
Research Article
J Blood Disord. 2019; 6(1): 1055.
Intra-Cerebrospinal Fluid Chemotherapy is New Direction in Pediatric Solid Tumors in Developing Countries
Babak A1, Zareifar S2*, Pouria S3 and Somayeh L1
1Department of Pediatric, Lorestan University of Medical Science, Iran
2Department of Pediatric, Shiraz University of Medical Science, Iran
33Department of Pediatric, Iran University of Medical Science, Iran
*Corresponding author: Soheila Zareifar, Department of Pediatric, Shiraz University of Medical Science, Shiraz, Iran
Received: December 04, 2019; Accepted: December 30, 2019; Published: December 31, 2019
Abstract
Introduction: Intra-cerebrospinal fluid (CSF) chemotherapy defined chemotherapy drugs administration in the brain/spine fluid compartment for leptomeningeal metastases treatment in infilterative tumors such as leukemia and lymphoma routinely. This method was administered for adults since 20 years ago but there is a limited experience in children.
Material and Method: We used electronic search on the online medical database "PubMed" and other motor search-engines such as "Google Scholar” and selected all of articles about intra-CSF chemotherapy regimens in solid tumors.
Results: We collected original articles and review articles over 20 years, on the target .All of selected articles include intrathecal chemotherapy regimens in solid tumors in pediatrics and adult patients with leptomeningeal metastasis.
Conclusion: We introduce several intra-CSF chemotherapy regimens in solid tumors. This approach can be used in pediatrics with radiation limitation.
Keywords: Intrathecal chemotherapy; Leptomeningeal metastases; Solid tumors
Introduction
Intra-cerebrospinal fluid (CSF) chemotherapy is chemotherapy drugs administration in the brain/spine fluid compartment, which can treat leptomeningeal metastases of infiltrative tumors (leukemia and lymphoma) since some years ago, but intra-CSF chemotherapy drugs administration during pediatric solid tumors treatment is unusual and there are limit experiences. This is a new insight and approach for fighting against leptomeningeal metastasis of solid tumors in cancerous children. This technique is available by 2 methods: 1.intratechally 2. intraventricular through ommaya reservoir. Co-administration of this technique with conventional chemotherapy in metastatic embryonic tumors among younger kids has good outcome. In this technique is tried to supply excessive doses of chemotherapy to the coating regions of the brain and cover sanctuary sites [1-3].
Intrathecal chemotherapy destroys subclinical leptomeningeal deposits and kill tumor cells floating in the cerebrospinal fluid, and prevent tumor seeding [4].
Efficacy of single or combined intracerebrospinal (ICSF) chemotherapy is a challenging and controversial issue. Some trials have shown no distinction among single-agent Methotrexate and combined regimen. Combination regimens may be have greater neurotoxicity than single ICSF chemotherapy [5].
Use of intraventricular ommaya reservoir has better protective effect than intrathecal chemotherapy against tumor cells.
This modality of treatment provides high concentration of the drug on the site of the tumor and has more harmful effects on the normal brain [6,7].
Thiotepa and methotrexate (MTX) is the one of the oldest chemotherapy drugs has administered intra-CSF in solid or hematopoetic malignancies. Thiotepa is cleared from CSF within a few minutes and has survival curves similar to those of MTX, but it is lesser neurotoxic than MTX. Leukoencephalopathy secondary to intraventricular excessive dose of methotrexate is a problematic event after long-term intra-CSF administration of MTX, but decreasing methotrexate dose infused into the fourth ventricle has lesser neurological deficits or toxicity.
Recently, new agents introduced for intra-CSF chemotherapy. A few studies define intra-CSF chemotherapy does no longer extend patient survival and considerably increases associated neurotoxicity’s [8].
Material and Method
We searched the Medline (PubMed) electronic database by using a broad search strategy. The studies were identified by utilizing a combination of MeSH terms, such as intra-CSF chemotherapy, intratechal chemotherapy, solid tumors and leptomeningeal metastasis. Two reviewers independently screened the list of references to assess their eligibility for inclusion in consultation with another reviewer. Collection, spanning over 20 years, was searched, as were recent issues of journals in which articles on the subject are most often found (2017-1995). The inclusion criteria were as follows:
1- The articles address an appropriate and clearly focused question
2- The study was done to minimise bias
3- Identify all the relevant studies.
