
Special Article - Blood & Its Components
J Blood Disord. 2016; 3(1): 1036.
SCD in Saudi Children, Teens, and Adults: A Study in Health-Related Quality of Life
Ahmed AE1,2*, Alaskar AS1,2,3, McClish DK4, Ali YZ3, Malhan HM5, Aldughither MH6, Al-Suliman AM7 and Jazieh AR3
¹King Saud Bin Abdul-Aziz University for Health Sciences, Saudi Arabia
²King Abdullah International Medical Research Center, Saudi Arabia
³King Abdul-Aziz Medical City, Saudi Arabia
4Department of Biostatistics, Virginia Commonwealth University, USA
5King Fahad Hospital Central, Saudi Arabia
6National Anti-Corruption Commission, Saudi Arabia
7King Fahad Hospital, Saudi Arabia
*Corresponding author: Anwar E. Ahmed, Department of Epidemiology and Biostatistics, College of Public Health and Health Informatics, King Saud bin Abdulaziz University for Health Sciences, MC 2350, P.O.Box 22490 Riyadh, 11426, Saudi Arabia
Received: February 24, 2016; Accepted: March 22, 2016; Published: March 29, 2016
Abstract
Background: While there have been earlier studies assessing Saudi adolescents with Sickle Cell Disease (SCD), this study demonstrates that Health Related Quality of Life (HRQoL) scores were negatively associated with increasing age in the adolescent population with SCD. To date, no study has assessed HRQoL in Saudi children and adults with SCD. The aim of this study was to assess HRQoL in patients with SCD and to identify what age group was most commonly associated with poor HRQoL.
Methods: A multicenter, cross-sectional study was conducted on patients with SCD who visited the hematology outpatient clinics at King Fahad Hospital in Hofuf and King Fahad Central Hospital in Jazan, Saudi Arabia. Data were gathered using a self-administered questionnaire: patient demographics, symptoms/complications of SCD, and the RAND SF-36. Data were collected from 753 SCD patients (68 children age = 14 years, 56 teenagers age 15-17 years, 290 young adults age 18-25 years, 233 intermediate-aged adults age 26-36 years, and 106 older adults age = 37 years).
Results: The ages ranged from 9 to 75 years with a mean of age of 26.4±10 years. As compared to children, young, intermediate-aged and older adults with SCD reported quite worse physical role functions and bodily pain. Better social function was noted in children than the intermediate-aged adults and older adults.
Conclusion: The current investigation noted age differences in HRQol, particularly between children and adults. Future studies are warranted to focus on the reasons of age differences in HRQoL and on the very poor HRQol of older adults.
Keywords: Sickle cell disease; Quality of life; HRQoL; Children; Teenagers; Adults
Abbreviations
ANOVA: Analysis of Variance (Fisher); HRQOL: Health Related Quality of Life; SCD: Sickle Cell Disease; SF-36: Short Form (36) Health Survey; WHOQOL-BREF: World Health Organization Quality-of-Life Scale
Introduction
Sickle Cell Disease (SCD) is considered the most common genetic disease in the eastern, western, and southern regions of Saudi Arabia [1-6]. Specifically, it is mainly spread throughout several cities: Hufuf, Qatif, and Jazan. The frequency of the SCD gene in the Hofuf region has reached 25% [4]. SCD patients tend to report fever, swelling of the hands and feet, need for blood transfusions, pulmonary hypertension, acute joint necrosis, or severe abdominal pain [7-9]. SCD complications and symptoms usually appear in childhood and last a lifetime. However, these SCD complications and symptoms could increase considerably as age increases. The average number of complications tends to increase with age in adults SCD patients [10].
SCD patients have been shown to report poorer Health Related Quality of Life (HRQoL) compared with general populations, or patients with other chronic non-communicable diseases [10-13]. Several studies have addressed HRQoL in children or adolescents within SCD populations [12,14]. HRQoL among Saudi adolescents with SCD, aged 14-17, was assessed using the RAND Short Form (36) Health Survey (SF-36). The authors noted that poorer HRQoL has been shown to correlate with age in adolescents with SCD [14]. However, a study that utilized Pediatric Quality of Life InventoryTM (PedsQL™) Generic Scales to assess physical, social, emotional, and school functioning in children and adolescents (ages between 8-18 years), found no significant differences in PedsQL between age groups [12]. The discrepancy between the two studies could be due to the sampling populations, whereas the latest study involved children.
