
Special Article - Leukemia
J Blood Disord. 2015; 2(3): 1033.
Blastic Plasmacytoid Dendritic Cell Neoplasm: Report of a Case with Atypical Cytology and Immunophenotype
Lamar E¹*, Roggy A2,3*, Le Calvez G¹, Marion V¹, Buors C1,4, Quintin-Roue I5, Costa S5, Morel F4,6, Brenaut E4,7, Tempescul A8, Biichle S3, Garnache- Ottou F2,3 and Ugo V1,4
¹Centre Hospitalier Universitaire de Brest, Laboratoire d’Hématologie, France
²EFS Bourgogne-Franche Comté, Laboratoire d’Hématologie, France
³Université de Franche Comté, France
4Université de Brest, France
5Centre Hospitalier Universitaire de Brest, Service d’Anatomie Pathologique, France
6Centre Hospitalier Universitaire de Brest, Laboratoire de Cytogénétique, France
7Centre Hospitalier Universitaire de Brest, Service de Dermatologie, France
8Centre Hospitalier Universitaire de Brest, Service d’Hématologie Clinique, France
*Corresponding author: Lamar E, Laboratoire d’Hématologie, CHU Brest, Avenue Tanguy Prigent, 29609 Brest, France
Roggy A, Laboratoire d’Hématologie, EFS Bourgogne- Franche Comté, 8 rue du Docteur François-Xavier GIROD (BP 1937), 25020 Besançon Cedex, France
Received: August 28, 2015; Accepted: November 16, 2015; Published: November 26, 2015
Abstract
Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) or Plasmacytoid Dendritic Cell Leukemia (pDCL) is a rare and aggressive malignancy, whose diagnosis is mainly based on phenotypic characterization of the blastic cells with CD4+/CD56+ antigens. We report a BPDCN case with a blastic population expressing CD4 with no CD56 expression. In cytology, the classical “pearl necklace” aspect was missing. The diagnosis was made thanks to the specific BPDCN markers, namely CD303 (BDCA2), CD304 (BDCA4), and cTCL1. Biologists should be aware of this atypical presentation.
Keywords: Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN); Leukemia; Plasmacytoid dendritic cell; Diagnosis; Immunophenotyping
Case Report
Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN), also called Plasmacytoid Dendritic Cell Leukemia (pDCL), is a rare and aggressive hematological disease described as a subtype of acute leukemia in the 2008 World Health Organization classification [1]. The diagnosis is often delayed because of an indolent initial clinical presentation, most frequently with asymptomatic solitary or multiple skin lesions, which usually progress towards aggressive systemic dissemination. The diagnosis is mainly based on phenotypic characterization of the blastic cell population expressing CD4+ and CD56+ antigens, in the absence of myeloid and lymphoid markers. However, phenotypic variations can be observed [2,3]. The blasts’ morphology can also be suggestive, usually with a large amount of cytoplasm, sometimes forming pseudopodia, with faint basophilia, no granulation and a peculiar arrangement of small vacuoles along the cytoplasmic outline with a “necklace” aspect [4].
