Review Article
Austin J Cancer Clin Res 2014;1(2): 1009.
Solid Tumors of the Pancreas: A Review
Yasser Zakaria1 and Safiye Kafadar* 2
1Radiology Department, Damiette Cancer Instute Damiette, Egypt
2Radiology Department, Harput State Hospital Elazig, Turkey
*Corresponding author: Safiye Kafadar, Radiology Department, Harput State Hospital Elazig, Turkey
Received: March 12, 2014; Accepted: May 13, 2014; Published: May 14, 2014
Abstract
Pancreatic cancer is one of the most aggressive human malignancies. The prognosis for patients diagnosed with pancreatic cancer has remained extremely poor. Although there are a wide variety of pancreatic neoplasms, the majority of them are epithelial tumors and 90% of these are ductal adenocarcinomas and their variants. Multi–detector computed tomography (MDCT) has been widely accepted as the imaging technique of choice for diagnosing and staging pancreatic cancer, as it gives information about localization, size and extension of tumor, while being non–invasive.
Keywords: Solid pancreatic tumor; Multi–detector computed tomography
Introduction
Pancreatic cancer is one of the most aggressive human malignancies. It represents the fourth most frequent cause of cancerrelated death and the second most frequent cause, after colorectal cancer, when considering digestive tract cancers alone.
Despite all the progress in the fight against other cancers in recent years, the prognosis for patients diagnosed with pancreatic cancer has remained extremely poor. It is characterized by rapid local spread; persistent invasion of surrounding structures and the early creation of distant metastases with only about 15–20% of the patients have resectable disease at the time of presentation.
Multi–detector computed tomography (MDCT) has been widely accepted as the imaging technique of choice for diagnosing and staging pancreatic cancer, as it gives information about localization, size and extension of tumor, while being non–invasive. A recent meta–analysis showed CT to be 91% sensitive and 85% specific for tumoral detection.
MDCT has the capability to improve selection of patients who may benefit from tumor resection, so that significant perioperative morbidity and mortality of unnecessary laparotomies can be avoided.
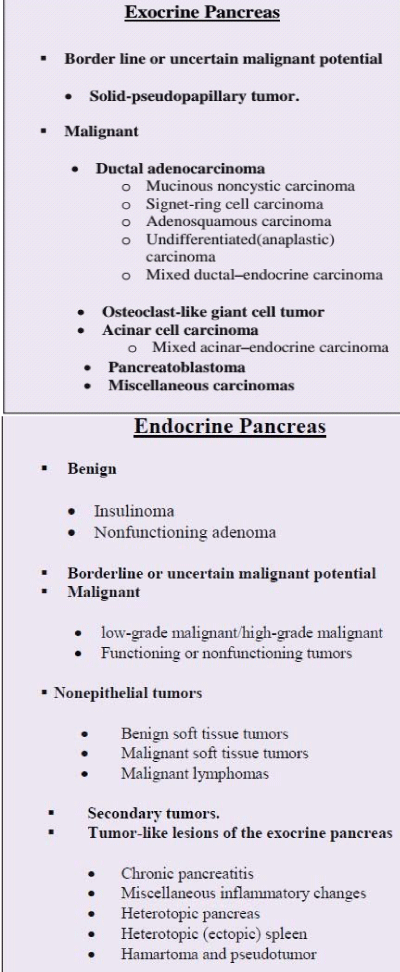
Although there are a wide variety of pancreatic neoplasms, the majority of them are epithelial tumors and 90% of these are ductal adenocarcinomas and their variants (e.g., adenosquamous carcinomas and undifferentiated carcinomas). Ductal adenocarcinoma is highly invasive and often elicits a desmoplastic reaction. Neoplasms originating outside the exocrine ductal epithelium are rare. They include pancreatic endocrine tumors and metastasis [1] (Table 1).
Table 1: Classification of Solid pancreatic tumors (according to WHO) [5].
Pancreatic Adenocarcinoma
Pancreatic adenocarcinoma remains the fourth leading cause of cancer–related death and is one of the most aggressive malignant tumors with an overall 5–year survival rate of less than 4%. It is the most common pancreatic exocrine neoplasm and accounts for 75%– 85% of all pancreatic malignancies. Despite all the progress in the fight against other cancers in recent years, the prognosis for patients diagnosed with pancreatic cancer has remained extremely poor. It is characterized by rapid local spread, persistent invasion of surrounding structures and the early creation of distant metastases [2].
