
Research Article
J Blood Disord. 2015;2(2): 1026.
Pseudotumors of the Limbs in Patients with Hemophilia
Caviglia H1,2*, Landro ME¹, Galatro G1,2, Candela M² and Neme D²
¹Department of Orthopaedic Surgery and Traumatology, Fernández General Hospital, Bs. As.-Argentina
²Haemophilia Foundation, Bs. As.-Argentina
*Corresponding author: Caviglia HA, Department of Orthopaedic Surgery and Traumatology, Fernández General Hospital, Bs. As.-Argentina,
Received: April 16, 2015;Accepted: May 18, 2015; Published: May 25, 2015
Abstract
Introduction: The haemophilic pseudotumour is a truly encapsulated hematoma, which tends to progress and produce different clinical symptoms depending on its anatomic location. Efforts must be made to prevent pseudotumors by ensuring that all patients receive adequate treatment for their muscle and intra-osseous hematomas. The objective of this paper is to show the evolution of the treatment of pseudotumors at our center over 47 years (1967- 2014).
Patients and Methods: Between 1967 and 2014 forty-nine patients were treated for sixty four pseudotumors. Forty-six were hemophilia type A (93.8%) and three type B (6.2%). Thirty-nine (79.6%) patients had single pseudotumors and ten (20.4%) had multiples pseudotumors. The average age of the patients was 26 years old. Nine were factor VIII or IX inhibitor patients. Forty-five (70%) pseudotumors were located in bones and nineteen (30%) were soft tissue pseudotumors.
Results: The frequency of hemophilic pseudotumors in our center has shown a gradual reduction from 2.6% in 1971 to 0.25% in 2014.Sixty percent of patients treated with radiation therapy evolved favorably. Five amputations were successfully performed. Sixteen resections of the pseudotumor and its pseudocapsule were performed. Four patients died and two patients developed a fistula after pseudotumor resection. Two patients responded to conservative treatment and did not require surgery. Mini-invasive surgery (percutaneous method) was performed on the other thirty-two patients with thirty-eight pseudotumors. Mini-invasive surgery failed in three patients. We did not observe any bleeding or infections due to surgery.
Conclusion: We believe that pseudotumors should be treated with minimally invasive technique (suction and refilling) with the proper hemostatic coverage.
Keywords: Pseudotumors; Haemophilia; Prophylaxis; Radiation; Amputation; Conventional treatment; Percutaneous treatment
Introduction
The hemophilic pseudotumour is a truly encapsulated hematoma, which tends to progress and produce different clinical symptoms depending on its anatomic location. Therefore, it is a clinical entity rather than a specific pathological lesion [1]. Pseudotumor growth can lead to destruction of soft parts, bone erosion or neurovascular complications due to compression [2]. These lesions were first described by Starker in 1918. He stated that pseudotumors are, in hemophiliacs, clinical entities and not specific pathological injuries [3].
Hemophilic pseudotumours only occur in one-two% of patients with severe hemophilia [4,5]. Nevertheless, when patients do not received adequate treatment the hemorrhage episodes, pseudotumor incidence increases. They can also occur in patients with moderate or mild disease [6]. This paper shows that with proper treatment of bleeding disorders, the incidence of pseudotumors is reduced.
Radiation therapy was the conservative treatment used in the early eighties. This treatment caused a large number of pathological fractures. At that time, conventional surgical treatment was resection of the pseudotumor and its pseudocapsule. Amputation was used when the pseudotumor was very invasive and there was no other option [7].
In 1982, Fernandez-Palazzi et al. developed a new technique for treating early cysts. The procedure consists of locating the cyst by means of an image intensifier, puncturing it with a trocar, and finally, aspiration of the cavity content and filling with fibrin seal [7]. In 1990 the Mariano R. Castex Investigations Institute of Argentinaás National Academy of Medicine decided to treat the largest cysts and pseudotumors with aspiration and then fill the cavity with bone graft in bone pseudotumors and with spongostan® or fibrin seal in soft ones [7].
