immune system from the MSC-mediated inhibition.
In addition, aminobisphosphonates (N-BP) as zoledronic acid can switch
MSC behaviour from the classical inhibition to stimulation. Indeed, the level
of mRNA transcription for TGFβ and its secretion decreases after priming of
MSC with zoledronate while transcription and secretion of IL15 increases.
This indicates a switch from the prevalence of an inhibiting cytokine as TGFβ
to that of an activating factor as IL15. This imbalance triggers the switch of
γδ+ Vδ2+ T lymphocytes from the production of the inhibiting cytokine IL10
to the secretion of anti-tumor cytokines as IFNγ and TNFa. Also, IMiDs as
thalidomide and pamilomide can trigger immune system activation possibly
influencing the stromal cell behaviour.
In vivo therapeutic stimulation of immune response with aminobisphosphonates
At present, MSC are not considered a target of therapy but it is conceivable that therapy with phosphoantigens or N-BP can actually activate anti-tumor response in the microenvironment. It is of note that these drugs may be used in two different ways a) to ex-vivo expand γδ T cell which are infused again in patients or b) to in vivo administration with low doses of IL2 to expand host γδ T cell. The repeated infusion of ex-vivo expanded γδ T cells in nude mice showed tumor growth arrest or anti-tumour effector functions of NK cells and γδ T lymphocytes [36,37]. Wilhelm and coworkers have demonstrated that pamidronate administered with low-dose IL-2 is well tolerated and induces a specific γδT cell expansion in non Hodgkin lymphoma and MM patients. Furthermore, γδT-cell proliferation was linked to the clinical response observed in these patients [38]. Several other studies have been performed mainly with patients suffering from solid tumors and promising results have been obtained (reviewed in [39]). It is of note that the treatment with NB-P induced a strong and specific expansion of TCRVγ9Vδ2 T lymphocytes producing IFN-γ and TNF-a, expressing FcγRIIIa (CD16) and triggering rituximab-mediated ADCC (reviewed in [40]). Importantly, the overall evaluation of clinical trials, present to date in the literature, would indicate that γδT cell-based immunotherapy appears more effective in hematological rather than in solid tumors.
Conclusion
MSC can play a role in the regulation of maturation of hematopoietic stem cell precursors and they can influence the growth and expansion of leukemic cells. MSC can down regulate the immune patrolling of the BM leading to leukemic escape and outgrowth. IMiDs and N-BP can trigger immune response acting on MSC leading to immune stimulation rather than immune suppression. At least for N-BP, this can happen through the imbalance between TGFβ and IL15 favoring the latter stimulating cytokine (Figure 2). This renders the microenvironment hostile to leukemic cell growth and triggers a positive loop to generate a Th1 efficient anti-leukemic response. It is mandatory to identify drugs with similar activities and characterize more precisely the phenotype of MSC to better target them avoiding unspecific effects.
Acknowledgement
This work was partially supported by funds from AIRC to MRZ (IG8727 and IG 12759) or to AP (IG15483) and from Compagnia di San Paolo n. 2012.0312 ID.ROL 891.
References
- Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014; 20: 833-846.
- Riminucci M, Remoli C, Robey PG, Bianco P. Stem cells and bone diseases: new tools, new perspective. Bone. 2015; 70: 55-61.
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505: 327-334.
- Tabe Y, Konopleva M. Advances in understanding the leukaemia microenvironment. Br J Haematol. 2014; 164: 767-778.
- Ribatti D, Basile A, Ruggieri S, Vacca A. Bone marrow vascular niche and the control of angiogenesis in multiple myeloma. Front Biosci (Landmark Ed). 2014; 19: 304-311.
- Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014; 35: 32-37.
- Roozen PP, Brugman MH, Staal FJ. Differential requirements for Wnt and Notch signaling in hematopoietic versus thymic niches. Ann N Y Acad Sci. 2012; 1266: 78-93.
- Chitteti BR, Cheng YH, Poteat B, Rodriguez-Rodriguez S, Goebel WS, Carlesso N, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010; 115: 3239-3248.