Most patients are 60–80 years of age, and males are affected twice as often as females. Of these tumors, 60%–70% are located inthe pancreatic head, 10%–20% in the body, and 5%–10% in the tail. Abdominal pain, weight loss, and jaundice are the main presenting complaints but generally occur late in the disease course. Prognosis is poor, with a 1–year survival rate of less than 20% and a 5–year survival rate of less than 5%. Non–resectable disease is seen at presentation in 75% of patients, with metastases (mainly to the liver and peritoneum) present in 85% of these patients [3].
Variants of ductal adenocarcinoma
Variants of ductal carcinoma are those neoplasms that present a specific histological differentiation pattern associated with a typical ductal adenocarcinoma component. These tumors, whose incidence ranges from 2% to 10%, show similar clinical and biological features to those of ductal adenocarcinoma [4].
A – Mucinous non cystic carcinoma: Macroscopically characterized by a gelatinous mass and better demarcated than the ductal adenocarcinoma. Its incidence is 1%–3% of all pancreatic cancers; sex and age at onset are similar to those of ductal carcinoma. This tumor is associated with a significantly better prognosis than ordinary ductal adenocarcinoma [5].
B – Adenosquamous carcinoma: It is a rare subtype, accounting for 1%–4% of exocrine malignancies. It has glandular and squamous cell component. It shows a high metastatic potential and the prognosis is worse than that of conventional adenocarcinoma. It does not present significant differences to a ductal adenocarcinoma and therefore, the two cannot be distinguished by imaging [4].
C – Undifferentiated (Anaplastic) carcinoma: Its incidence is 5%–7% of all pancreatic tumors. Its prognosis is poorer than that of conventional adenocarcinoma, with distant metastases frequently present at the time of the diagnosis. The preferential location is in the tail of the pancreas [5].
D– Osteoclast–like giant cell carcinoma: Subtype of ductal adenocarcinoma. This carcinoma is composed of malignant undifferentiated epithelial cells with round or spindle–shaped cells associated with non–neoplastic osteoclast–like giant cells. The clinical course is extremely aggressive and most patients do not survive 1 year. The macroscopic aspect is identical to that of anaplastic carcinoma [4].
MDCT findings
Typically, ductal adenocarcinoma is a hypoattenuating solid mass relative to the normally enhancing pancreatic parenchyma in the pancreatic and portal venous phases because of fibroblastic proliferation and decreased vascularity. Tumors in the head and body of the pancreas frequently cause pancreatic or biliary duct obstruction with upstream ductal dilatation and pancreatic parenchymal atrophy [1].
CT has an accuracy of 85%–95% for tumor detection, a positive predictive value of 89%–100% for unresectability, and a negative predictive value of 45%–79% for resectability. Most tumors are hypoattenuating, with a mean size of 3 cm (range, 1.5–10 cm; average size in the pancreatic head, 2.5–3 cm; average size in the body and tail, 5–7 cm) [3].
A sensitivity of 84% and a specificity of 98% for invasion are reported if the tumor is contiguous with more than 50% of the vessel circumference. Other features suggesting vascular invasion include vessel deformity, thrombosis, and development of collateral vessels.The “tear–drop sign” refers to an alteration of the superior mesenteric vein from its normal round shape to a teardrop shape on axial images secondary to tumor infiltration or peritumoral fibrosis. Cystic necrotic degeneration, an uncommon feature of adenocarcinoma, is present in 8% of cases. Metastases are most commonly found in the liver andperitoneum. CT has an accuracy of 85%–95% for tumor detection, a positive predictive value of 89%–100% for unresectability, and a negative predictive value of 45%–79% for resectability [3].Surgical resection remains the only potentially curative treatment but it is only possible for 15%–20% of patients with pancreatic adenocarcinoma. About 40% of patients have locally advanced nonresectable disease. The remaining patients have metastatic disease. Therefore, about 80%–85% of patients are treated palliatively or neoadjuvantly. Consequently, accurate staging is absolutely necessary to differentiate the resectable patients from the unresectable and new imaging modalities play the critical role in making this differentiation. Multidetector computed tomography (MDCT) has been widely accepted as the imaging technique of choice for diagnosing and staging pancreatic cancer [2].