Also in 1982, Fernandez Valderrama et al., described pseudotumor capsules as defensive fibrous tissue which resists the advance of hematoma [8] Therefore, at our center, the Hemophilia Foundation in Buenos Aires-Argentina, we considered capsule resection unnecessary. In the 1990’s bone pseudotumor cavities were filled with lyophilized bone apatite. Currently we use hydroxyapatite coralline which is easier to work with and to find and also less expensive.
The objective of this paper is to show the evolution of the treatment of pseudotumors at our center over 47 years. We demonstrate that mini-invasive techniques and proper treatment reduce the numbers of pseudotumors in patients with hemophilia.
Patients and Methods
At the Hemophilia Foundation in Buenos Aires, Argentina, forty-nine patients were treated for sixty-four pseudotumors between 1967and 2014. Forty-six were hemophilia type A (93.8 %) and three type B (6.2 %). Thirty-nine (79.6 %) patients had single pseudotumors and ten (20.4 %) had multiple pseudotumors (simultaneous or successive). The average age of the patients was 26 years old (6-75, median 23). Nine were factor VIII or IX inhibitor patients.
There was an average delay of 22 months between the appearance of the pseudotumor and the start of treatment (3-284months, median 14 months).
Forty-five (70%) pseudotumors were located in bones: fourteen (23.4%) in the femur, twelve (18.8%) in the tibia, six (9.4%) in the calcaneus, three (4.7%) in the ulna, three (4.7%) in the phalanx, three (4.7%) in the tarsal-metatarsal, one (1.6%) in the talus, one (1.6%) in the radius, one (1.6 %) in the cuboid, one (1.6%) in the fibula. Nineteen (30%) were soft tissue pseudotumors: ten of these were located in the thigh (15.6%), two (3.1%) in the leg, two (3.1%) in the knee, two (3.1%) in the foot, one (1.6%) in the gastrocnemius muscle and one (1.6%) in the arm Graph 1.
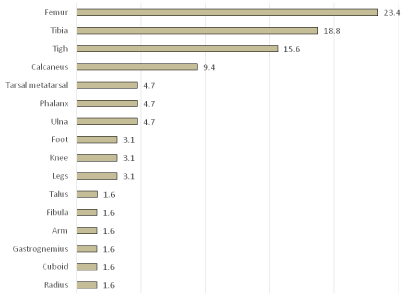
Graph 1: Location of the pseudotumors in our series.
The upper limb was compromised in eight cases (12.5%) and the lower limb was affected in fifty-six cases (87.5%).
Size and content of all lesions was assessed with X-rays, CT scans, or Magnetic Resonance Imaging (MRI) at baseline.
Treatment evolved over time. Until 1982, pseudotumors were treated with radiation, amputation and conventional surgery. Miniinvasive surgery has been used since 1982. A series of steps are followed prior to surgery:
Conservative treatment
Patients receive Buenos Aires Protocol (which will be defined below) as conservative treatment of their pseudotumors for 6 weeks Then X-rays or new MRIS of each pseudotumor are used to assess the response to treatment. Surgery is performed on all patients whose pseudotumors have not reduced to less than half of their original size [9] Graph 2.
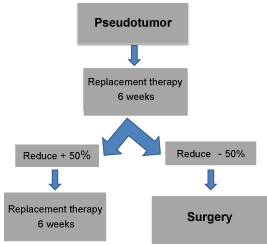
Graph 2: Buenos Aires Protocol Indications. Every pseudotumor treated with
preoperative replacement therapy that does not reduce by 50 % in size after
6 weeks, or 25% after 12 weeks or is still present after 18 weeks should be
surgically removed [14].
Preoperative planning
An MRI (Magnetic Resonance Image) is used to study the pseudotumor cavities and communication between them in order to decide the number of approaches and the location of the incisions needed to achieve effective aspiration of the pseudotumor [9].