In adult populations, increases in age tend to reduce all SF-36 scores, which indicate poor quality of life [10]. Another study found that elderly thalassemia patients tend to report poor HRQoL [15]. Sanders et al. examined age differences in pain, coping and healthcare utilization in adults with SCD (18-62 years). They concluded that age plays an important role in healthcare utilization and coping of the SCD patients [16]. According to their study younger SCD patients (= 36 years old) tend to cope by ignoring pain. However, there were no differences in psychological distress or social support across the age groups [16]. Edwards et al. examined the role of self-efficacy of 147 African American adults with SCD (18 years or greater). It was noted that age tends to relate negatively with physical symptoms [17]. A recent study by our group of data collected from Saudi Arabia on SF- 36 in adult patients with SCD has suggested no age differences were found in SF-36 subscales [18].
All the above-mentioned literature focused on children or adults; however they lack the ability to assess and compare HRQoL across various age groups that include both children and adults. There have been a few such studies involving samples from children and adults for the reasons of assessment and comparison of the HRQoL of SCD patients in various age groups [19-21]. A study conducted at King Abdulaziz University Hospital, Saudi Arabia assessed HRQoL in patients with SCD aged between 2-48 years using the WHOQOLBREF: World Health Organization Quality-of-Life Scale (WHOQOLBREF) [18]. This study observed differences in WHOQOL-BREF among various age groups with children patients (2-12 years) tending to be happier than the adults patients (18 years or older) (74% versus 42%) [19]. A study conducted in Alagoas, Brazil on 40 SCD patients aged 12 to 43 years showed that the adults rather than adolescents exhibited significantly lower SF-36 scores in several domains [20].
The hypothesis tested in this study is whether any component of SF-36 subscales is significantly poorer/worse in certain age groups. To the best of our knowledge, no finding has been reported on the comparison of HRQoL across children, teenagers, and adults. The aim of the current study was to assess these differences in a large sample of people with SCD in Saudi Arabia.
Methods
A sample of 753 patients with sickle cell disease was recruited between October 1, 2014 to February 29, 2015 from the hematology outpatient clinics of two hospitals: King Fahad Hospital in Hofuf and King Fahad Central Hospital in Jazan, Saudi Arabia. The study participants were patients with SCD of age 9 years old or greater where the patient had the ability to read and write. The current study was approved by the Ministry of Health, Kingdom of Saudi Arabia and King Abdullah International Medical Research Center, Research Protocol - RC12/127/R. The ethical approval for this study was gained by verbal consent with the IRB Log No-15-247E. Permission and assent was obtained from parents of all children and teenagers involved in the study (9 to 17 years old). Adult SCD patients who verbally consented to participate in the study were asked to complete the survey. No identifiable data gathered from participants. Sample characteristics were collected, such as age/year, gender (male/female), and engaging in regular exercise (yes/no). Clinical characteristics were also collected such as family history of anemia (yes/no), whether the spleen had been removed (yes/no), any history of blood transfusions (yes/no), the number of visits to the Emergency Department (ED) within the past six months (<3 or =3 visits), and the presence of chronic diseases other than SCD (yes/no). Symptoms and complications of SCD during the past six months were collected, such as swelling (yes/no), fever (yes/no), and skin redness (yes/no).
Study instrument
In the present study we utilized the Medical Outcome Study 36- Item Short Form (SF-36) [21]. The SF-36 was found to be reliable across various age groups [21]. The Arabic-translated version of RAND SF-36Health Related Quality of Life Questionnaire [22] was used to evaluate Health Related Quality of Life in our population. The SF-36 questionnaire consists of 8 subscales that assess perceptions and feelings with regard to physical function (ten items); emotional role functions (three items); vitality (four items); physical role health (four items); emotional wellbeing (five items); bodily pain (two items); social function (two items); and general health perceptions (five items). The SF-36 subscales were scored according to RAND Health scoring. Each subscale has a single summary variable ranged from 0 = least favorable health state to 100 = most favorable health state. The reliability of the SF-36 has been assessed in a pilot study of eighty SCD patients. The reliability analysis showed acceptable internal consistency with Cronbach alpha (a) ranged from 0.6 to 0.86. The reliability analysis showed high internal consistency for vitality (a = 0.79); physical function (a = 0.81); bodily pain (a = 0.84); physical role health (a = 0.84); and emotional role functions (a = 0.86). The other three domains had a lower than the ideal range: general health (a = 0.60); social function (a = 0.67); and emotional wellbeing (a = 0.67).