4- There are enough similarities between the studies selected to make combining them reasonable.
5- Publication date between 1995- 2017
6-Article in English
7- Peer-reviewed journals. In addition, an extensive personal literature.
Inclusion Criteria
Finally, 31 main articles were founded to form the basis for this mandatory narrative review.
Results
A total of 31 publications (22 include and 67 exclude) during 1995-2017, met eligibility criteria for this review was based on successful experiences for intra-CSF chemotherapy on solid tumors.
We found some chemotherapy drugs for intra-CSF chemotherapy for solid tumors treatment in the literature and suggest two standard intrathecal chemotherapy regimens for pediatric solid tumors.
Discussion
Majority of intra-CSF chemotherapy regimens in solid tumors were suggested for adult and pediatric during two decades.
Traditionally, intra-CSF drug regimen include 3 chemotherapeutic drugs (Methotrexate, Cytosine Arabinoside, and Thiotepa) administered through intratechal or intraventricular pathway [9].
Cytararabine is not effective for solid tumors but is useful in leukemic and lymphomatous meningitis. It is now available in liposome-encapsulated form (DepoCyt) that can be administered every 2 weeks rather than 2-3 times a week and results in a longer time to disease progression and higher quality of life than therapy with MTX [10].
Thiotepa can be use as the second line agent after MTX and cytarabine [11].
Intraventricular methotrexate is used for non-disseminated tumors that were absolutely resected. Five-year free survival after the addition of methotrexate is envisioned approximately 60%. Just 3-5% of systemically administered MTX penetrates the blood-brain barrier (BBB) at a half of-clearance time of 6 hours. Single intraventricular injection of MTX (6.25 mg/m2) can achieve healing awareness inside the lumbar area for 48 hours at a minimal systemic absorption (<0.1 µM ) [12].
In brain tumors, intraventricular methotrexate therapy was more acceptable and feasible and mostly well tolerated. In this method, infections were the most frequent complication. A higher cumulative dose of intraventricular methotrexate was associated with better survival [13].
IC: Intrathecal chemotherapy; RT: Radiation Therapy; KPS: Karnofsky Performance Status; MTX: Methotrexate; DXM: Dexamethasone
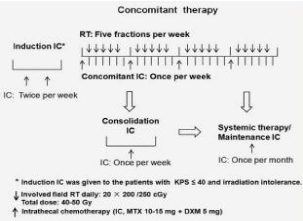
Figure 1: Protocol schema.
IC: Intrathecal chemotherapy; RT: Radiation Therapy; KPS: Karnofsky
Performance Status; MTX: Methotrexate; DXM: Dexamethasone

Figure 2: BCCA Protocol Summary for Solid Tumors using Intrathecal Methotrexate and/or Thiotepa and/or Cytarabine.
In meningeal carcinomatosis with concomitant parenchymal brain metastasis, management of repeated courses of intrathecal chemotherapy (ITC) consistent with the following alternated weekly time is suggested: day 1: thiotepa 10 mg, methotrexate 15 mg, hydrocortisone 30 mg; day five: cytarabine (Ara-C) 70 mg, methotrexate 15 mg, hydrocortisone 30 mg. folinic acid 15 mg became given orally, every six hours after methotrexate on days 2-3 and 6-7 confirm the controversial role of ITC [14].
The position of intrathecal/intraventricular chemotherapy in number one CNS lymphoma (PCNSL) is not described. MTX, Ara-C and corticosteroids were given via lumbar or ventricular (through a subgaleal reservoir) routes as a part of systemic chemotherapy regimens: MTX 12 mg two times per week became given by using lumbar path in trials ,but intraventricular packages offer decrease day by day doses to attain sustained CSF tiers [15].
Some chemotherapy or biologic agents for leptomeningeal carcinomatosis include:
Mafosfamide is a shape of Cyclophosphamide that is active intrathecally and has little neurotoxicity aside from complications. Mafosfamide at a dose of 20 mg once or twice weekly is administered until remission is achieved, follow by weekly administrations as consolidation therapy, and every 3 to 4 weeks thereafter for maintenance therapy [16].
Rituximab has been given intrathecally and is likewise prescribed (from lymphoma only). Inta-CSF dose of Rituximab ranged from 10 to 40 mg. An initial dose of 10 mg became used most usually and systematically escalated to clinical response or affected person tolerance. Rituximab doses encompass: 10 mg, 25 mg or 50 mg were administered diluted in normal saline (Nacl 0.9%) or undiluted as immediately drug throughout a period of 1 minute to 5 minutes [17].