Hematological care
Before orthopedic surgery, pre-operative recovery measure was performed in all patients without inhibitors. The target factor (VIII/ IX) before surgery was between 80% and 100%. The same levels were maintained by continuous infusion for three days. Levels between 60-80% were used for the next two days and levels near 50% for the last three-four days. After that, one bolus infusion was performed per day to complete seven-fifteen days of treatment. Then secondary prophylaxis was indicated.
In patients with inhibitors, an initial dose of recombinant factor VIIa (rFVIIa) 150-200 μg/kg was infused immediately before surgery. The following doses were 90 μg/kg every two hours for two days, then every three hours for the next three days and every four hours for the last three days. Patients received a high daily dose during the following three to seven days and finally secondary prophylaxis was prescribed.
Mini invasive surgical technique
Under general anesthesia, a small incision was made in each cavity of the pseudotumor, mechanically evacuating it, washing it with saline solution and aspiration of the contents. Clots were removed with graspers without damaging the inner surface of the pseudotumour. Bone pseudotumor cavities were filled with hydroxyapatite coralline granules, and soft tissue pseudotumor cavities were filled with spongostane®. The wound was closed with separate nylon stitches. All procedures were performed by the same multidisciplinary team at the same hospital.
Results
The frequency of haemophilic pseudotumor was 2.6% in 1971 and 1.3% at the end of the eighties [6]. This percentage has undergone a gradual reduction to 0.25% in 2014 Graph 3.
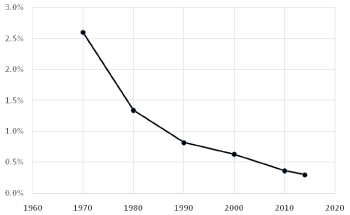
Graph 3: Prevalence of pseudotumor in Patients with Haemophilia (PWH) in
our study. The frequency of hemophilic pseudotumor has shown a gradual
reduction from 2.6% in 1971 to 0.25% in 2014.
Radiation therapy was used on four patients with five pseudotumors (doses varied between 2000 and 5000 rads). Three (60%) of the pseudotumors responded favorably and two (40%) did not. Pseudotumors refractory to treatment were larger and more soft tissue was involved. Pathological fractures occurred in two patients with femur pseudotumor after radiation therapy, resulting in over five centimetre of limb shortening in each. These negative results led our center to abandon radiation therapy.
Five amputations were successfully performed (one phalanx, three femur, one tibia).
Sixteen resections of the pseudotumor and its pseudocapsule were performed. Seven pseudotumors were located in the thigh, two in the foot, two in the calf, one in the femur, one in the tibia, one in the ulna, one in the phalanx, and one in the tarsal-metatarsal.
The four patients with thigh pseudotumors died (25%). Two of them had skin necrosis and two had fistula after pseudotumor resection, both in the calcaneus bone.
Since 1982, forty pseudotumors have been treated conservatively, thirty-eight of which later continued to mini invasive surgery. Two patients (5%) (with one pseudotumor each) responded to conservative treatment and did not require surgery. One of them was an inhibitor patient with a soft tissue pseudotumor in the arm, and the other had a bone pseudotumor of the femur. These pseudotumors were the most recently formed (3 months).
Mini-Invasive surgery was performed on the other thirty-two patients with thirty-eight pseudotumors since their pseudotumors were not reduced to less than half of their original size with conservative treatment. We did not observe any bleeding or infections due to surgery.
Mini-invasive surgery failed in three patients (7.8%). One was a patient with an old pseudotumor (284 months) in the thigh. It was not possible to collapse the pseudotumors cavity after aspiration of the content because the walls were fibrous and rigid. The patient required additional surgery. Conventional technique, resection of the pseudotumor and its pseudocapsule, was successful. The second patient, with a 53-months-old pseudotumor, had a bone pseudotumor of the distal femur with severe soft tissue damage. It was aspired, but it was impossible to adequately pack the cavity or collapse the soft tissues. The patient had had a total knee replacement, which also included the distal end of the femur. The third patient had had a calcaneus pseudotumor in his childhood and had been treated with radiation therapy. The original pseudotumor developed cavities, and he later developed a second pseudotumor. He began treatment after a thirty-eight-months-old pseudotumor caused his calcaneus bone to disappear. The patient had surgery but it was not possible to collapse the cavity. A second surgery was carried out after embolization, finally healing the pseudotumor.