Statistical analyses
Data were analyzed using IBM Statistical Package for Social Sciences (SPSS®) version 23 (Chicago, Illinois, USA). On the basis of age, SCD patients were classified into five groups: children age = 14 years, teenagers age 15-17 years and adults (young adults age 18-25 years, intermediate-aged adults age 26-36 years, and 106 older adults age = 37 years). Some of these cut-off points of age-groups were used in a previous study [16]. Frequency and count were used to summarize the sample characteristics (Table 1). Chi-square linear-by-linear tests were performed for associations between symptoms/complications of SCD and age spectrum (Table 1). ANOVAs (Analysis of Variance) were performed to compare quality of life subscales across various age groups (Table 2 and Figure 1). When appropriate, Tukey’s multiple comparison procedure was applied for age group comparison with significant level at 0.05.
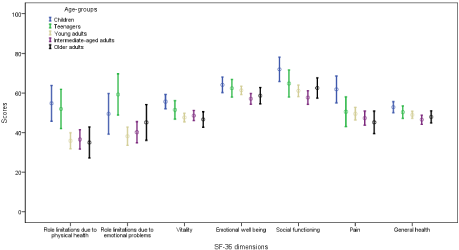
Figure 1: Differences in health-related quality of life between age-groups by the SF-36 dimensions: 95% confidence intervals.
Overall
Children
Teenagers
Young adults
Intermediate-aged adults
Older adults
=14 yrs
15-17 yrs
18-25 yrs
26-36 yrs
v37 yrs
N=753
68(9%)
56(7.4%)
290(38.5%)
233(30.9%)
106(14.1%)
Characteristics
Levels
n
%
n
%
n
%
n
%
n
%
n
%
P
Gender
Male
439
58.5
38
57
27
48
172
59
131
57
71
68
0.159
Female
311
41.5
29
43
29
52
118
41
101
44
34
32
ED visits
=3
422
67.1
31
57
30
65
172
69
137
69
52
63
0.486
Obese
Yes
50
8.8
4
9.8
2
5
13
6.4
14
7.3
17
19
0.024*
Fever
Yes
427
58.4
27
42
35
64
163
58
146
65
56
54
0.106
Redness
Yes
237
32.5
17
27
16
30
85
30
83
37
36
35
0.065
Swelling
Yes
337
46.1
22
34
28
51
113
40
128
57
46
45
0.019*
Blood transfusion
Yes
609
82.0
45
67
44
79
242
84
196
86
82
78
0.048*
Family history of anemia
Yes
658
89.8
60
91
44
82
255
90
206
90
93
90
0.549
Family support
Yes
680
93.4
67
100
53
98
266
95
209
93
85
84
0.001*
Chronic disease other than SCD
Yes
116
15.9
8
12
6
11
37
13
38
17
27
26
0.005*
Regular exercise
Yes
254
34.3
24
36
22
39
104
36
74
32
30
29
0.171
Spleen removed
Yes
130
17.5
11
17
6
11
45
16
47
20
21
20
0.144
*. Chi-square linear-by-linear is significant at a=0.05.
Table 1: Symptoms/complications of Sickle Cell Disease in relation to age groups.
Children
=14 yrs
68(9%)
Teenagers
15-17 yrs
56(7.4%)
Young adults
18-25 yrs
290(38.5%)
Intermediate-aged adults
26-36 yrs
233(30.9%)
Older adults
=37 yrs
106(14.1%)
Mean
SD
Mean
SD
Mean
SD
Mean
SD
Mean
SD
P
Physical functioning
60.2
22.3
56.3
22.1
56.0
23.2
54.8
23.8
53.5
22.7
0.446
Role limitations due to physical health
54.8
35.9
51.9
36.0
35.8
34.2
36.5
37.0
34.9
39.1
0.001*
Role limitations due to emotional problems
49.5
41.1
59.3
38.1
38.1
38.5
40.2
40.2
45.1
46.1
0.004*
Vitality
55.6
14.8
51.4
16.4
47.6
18.4
48.5
19.3
46.6
20.2
0.016*
Emotional well being
64.1
15.6
62.4
16.3
61.3
17.8
57.0
20.5
58.6
21.0
0.026*
Social functioning
71.9
25.0
64.8
25.2
61.1
24.2
57.7
25.4
62.5
26.1
0.002*
Pain
61.8
28.1
50.5
27.8
49.5
27.4
47.3
27.1
45.1
29.4
0.002*
General health
52.8
11.0
50.3
11.4
48.9
14.6
46.5
17.0
47.9
15.1
0.039*
*. ANOVA test is significant at a=0.05.