We report the case of a 66 year old Caucasian male who was hospitalized in the dermatology unit for multiple cervical adenopathies and cutaneous lesions. One month previously, a lesion had appeared on the right cheek and had grown rapidly. On examination, he presented a large infiltrated purplish lesion with a diameter of about 5 cm on the cheek, which was not painful. Numerous ecchymotic lesions on the top of the trunk appeared about two weeks later (Figures 1A & 1B). The blood count was normal, except for macrocytic anemia (hemoglobin (Hb) 11.5 g/dL, mean corpuscular volume (MCV) 103.8 fL). After having excluded infection, the skin lesions were biopsied. Histology and immunohistochemistry studies showed proliferation of hematological cells that were CD45+, CD20- CD3-, CD138-, with cCD79 a weakly expressed by a few large cells, consistent with possible large B-cell lymphoma. Cervical lymph node biopsy was performed and also showed proliferation of undifferentiated cells expressing CD45 and weakly expressing CD79a. The proliferation cell fraction as detected by Ki67 was 50% and the diagnosis of large B-cell lymphoma was retained. The patient was treated by COP-CDEA chemotherapy. A few months later, before the third chemotherapy course, the patient was in poor condition, presenting new cervical lymphadenopathies, hemorrhagic syndrome with ecchymosis, new skin lesions, neurological symptoms and respiratory distress. Laboratory findings showed anemia (Hb: 9 g/dL), thrombocytopenia (50 G/L) and leukocytosis (85.8 G/l) with 83% blast cells. Morphology was heterogeneous, with some poorly differentiated large-to-intermediate blasts and abundant basophilic cytoplasm; some cells containing azurophilic granulation; few blasts showing large pink granules (pseudo-Chediak-Higashi granules); and some smaller blasts presented pseudopodia with or without small granulations. No Auer rods or dysplasia were observed. Bone marrow aspirate revealed a massive infiltration of blast cells with more nuclei irregularities than in blood cells (Figure 2A). Analysis of cerebrospinal fluid showed large blastic infiltration.
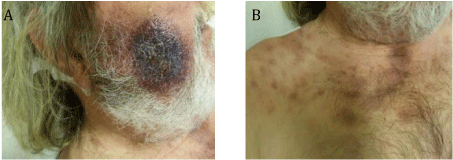
Figure 1: 1A: Cutaneous lesions of the cheek.
Figure 1B: Ecchymotic lesions in necklace.
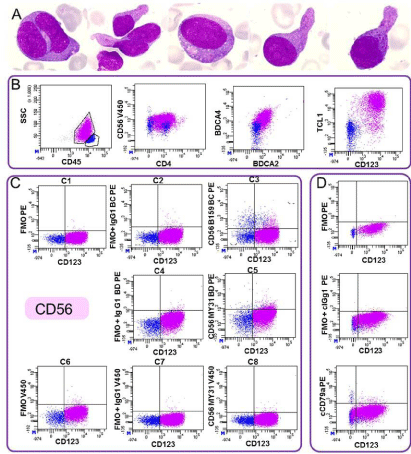
Figure 2: Bone marrow examination showed blasts, with bilobed nuclei, azurophilic granulation, large pink granules, rare small vacuoles and/or pseudopodia.
2B: Flow cytometry analysis performed on blood revealed a blastic population (pink) CD45lowCD4+ CD56- with expression of dendritic plasmocytoid markers :
CD303 (BDCA2), CD304 (BDCA4), cTCL1 and CD123. CD45+ lymphocytes are in blue. 2C: Absence of CD56 expression was confirmed with three different anti-CD56 antibodies (C3, C5, C8) and FMO control that were performed with no antibodies
in the channel or with an isotype control. C1: CD45V500/CD4APC-H7/CD123PC7/_PE/ ; C2: CD45V500/CD4APC-H7/CD123PC7/IgG1 BCPE; C4: CD45V500/CD4APC-H7/CD123PC7/
IgG1 BD PE; C6: CD45V500/CD4APC-H7/CD123PC7/_V450/; C7: CD45V500/CD4APC-H7/CD123PC7/IgG1V4502D: The same analysis was performed for cCD79a evaluation.