Discussion
The best way to prevent pseudotumors is to treat bleeding episodes. When pseudotumors do appear they can be surgically removed with proper surgical techniques and hemostatic coverage.
Over the past forty-seven years we have observed a high number of pseudotumors in patients with hemophilia type A (93.75%) and also a high frequency of patients who developed more than one pseudotumor in their lives (21.74%). In the last 15 years, the majority of patients treated for pseudotumors at our center, have been inhibitor patients. The lower limbs are affected in 87.5% of cases, of which 36% of cases involve the thigh and 28.88% of cases involve the femur. The way muscles attach to the femur makes it susceptible to periosteal detachment and hematomas adjacent to the bone.
The frequency of pseudotumors at our centre was 2.6% in 1971. This percentage is currently 0.25%.This decrease is due to the availability of replacement therapy. The Hemophilia Foundation was founded seventy years ago and created a network of centers across the country which are coordinated by hematologists and provide training. The Argentinean Ministry of Health starting reimbursing patients under 18 years old for prophylaxis in 2000, thus closing the circle of treatment. A retrospective study published by Xue et al. in 2011 about a single center experience in China also showed a low number of pseudotumors among hemophilia patients. Of 1248 patients with hemophilia diagnosed between January 1983 and October 2004, only fourteen had pseudotumors (1.12%) [10].
We have treated pseudotumors with radiation therapy, amputation, conventional surgery involving resection of the pseudotumor and pseudocapsule, and mini invasive surgery.
Radiation therapy was used in four patients with five pseudotumors, with a 40% failure rate and 40% post-treatment pathologic fractures, especially in bones like the femur which are subject to loading [6]. Pseudotumors refractory to treatment were larger and more soft tissue was damaged [6]. A study published in 2010 at Nepal Medical College by Rijal et al. used this technique in a calcaneal pseudotumor with an ulcer and recommends its use [11]. We only recommend radiation therapy to treat pseudotumors found in anatomical regions that are not accessible to surgical treatment.
Amputation was successfully performed in five cases. This method of treatment solves the problem but implies a partial or total loss of the affected limb and leads to marked loss of function, especially when amputation is very proximal in the thigh, preventing the use of prosthetics.
The resection of pseudotumors and pseudocapsules was performed in sixteen patients. The thigh was affected in eight patients. One had a bone pseudotumor and seven had soft tissue pseudotumors. Two patients died after resection of pseudotumors due infection and sepsis. The first case was a resection of a soft tissue pseudotumor. The patient developed a post-surgical skin necrosis and died forty-four months later due to sepsis. The second case was a pseudotumor that destroyed most of the femur, which also showed extensive necrotic skin plate caused by the tension of the skin over the hematoma. The patient died of necrotizing fasciitis. Total mortality for the procedure was 12.5% of all patients, 25% of patients with thigh pseudotumors. Rodriguez-Merchan et al. similary reported a mortality rate of 20% for pseudotumorsin the thigh [2]. A study published in 2015 by Zhai et al. covers eight pseudotumors with high incidence of wound infection, coagulation factor inhibitor formation, pseudotumor recurrence, and fracture non union [12].
Two of our patients had fistula after calcaneus pseudotumor resection. The calcaneus pseudotumor is one of the most difficult to treat, because it is very aggressive and expands rapidly destroying the bone’s thin walls. The resulting skin tension can produce skin necrosis and infection. Postoperative fistula is common in this location because after the resection of the pseudotumor and its pseudocapsule the soft tissues are not collapsible and new bleeding feeds the fistula.
Only two (4%) of our patients were cured with conservative treatment. Both patients had had pseudotumors for a short time (three months) far below the average of our study population. The other forty-seven patients (96%) did not respond to conservative treatment and required surgery. Of the thirty-eight patients who had mini-invasive treatment, twelve (31.5%) had thigh pseudotumors, eight had bone pseudotumors (21%) and four had soft tissue pseudotumors (10.52%). No complications were observed with miniinvasive technique.