Table 2: Characterizes differences in HRQoL of patients with SCD across the age groups (N=753).
Results
Seven hundred fifty-three (N=753) SCD patients were included in the study. Age ranged from 9 to 75 years (mean age 26.4±10 years). The SCD patients were distributed as the following, 68 in children group, 56 in teenagers group, and 290 in young adults group, 233 in intermediate-aged adults group, and 106 in older adults group. The majority (89.8%) of the SCD patients reported family history of anemia, 82% reported history of blood transfusions, and 93.4% reported family support. The most commonly reported symptoms were: fever (58.4%), swelling (46.1%), and skin redness (32.5%). Removal of the spleen was reported by 17.5% of the SCD patients. Symptoms and complications of sickle cell disease in relation to the age spectrum are reported in (Table 1). Swelling and blood transfusion were significantly less common in children with SCD compared with other age groups (teenagers, young adults, intermediate-aged adults, and older adults). However, obesity and chronic diseases other than SCD were reported more frequently in the older adults with SCD than in other age groups (children, teenagers, young adults, and intermediate-aged adults). Family support was found to be less common in older adults with SCD compared with other age groups (children, teenagers, young adults, intermediate-aged adults). Gender, ED visits, fever, skin redness, and regular exercise were not significantly associated with age (Table 1).
(Table 2) characterizes the differences in HRQoL of patients with SCD across the age spectrum. According to our study, there were statistically significant age group differences on physical role function (F =5.4, P=0.001), emotional role functions (F =3.4, P=0.009), vitality (F =2.6, P=0.038), emotional wellbeing (F=2.9, P=0.022), social function (F =4.8, P=0.001), bodily pain (F =3.8, P=0.004), and General health (F =2.5, P=0.039), (Figure 1). No differences in physical function were noted between the various age groups. Multiple comparisons of various age groups revealed that children and teenagers with SCD had significantly higher scores on physical role functions than adults groups (young adults, intermediate-aged adults, and older adults). More specifically, Saudi children with SCD tend to experience better physical role functions as compared to young adults, intermediateaged adults, and older adults (observed difference: 18.9, 18.3, and 19.8, respectively). Teenagers reported better emotional role functions as compared to young adults and intermediate-aged adults (observed difference: 21.1 and 19.1, respectively). More vitality was found in children than the young adults and older adults (observed difference: 8.0 and 9.0, respectively). Better social function was noted in children than the young adults and intermediate-aged adults (observed difference: 10.8 and 14.2, respectively). Saudi children with SCD tend to report less bodily pain as compared to young adults, intermediateaged adults, and older adults (observed difference: 12.3, 14.5, and 16.7, respectively). Children tend to report better social function than young adults and intermediate-aged adults (observed difference: 10.8 and 14.2, respectively). General health scale was significantly better in children than the intermediate-aged adults (observed difference: 6.3).
Discussion
The purpose of this investigation was to assess the HRQoL in Saudi patients with SCD within various age groups across a wide spectrum of ages. In the present study we considered five age groups: children (= 14 years), teenagers (15-17 years), young adults (18-25 years), and intermediate-aged adults (26-36 years), and older adults (= 37 years). We evaluated age differences in HRQoL for patients with SCD in a large multicenter cross-sectional study. Based on our study, adults with SCD generally tend to report poor HRQoL than children or teenagers. Research performed in Saudi Arabia has shown that the HRQoL in the adolescent population with SCD tends to get worse as the age of the adolescent increases [14]. On the other hand, two studies reported HRQoL in the adult population with SCD, and both demonstrated that age has a negative effect on HRQoL- as age increases patients tended to report a poorer quality of life [10,15]. No significant differences in HRQoL subscales were found in terms of adult age groups (Older age: Yes/No) in a study conducted by our group [18]. However, little is known about the comparison of HRQoL in children and teenagers as compared to older adults, or between more subgroups of adult patients (young adults, intermediate-aged adults, and older adults) with SCD. In addition to SCD adult data [18], the current study incorporated data from children and teenagers in the analysis for the purpose of age comparisons. Two studies involved samples from children and adults, but their patients’ ages ranged between 2-48 years [19] and between 12-43 years [20]. Although no elderly patients were included in their studies, both studies revealed that old patients exhibited poorer quality of life. Our population had ages ranging from 9 to 75 years, and thus may be considered an extension of their study.