- Poggi A, Musso A, Dapino I, Zocchi MR. Mechanisms of tumor escape from immune system: role of mesenchymal stromal cells. Immunol Lett. 2014; 159: 55-72.
- Pacini S, Petrini I. Are MSCs angiogenic cells? New insights on human nest in-positive bone marrow-derived multipotent cells. Front Cell Dev Biol. 2014; 2: 20.
- Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta. 2013; 1836: 321-335.
- Yamazaki S, Iwama A, Takayanagi S, Eto K, Ema H, Nakauchi H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009; 113: 1250-1256.
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110: 3499-3506.
- Krampera M. Mesenchymal stromal cell 'licensing': a multistep process. Leukemia. 2011; 25: 1408-1414.
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004; 103: 4619-4621.
- Musso A, Zocchi MR, Poggi A. Relevance of the mevalonate biosynthetic pathway in the regulation of bone marrow mesenchymal stromal cell-mediated effects on T-cell proliferation and B-cell survival. Haematologica. 2011; 96: 16-23.
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006; 107: 367-372.
- Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008; 26: 562-569.
- Franquesa M, Hoogduijn MJ, Bestard O, Grinyo JM. Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol. 2012; 3: 212.
- Najar M, Rouas R, Raicevic G, Boufker HI, Lewalle P, Meuleman N, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy. 2009; 11: 570-583.
- Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007; 92: 881-888.
- Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005; 90: 516-525.
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105: 1815-1822.
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006; 24: 386-398.
- Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014; 32: 1713-1723.
- Chanan-Khan AA, Swaika A, Paulus A, Kumar SK, Mikhael JR, Rajkumar SV, et al. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J. 2013; 3: e143.
- Gozzetti A, Candi V, Papini G, Bocchia M. Therapeutic advancements in multiple myeloma. Front Oncol. 2014; 4: 241.
- Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003; 102: 1389-1396.
- Musso A, Catellani S, Canevali P, Tavella S, Vene R, Boero S, et al. Aminobisphosphonates prevent the inhibitory effects exerted by lymph node stromal cells on anti-tumor Vd 2 T lymphocytes in non-Hodgkin lymphomas. Haematologica. 2014; 99: 131-139.
- Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, et al. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007; 447: 482-486.
- Zingoni A, Ardolino M, Santoni A, Cerboni C. NKG2D and DNAM-1 activating receptors and their ligands in NK-T cell interactions: role in the NK cell-mediated negative regulation of T cell responses. Front Immunol. 2013; 3: 408.
- Poggi A, Zocchi MR. Stress immunity in lymphomas: mesenchymal cells as a target of therapy. Front Biosci (Landmark Ed). 2014; 19: 281-290.
- Zocchi MR, Catellani S, Canevali P, Tavella S, Garuti A, Villaggio B, et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D ligands recognition in Hodgkin lymphomas. Blood. 2012; 119: 1479-1489.
- Poggi A, Zocchi MR. How to exploit stress-related immunity against Hodgkin's lymphoma: Targeting ERp5 and ADAM sheddases. Oncoimmunology. 2013; 2: e27089.
- Filipazzi P, Burdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012; 22: 342-349.
- Zheng BJ, Chan KW, Im S, Chua D, Sham JS, Tin PC, et al. Anti-tumor effects of human peripheral gammadelta T cells in a mouse tumor model. Int J Cancer. 2001; 92: 421-425.
- Lozupone F, Pende D, Burgio VL, Castelli C, Spada M, Venditti M, et al. Effect of human natural killer and gammadelta T cells on the growth of human autologous melanoma xenografts in SCID mice. Cancer Res. 2004; 64: 378-385.
- Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003; 102: 200-206.
- Fournie JJ, Sicard H, Poupot M, Bezombes C, Blanc A, Romagne F, et al. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013; 10: 35-41.
- Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: A systematic review of clinical trials. Oncoimmunology. 2014; 3: e27572.