Editorial
Austin J Cancer Clin Res 2014;1(1): 1005.
Cancer Vaccines: A Ray of Hope
Lipika Chablani
Department of Pharmaceutical Sciences, Wegmans School of Pharmacy, USA
*Corresponding author: Lipika Chablani , Department of Pharmaceutical Sciences, Wegmans School of Pharmacy, St. John Fisher College, Rochester, NY 14618
Received: December 17, 2013; Accepted: January 10, 2014; Published: January 12, 2014
As we put forth the inaugural issue of Austin Journal of Cancer and Clinical Research, I would like to take this opportunity to welcome the readers and encourage fellow peers in academia, industry, government and related healthcare professionals to contribute their research work and expert reviews to the journal.
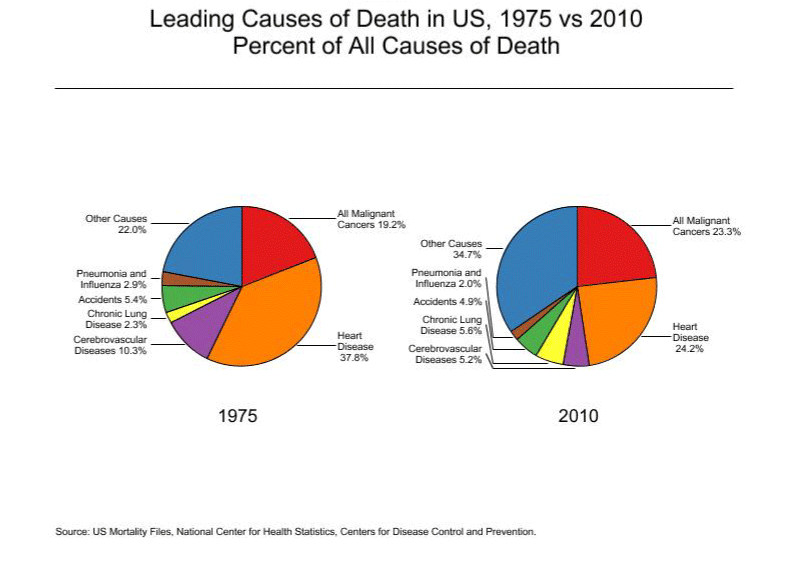
Recent cancer statistics review by Surveillance, Epidemiology, and End Results (SEER) Program by National Cancer Institute (NCI) shows that cancer is the second most leading cause of death after heart diseases. Cancer incidence has grown from 19.2% to 23.3% from 1975 to 2010 (Figure 1)[1]. Lung cancer remains to be the most fatal form of cancer followed by colorectal, breast and prostate cancer in the country (Table 1)[1]. Regardless of several treatment options, cancer remains to be a unique challenge for both patients and the healthcare providers. Several treatment options are available to address this disease now. Chemotherapy, surgery, radiation therapy are still the mainline of treatment plan for cancer patients. Along with these therapies, immunotherapy is being explored as a combination therapy. Immunotherapy allows utilization of patient’s own immune system to combat the disease and⁄or assist in avoiding a relapse.
Figure 1: Leading causes of death in US. SEER cancer statistics review 1975-2010.
Common Types of Cancer
Estimated New Cases 2013
Estimated Deaths 2013
All Cancer Sites
1,660,290
580,350
1.
Lung and Bronchus Cancer
228,190
159,480
2.
Colon and Rectum Cancer
142,820
50,830
3.
Breast Cancer
232,340
39,620
4.
Prostate Cancer
238,590
29,720
5.
Non-Hodgkin Lymphoma
69,740
19,020
6.
Bladder Cancer
72,570
15,210
7.
Kidney and Renal Pelvis Cancer
65,150
13,680
8.
Melanoma of the Skin
76,690
9,480
9.
Endometrial Cancer
49,560
8,190
10.
Thyroid Cancer
60,220
1,850
Table 1: Estimated number of new cancer cases and deaths in 2013 (SEER stat fact sheet).
Cancer research and clinical trials are one of the most challenging ones attributed to the nature of the disease state. This editorial is devoted to those, who have dedicated their careers to develop various immunotherapeutic approaches leading to the evolution of cancer vaccines, providing a ray of hope to cancer patients. These cancer vaccines are targeted to boost the immune response of the host further protecting them from the challenges posed by cancerous cells. Unlike vaccines for infectious diseases, a cancer vaccine is targeted against host’s own cells. Thus, identification and isolation of such cancer antigens is not only difficult but also unique for the patient at times.
Cancer vaccines are classified into two categories: prophylactic and therapeutic. Prophylactic cancer vaccines are preventive vaccines, currently there are two such vaccines available against cervical cancer. Cervical cancer is caused by human papilloma virus (HPV) and currently two FDA approved vaccines, Gardasil® and Cervarix®, are available for its prevention. On the other hand, therapeutic vaccinesare administered post cancer diagnosis and are for treatment purposes. Currently, Sipuleucel–T (Provenge® by Dendreon Corporation) is the only FDA approved therapeutic cancer vaccine. Sipuleucel–T is a therapeutic cancer vaccine for metastatic prostate cancer in males. Treatment with Sipuleucel–T is individualized; it involves isolation of patient’s immune cells using leukapheresis and then activation of their antigen presenting cells (APCs) ex vivo with prostatic acid phosphatase and granulocyte–macrophage colonystimulating factor (PAP–GM–CSF). PAP is the prostate cancer antigen, which is overexpressed in prostate cancer patients and activation of APCs with PAP in presence of an immunostimulant (GM–CSF) triggers an immune response. These activated APCs are infused back into the patient leading to an immune response against the cancerous cells expressing PAP antigen. These patients receive three such treatments in an interval of two weeks each to obtain the therapeutic effect. Sipuleucel–T based clinical trials have resulted in an increase in survival rate of men suffering from metastatic prostate cancer by an average of four months. Various limitations of being eligible for the therapy, the cost and effort required to conduct the procedure, reduced the number of patients being benefited by this therapeutic vaccine.
Considering the potential of these cancer vaccines, various ongoing clinical trials are utilizing different approaches to develop cancer vaccines.Some of the antigen sources for these vaccines are:
Considering the potential of these cancer vaccines, various ongoing clinical trials are utilizing different approaches to develop cancer vaccines.Some of the antigen sources for these vaccines are:
- Viral antigens
- Whole cell lysates of cancer cells⁄weakened cancer cells
- Protein⁄carbohydrate⁄glycolipid⁄ganglioside based cancer antigens derived from cancer cells
- DNA⁄RNA fragments responsible for tumor associated antigens (TAAs)
Various immunostimulants⁄adjuvants have been employed to enhance the immune response against these antigens, some examples include: granulocyte–macrophage colony–stimulating factor (GMCSF), Bacillus Calmette–Guérin (BCG), cytosine phosphate–guanine (CpG), interleukin–2⁄ interleukin–12, mono–phosphoryl lipid A (MPL), etc[2].
With several promising cancer vaccine based clinical trials in the pipeline, we continue to explore the potential of developing another line of therapy for cancer patients. With this, I congratulate Austin Journal of Cancer and Clinical Research for the inaugural issue and encourage the readers to contribute to the scientific community through the journal.
References
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, et al. SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, based on November 2012 SEER data submission, posted to the SEER web site; April 2013.
- National Cancer Institute Cancer Vaccine Factsheet. Available at: https://www.cancer.gov/cancertopics/factsheet/Therapy/cancer-vaccines