Our findings revealed that there were statistically significant differences in HRQoL subscales by age groups. According to our study, various adult aged groups tend to report poorer HRQoL then children and/or teenagers. This finding is consistent with the results of several studies, which have shown that HRQoL subscales tend to decrease with age, meaning older patients reported poorer HRQoL than the younger age group [10,11,14,15,19,20]. For instance a study reported that as the age of adolescents with SCD increases by one year, the average of several SF-36 domains tends to decrease [14]. In our study, the physical role function and bodily pain were strikingly worse in adults. It was noted that bodily pain scores decreased monotonically with age. Children tended to report less bodily pain than the young adults, intermediate-aged adults, and older adults. Saudi children with SCD tend to experience better social functioning, general health as compared to young adults and intermediate-aged adults. It is interesting to note that the teenagers group has the highest values for emotional role functions. According to our study, children with SCD were less likely to report SCD related-complications such as swelling and blood transfusion as compared to adult patients with SCD. Our study agrees with the findings of a previous study, which concluded adult patients exhibited a higher number of SCD relatedcomplications [10].
Several limitations of the current study were noted. Sample selection was based on purposive sampling techniques but not random sampling. The target SCD population was from outpatient clinics but not community-based, as we only surveyed SCD patients who attended outpatient clinics for medical care. Thus, the chance of being part of the study was high in SCD patients who actively attend outpatient clinics. SCD patients who did not have ability to read and write were excluded from the sample. One might hypothesize that these patients might have somewhat poorer quality of life than those who don’t seek medical care, but there is no reason to believe that age effects would vary. Accordingly, our study could be generalizable only in SCD patients who attend outpatient clinics for medical care. The findings were based on self-reported data, and associations do not confirm causality. Some of the sub-samples may have been small to detect important differences between age-groups. The SF-36 is suitable for self-report to individuals who are age 14 years and older, while our population included age 9 years and older. However, several strengths of this study should be noted. The study was able enroll SCD patients from two centers in Saudi Arabia, Hofuf and Jazan, and was able to recruit a large sample size of 753 SCD patients. The study covers a broad age range of patients with SCD. We compared HRQoL of SCD patients in various age groups (children, teenagers, young adults, intermediate-aged adults, and older adults) with ages ranging from 9 to 75 years. This is the first investigation nationally and internationally to report age-specific HRQoL in patients with SCD that encompassed such as wide range of ages.
Conclusion
Differences in HRQoL subscales appeared to depend on age in patients with SCD. Adults with SCD reported poorer physical role functions and worse bodily pain than children with SCD. Adults with SCD reported significantly more SCD-related swelling and blood transfusion than children. It is highly recommended that hematologists routinely assess the quality of life of adult patients and SCD-related complications, since they tend to exhibit poorer quality of life. Additionally, future studies are warranted to focus on the reasons for the HRQoL differences shown between the five age groups with SCD.
Acknowledgement
The authors would like to thank the Ministry of Health, Kingdom of Saudi Arabia for approving this study. The authors would also like to thank King Abdullah International Medical Research Center for approving and funding this research. The authors also acknowledge the efforts of Huda Salman Abdulla Al-Salman, Zakaria Ali Sharidah, Anas Walid Alhakim, Asim Mohamed Walid Mohamed Aldorrah, and Omar Abdulaziz who have been involved in collecting the data for this research.
References
- Lehmann H, Maranjian G, Mourant AE. Distribution of sickle-cell hemoglobin in Saudi Arabia. Nature. 1963; 198: 492-493.
- el-Hazmi MA. Clinical and haematological diversity of sickle cell disease in Saudi children. J Trop Pediatr. 1992; 38: 106-112.
- el-Hazmi MA, Warsy AS. Appraisal of sickle-cell and thalassaemia genes in Saudi Arabia. East Mediterr Health J. 1999; 5: 1147-1153.