The age of a pseudotumor is a relevant factor. Our experience shows that when the pseudotumor is young it is more likely to respond to conservative treatment than when it is older.
Surgery failed in three patients (7.8%). These patients pseudotumors averaged 125 months old (38 -284) far above the average for the population studied. It was impossible to adequately pack the cavity or collapse the soft tissues in old pseudotumors. In these cases it is better to perform resection of the pseudocapsule.
We believe that pseudotumors should be treated with minimally invasive technique (suction and refilling) with the proper hemostatic coverage, and with a multidisciplinary team working together. Pseudotumor surgery in patients with hemophilia is possible and can improve the quality of life of these patients [13].
Conclusion
As has been said by Dr. Tezanos Pinto “the best treatment for pseudotumors is prevention by means of early substitution treatment of bleeding episodes” [6].
We believe that the percutaneous method is a non-aggressive and improved treatment of this pathology. We think efforts should be made to prevent pseudotumors by ensuring that all patients receive adequate treatment for their bleeding episodes.
References
- Gilbert M. Characterizing the haemophilic pseudotumor. Ann N Y AcadSci. 1975; 240: 313-315.
- Rodriguez- Merchan EC. The haemophilicpseudotumour. IntOrthop. 1995; 19: 255-260.
- Starker L. Kochenusurdurchein homophiles subperiosta Hämaton. Mitt Grenzgeb Med Chir. 1918; 31: 381–415.
- Gunning A. The surgery of haemophilic cysts. In: Bigs R, MacFarlane RG. Treatment of haemophilia and other coagulation disorders. Oxford: Blackwell Scientific. 1966.
- Rodriguez -Merchan EC. Aspects of current management: orthopaedic surgery in haemophilia. Haemophilia 2012; 18: 8-16.
- Tezanos Pinto M, Nieto R, Perez Bianco R. Hemophilic pseudotumor: A case report of 25 cases. In: JM Lascherand, CM Kessiler, editors. Hemophilia and von Willebrand´s disease in the 1990s. Elsevier science publishers. 1991; 165-178.
- Rodriguez- Merchan EC, Goddard NJ and Lee CA. Musculoskeletal Aspects of Haemophilia. Blackwell Science Ltd. In: Caviglia HA, Fernandez-Palazzi F, Galatro G, editors. Chapter 15: Percutaneous treatment of haemophilic pseudotumors. 2000.
- Fernández de Valderrama JA, Matthews JM. The haemophilic pseudotumor or haemophilic subperiosteal haematoma. J Bone Joint Surg. 1965; 47: 256-265.
- Caviglia H, Perez Bianco R, Tezanos Pinto M. Therapeuthic Algorithms of muscular skeletal complications of haemophilia. Editorial Akadia (Bs As). Chapter 15: Pseudotumores. 2006; I65-I68.
- Xue F, Sun C, Sui T, Zhang L, Jiang L, Yang R. Hemophilic pseudotumor in Chinese patients: a retrospective single-centered analysis of 14 cases. Clin Appl Thromb Hemost. 2011; 17: 279-282.
- Rijal L, Neogi DS, Ansari MT, Khan SA, Yadav CS. Hemophilic pseudotumor--is there a role of radiotherapy? Literature review and a case report. Nepal Med Coll J. 2010; 12: 193-197.
- Zhai J, Weng X, Zhang B, Peng HM, Bian YY, Zhou L. Surgical management of haemophilic pseudotumor complicated by destructive osteoarthropathy. Blood Coagul Fibrinolysis. 2015.
- Caviglia H, Candela M, Landro ME, Douglas Price AL, Neme D, Galatro GA. Haemophilia Pseudotumours in patients with inhibitors. Haemophilia. 2015.
- Caviglia H, Solimeno L. Percutaneous Treatment of HemophilicPseudotumors. Capítulo. Orthopedic Surgery in Patients with Hemophilia. Springer-Verlag Italia. 2008; 34: 249-254.