Trastuzumab has been given intrathecally to deal with leptomeningeal (LC) for breast cancer. First ventricular injection of 50 mg of trastuzumab is administered (cycle 1) and, after a 21-day interval, trastuzumab is injected at a dose of 50 mg inside the ventricle and at a dose of 6 mg/kg intravenously (cycle 2; intravenous). The timing and the doses of later ventricular injections were set up in keeping with the pharmacokinetic profile of trastuzumab concentrations inside the CSF. On days 1, 2, 4, 7, and 14 of those first cycles, trastuzumab concentrations had been measured with the aid of enzyme-linked immunosorbent assay (ELIZA) in lumbar and ventricular CSF, and in peripheral blood [18].
There are some case reports of LC of non-small cell lung cancer (NSCLC) or breast cancer responding to intrathecal gemcitabine, trastuzumab, letrozole, and tamoxifen [19].
Immunotoxins, which includes monoclonal antibodies coupled with a protein toxin or radioisotope, appear powerful and are being studied. Compartmental intrathecal antibody-based radioimmunotherapy (cRIT) administration in recurrent metastatic central nervous system (CNS) neuroblastoma following surgical operation is one of the theses alternative techniques.
The cRIT-containing salvage regimen incorporating intrathecal I-131 monoclonal antibodies (MoAbs) targeting GD2 or B7H3 following surgical procedure and radiation or 1 or 2 monthly injections 131I-8H9 (10–60 mci/injection).
Furthermore, unwell-described delayed neurotoxicity turned into not able to be differentiated from disorder progression.
Furthermore, ill-defined delayed neurotoxicity was unable to be differentiated from disease progression.
Protocol schema intratechal chemotherapy treating leptomeningeal metastasis from solid tumors with adverse prognostic factors
The regimen consisted of IC via lumbar punctures,
Induction IC (MTX 12.5–15 mg, plus dexamethasone 5 mg, twice per week): Then these patients were allowed to receive concomitant therapy upon neurologic improvement and radiotherapy tolerance. Supporting therapy was given to patients with low KPS score.
Concomitant therapy: Include consolidation IC and fractional RT
(MTX 12.5–15 mg, plus dexamethasone 5 mg, once per week, 4 weeks in total) and IF-RT: Radiotherapy consisted of fractionated, conformal radiation given at a daily dose of 2 Gy. The planning volume consisted of sites of symptomatic disease, bulky disease observed on MRI, including the whole brain and basis cranii received 40 Gy in 20 fractions and/or segment of spinal canal received 40–50 Gy (the above segments of the first lumbar vertebra were given 40 Gy in 20 fractions; the first lumbar vertebra and the inferior segments were given 40/50 Gy in 20 fractions) (Figure 1).
Patients with KPS of ≤ 40 and irradiation intolerance were required to receive,
Protocol schema. IC: intrathecal chemotherapy; RT: radiation therapy; KPS: Karnofsky performance status; MTX: methotrexate; DXM: dexamethasone.
Subsequent treatment was recommended after concomitant therapy.
Consolidation IC (MTX 12.5–15 mg, plus dexamethasone 5 mg) was recommended once per week
The total cycles of IC including the induction therapy, concomitant therapy and consolidation therapy should be <8 times within 2 months
Maintenance IC (MTX 12.5–15 mg, plus dexamethasone 5 mg) was recommended once per month: After concomitant therapy and/or consolidation therapy to patients with stable systemic disease or longer expected survival. The patients with active systemic disease were proposed to systemic therapy (chemotherapy or molecular target therapy) according to the NCCN guidelines of related tumors.
BCCA Protocol Summary for Solid Tumors using Intrathecal Methotrexate and/or Thiotepa and/or Cytarabine: Maximum 2 intrathecal chemotherapy treatments weekly (e.g., Monday and Thursday). Give one drug each treatment. ƒ Drugs may be alternated (e.g., methotrexate alternating with thiotepa for a maximum of 2 intrathecal chemotherapy treatments per week) or single agents may be used. Methotrexate and thiotepa are most commonly used in breast cancer, cytarabine in lymphomas (see protocol LYIT). However, the oncologist may use any of the above depending on the clinical situation. The drugs are generally given twice a week for 2-4 weeks and then once weekly for a total of 10-12 treatments if there is a response (improved symptoms and decreased malignant cells in CSF). If there is no response, radiation or supportive care only are options for treatment (Figure 2).