BC: Beckman Coulter Immunotech, Miami, FL, USA; BD: BD Biosciences, San Jose, CA, USA; V500: Horizon V500; APC-H7: allophycocyanin-H7; PC7:
phycoerythrin-cyanin-7; PE: phycoerythrin, V450: Horizon V450
Flow cytometry analysis performed on a blood sample revealed a blastic population (CD45low, CD34-) lacking specific lineage markers (intracytoplasmic (c) CD3, cCD22, cCD79a, CD19, MPO, CD14), expressing CD4 and CD7, without expression of other T-cell associated antigens (CD5, CD2, CD8 negative). Cells also expressed CD36, but no other myeloid-associated antigens (CD13, CD33, CD117 negative). Other non-specific markers were present such as HLA DR, CD38, CD71 and CD123. The expression of CD4 and CD123 without expression of strong lineage markers suggested possible blastic plasmacytoid Dendritic Cell (pDC) origin and to this end, CD56 expression was tested. The cells did not express CD56 and this was confirmed with 3 different clones and Fluorescence Minus One (FMO) control (Figure 2C). Despite this marker being negative, we investigated expression of specific pDC markers [2,3], namely CD303 (BDCA2), CD304 (BDCA4), and cTCL1. These markers were positive, allowing the diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) according to the 2008 World Health Organization classification (1) (Figure 2B). The karyotype was complex: 46, XY, add(8)(q24), del(9)(q33), del(12)(p1?), -13, add(17) (p11), +mar[cp21]/ 6, XY[1] with TP53 deletion by FISH. All these abnormalities are recurrent in BPDCN [5].
An acute lymphoid leukemia-type treatment was introduced, but the patient died shortly after from invasive pulmonary aspergillosis.
This case of BPDCN is interesting for three reasons. In the absence of classical lineage marker expression, the low staining of cCD79a by immunohistochemistry led to a misdiagnosed B lymphoma. Flow cytometry using 2 different antibodies confirmed the absence of CD79a expression in peripheral blasts (Figure 2D). The expression of cCD79a is unusual in BPDCN. In a French network, we have compiled 72 BPDCN cases, and only one case expressed a low level of cCD79a (F. Garnache-Ottou, unpublished data). As previously described in T-cell acute lymphoblastic leukemia, cCD79a must not be considered as a B-specific lineage marker [6]. The absence of CD56 expression is remarkable in the context of BPDCN diagnosis, although it has previously been described [3,7]. In this case, pDC markers were essential for the diagnosis. Lastly, the cytology was particular because it lacked the typical “pearl necklace” aspect, and also because of the unusual presence of azurophilic granulations (Figure 2A). To the best of our knowledge, this is the first report to describe such granulations in BPDCN cells. In conclusion, biologists should be aware of this atypical presentation, in order to avoid any delay in the diagnosis of BPDCN.
References
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasm s and beyond: evolving concepts and practical applications. Blood. 2011; 117: 5019-5032.
- Angelot-Delettre F, Biichle S, Ferrand C, Seilles E, Gaugler B, Harrivel V, et al. Intracytoplasmic detection of TCL1-but not ILT7-by flow cytometry is useful for blastic plasmacytoid dendritic cell leukemia diagnosis. Cytometry A. 2012; 81A: 718-724.
- Garnache-Ottou F, Feuillard J, Ferrand C, Biichle S, Trimoreau F, Seilles E, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009; 145: 624-636.
- Feuillard J, Jacob MC, Valensi F, Maynadié M, Gressin R, Chaperot L, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002; 99: 1556-1563.
- Leroux D, Mugneret F, Callanan M, Radford-Weiss I, Dastugue N, Feuillard J, et al. CD4+, CD56+ DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: a study of 21 cases by the Groupe Français de Cytogénétique Hématologique. Blood. 2002; 99: 4154-4159.
- Pilozzi E, Pulford K, Jones M, Müller-Hermelink HK, Falini B, Ralfkiaer E, et al. Co-expression of CD79a (JCB117) and CD3 by lymphoblastic lymphoma. J Pathol. 1998; 186: 140-143.
- Mitteldorf C, Bertsch HP, Baumgart M, Haase D, Wulf G, Schön MP, et al. Lacking CD56 expression in a relapsing cutaneous blastic plasmacytoid dendritic cell neoplasm after allogeneic bone marrow transplantation: FISH analysis revealed loss of 11q: CD56-negative BPDCN after BMT with loss of 11q. J Eur Acad Dermatol Venereol. 2011; 25: 1225-1229.