- Alhamdan NA, Almazrou YY, Alswaidi FM, Choudhry AJ. Premarital screening for thalassemia and sickle cell disease in Saudi Arabia. Genet Med. 2007; 9: 372-377.
- Alabdulaali MK. Sickle cell disease patients in eastern province of Saudi Arabia suffer less severe acute chest syndrome than patients with African haplotypes. Ann Thorac Med. 2007; 2: 158-162.
- Al-Qurashi MM, El-Mouzan MI, Al-Herbish AS, Al-Salloum AA, Al-Omar AA. The prevalence of sickle cell disease in Saudi children and adolescents. A community-based survey. Saudi Med J. 2008; 29: 1480-1483.
- Dahoui HA, Hayek MN, Nietert PJ, Arabi MT, Muwakkit SA, Saab RH, et al. Pulmonary hypertension in children and young adults with sickle cell disease: evidence for familial clustering. Pediatr Blood Cancer. 2010; 54: 398-402.
- Onyekwere OC, Campbell A, Teshome M, Onyeagoro S, Sylvan C, Akintilo A, et al. Pulmonary hypertension in children and adolescents with sickle cell disease. Pediatr Cardiol. 2008; 29: 309-312.
- Chiang EY, Frenette PS. Sickle cell vaso-occlusion. Hematol Oncol Clin North Am. 2005; 19: 771-784.
- Dampier C, LeBeau P, Rhee S, Lieff S, Kesler K, Ballas S, et al. Comprehensive Sickle Cell Centers (CSCC) Clinical Trial Consortium (CTC) Site Investigators. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol. 2011; 86: 203-205.
- McClish DK, Penberthy LT, Bovbjerg VE, Roberts JD, Aisiku IP, Levenson JL, et al. Health related quality of life in sickle cell patients: the PiSCES project. Health Qual Life Outcomes. 2005; 3: 50.
- Dale JC, Cochran CJ, Roy L, Jernigan E, Buchanan GR. Health-related quality of life in children and adolescents with sickle cell disease. J Pediatr Health Care. 2011; 25: 208-215.
- Bhagat VM, Baviskar SR, Mudey AB, Goyal RC. Poor health related quality of life among patients of sickle cell disease. Indian J Palliat Care. 2014; 20: 107-111.
- Amr MA, Amin TT, Al-Omair OA. Health related quality of life among adolescents with sickle cell disease in Saudi Arabia. Pan Afr Med J. 2011; 8: 10.
- Sobota A, Yamashita R, Xu Y, Trachtenberg F, Kohlbry P, Kleinert DA, et al. Quality of life in thalassemia: a comparison of SF-36 results from the thalassemia longitudinal cohort to reported literature and the US norms. Am J Hematol. 2011; 86: 92-95.
- Sanders KA, Labott SM, Molokie R, Shelby SR, Desimone J. Pain, coping and health care utilization in younger and older adults with sickle cell disease. J Health Psychol. 2010; 15: 131-137.
- Edwards R, Telfair J, Cecil H, Lenoci J. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001; 63: 850-858.
- Ahmed AE, Alaskar AS, Al-Suliman AM, Jazieh AR, McClish DK, Salamah MA, et al. Health-related quality of life in patients with sickle cell disease in Saudi Arabia. Health and Quality of Life Outcomes. 2015; 13: 183.
- Al Jaouni SK, Al Muhayawi MS, Halawa TF, Al Mehayawi MS. Treatment adherence and quality of life outcomes in patients with sickle cell disease. Saudi Med J. 2013; 34: 261-265.
- Vilela RQ, Cavalcante JC, Cavalcante BF, Araújo DL, Lobo Mde M, Nunes FA. Quality of life of individuals with sickle cell disease followed at referral centers in Alagoas, Brazil. Rev Bras Hematol Hemoter. 2012; 34: 442-446.
- Newnham EA, Harwood KE, Page AC. Evaluating the clinical significance of responses by psychiatric inpatients to the mental health subscales of the SF- 36. J Affect Disord. 2007; 98: 91-97.
- Coons SJ, Alabdulmohsin SA, Draugalis JR, Hays RD. Reliability of an Arabic version of the Rand-36 Health Survey and its equivalence to the US-English version. Medical Care. 1998; 36: 428-432.