Balm M reviewed the medical records of 126 patients with cytologically confirmed leptomeningeal carcinomatosis seen at the Mayo Clinic in Rochester, Minn, from 1983 to 1994h. He showed t Patients treated with intra-CSF chemotherapy also survived longer.
Conclusion
Embryonal brain tumors are similar in appearance and differentiation potential to neural stem and progenitor cells. For prevention of drug induced resistant in stem cells in embryonal tumors should treated by risk adjusted intravenous chemotherapy protocols and probably additional local chemotherapy through ommaya reservoir or intrathecally especially in radiation limited patients.
References
- Gwak HS, Lee SH, Park WS, Shin SH, Yoo H, Lee SH. Recent Advancements of Treatment for Leptomeningeal Carcinomatosis. J Korean Neurosurg Soc. 2015; 58: 1-8.
- Gwak HS, Joo J, Shin SH, Yoo H, Han JY, Kim HT, et al. Ventriculolumbar perfusion chemotherapy with methotrexate for treating leptomeningeal carcinomatosis: a Phase II Study. Oncologist. 2014; 19: 1044-1045.
- Shim Y, Gwak HS, Kim S, Joo J, Shin SH, Yoo H. Retrospective Analysis of Cerebrospinal Fluid Profiles in 228 Patients with Leptomeningeal Carcinomatosis : Differences According to the Sampling Site, Symptoms, and Systemic Factors. J Korean Neurosurg Soc. 2016; 59: 570-576.
- Grewal J, Saria M, Grewal HK, Kesari S. Neoplastic meningitis resulting from hematological malignancies: pharmacokinetic considerations and maximizing outcome. Clin Investig (Lond). 2011; 1: 1391-1402.
- Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012; 14: iv45-iv54.
- Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982; 49: 759-772.
- Siegal T. Which drug or drug delivery system can change clinical practice for brain tumor therapy? Neuro Oncol. 2013; 15: 656-669.
- Abstracts from the Thirteenth International Symposium on Pediatric Neuro-Oncology: June 29 - July 2, 2008: Chicago, Illinois, USA. Neuro Oncol. 2008; 10: 370-515.
- Ruggiero A, Conter V, Milani M, Biagi E, Lazzareschi I, Sparano P, et al. Intrathecal chemotherapy with antineoplastic agents in children. Paediatr Drugs. 2001; 3: 237-246.
- Kripp M, Hofheinz RD. Treatment of lymphomatous and leukemic meningitis with liposomal encapsulated cytarabine. Int J Nanomedicine. 2008; 3: 397-401.
- Rhun EL, Taillibert S, Chamberlain M. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013; 4: S265-S288.
- Sandberg DI, Rytting M, Zaky W, Kerr M, Ketonen L, Kundu U, et al. Methotrexate administration directly into the fourth ventricle in children with malignant fourth ventricular brain tumors: a pilot clinical trial. J Neuro-Oncology. 2015; 125: 133-141.
- Schlegel U. Primary CNS Lymphoma. Ther Adv Neurol Disord. 2009; 2: 93-104.
- Shapiro WR, Johanson CE, Boogerd W. Treatment modalities for leptomeningeal metastases. Semin Oncol. 2009; 36: S46-54.
- Villela L, García M, Caballero R, Borbolla-Escoboza JR, Bolaños-Meade J. Rapid complete response using intrathecal rituximab in a patient with leptomeningeal lymphomatosis due to mantle cell lymphoma. Anticancer Drugs. 2008; 19: 917-920.
- Park WY, Kim HJ, Kim K, Bae SB, Lee N, Lee KT, et al. Intrathecal Trastuzumab Treatment in Patients with Breast Cancer and Leptomeningeal Carcinomatosis. Cancer Res Treat. 2016; 48: 843-847.
- Gwak HS, Joo J, Kim SH, Yoo H, Shin SH, Han JY, et al. Analysis of Treatment Outcomes of Intraventricular Chemotherapy in 105 Patients for Leptomeningeal Carcinomatosis from Non–Small-Cell Lung Cancer . J Thoracic Onco. 2013; 8: 599-605.
- Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010; 97: 409-418.
- Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996; 53: 626-632.