Pancreatic Neuroendocrine Tumors
Pancreatic neuroendocrine tumors (NETs) were previously referred to as islet cell tumors because they were thought to have originated from the islets of Langerhans. However, new evidence suggests that these tumors originate from pluripotential stem cells in ductal epithelium. They account for 1%–5% of all pancreatic tumors, have equal gender distribution, and typically manifest in patients aged 51–57 years. Most cases are sporadic, but association with syndromes such as multiple endocrine neoplasia type 1, von Hippel–Lindau syndrome, neurofibromatosis type 1, and tuberous sclerosis has been observed. NETs are classified into functioning and nonfunctioning tumors. Functioning tumors produce symptoms related to excessive hormone production. They account for 15%–52% of all tumors and are subdivided according to the hormones they produce [3].
Tumor size is variable. In general, functioning tumors manifest early in the course of disease when they are small, due to the clinical manifestations of excessive hormone production. Nonfunctioning tumors manifest when they are large, due to mass effect. Risk of malignancy increases with tumor size (especially in tumors >5 cm), with 90% of nonfunctioning tumors being malignant at presentation [3].
MDCT findings
A variety of imaging appearances exist. Tumors tend to be multiple, especially when associated with syndromes such as multiple endocrine neoplasia type 1 and von Hippel–Lindau syndrome. Single lesions are seen in 90% of insulinomas, whereas multiple lesions are present in 20%–40% of gastrinomas. Tumor size is variable. In general, functioning tumors manifest early in the course of disease when they are small, due to the clinical manifestations of excessive hormone production. Nonfunctioning tumors manifest when they are large, due to mass effect. Risk of malignancy increases with tumor size (especially in tumors >5 cm), with 90% of nonfunctioning tumors being malignant at presentation [3].
Conventional pancreatic endocrine tumors are generally hypervascular and frequently demonstrate avid, homogeneous enhancement in the arterial phase. Imaging findings of a cystic NET are highly nonspecific, and a definitive diagnosis is generally difficult to establish. When cystic degeneration occurs, the presence of residual peripheral enhancement when seen can be helpful establishing the iagnosis of a cystic PET [6].
Solid Pseudopapillary Tumor
First described by Franz in 1959, solid–pseudopapillary tumor of the pancreas (SPT) is a rare, low–grade malignant tumor of unknown etiology accounting for 0.2–2.7% of all primary pancreatic tumors. It is also known as solid cystic papillary epithelial tumor, papillary cystic tumor, solid and cystic tumor, papillary–cystic neoplasm, Hamoudi or Franz tumor. SPT is most prevalent in women of younger age. Abdominal mass is the most common presenting symptom, with dyspepsia, early satiety, nausea, or vomiting being less common presenting symptoms. Up to 20% of patients are asymptomatic with tumors identified either incidentally on imaging or at operation for unrelated pathology [7].
MDCT findings
Typically, CT shows a well–encapsulated heterogeneous mass in the pancreas with both solid and cystic components. Thus, when these features are encountered in a young female patient, this neoplasm should be a strong diagnostic consideration [8].
Typically, SPT shows peripheral heterogeneous enhancement during the arterial phase and progressive non uniform enhancement thereafter, with enhancement generally being less than that of the normal pancreas. The main differential consideration is cystic NET. There are several features that can help distinguish between SPT and cystic NET. 1. Age at presentation. NETs rarely occur in patients younger than 30 years of age. 2. Tumor enhancement characteristics. NETs are more vascular and demonstrate either diffuse or ring like hyperenhancement. The pseudocapsule (composed of compressed pancreatic tissue and reactive fibrosis) has low attenuation at CT. Internal hemorrhagic and cystic degeneration is the hallmark of SPT due to the fragile vascular network of the tumor [3].
To the best of our knowledge, there have been three articles regarding spontaneous regression of SPTs of the pancreas and the patients were all pediatric patients. Nakahara et al. reported a case showing shrinkage of a tumor from 4.5 cm to 1.5 cm during a 10– year follow–up period, and Suzuki et al. reported on a case showing shrinkage of a tumor from 5 cm to a non–measurable size over a sixyear period. The authors explained that the natural shrinkage resulted from degenerative change, including hemorrhage and necrosis, followed by absorption. SPTs of these cases were primary pancreatic masses, which were relatively small (less than 5 cm) [8].
Pancreatoblastoma
Pancreatoblastoma is a rare pancreatic tumor, representing the most common pancreatic tumor in early childhood. It usually occurs in children in the first decade of life, although occasional neonatal and adult cases have been reported. The biologic behavior of Pancreatoblastoma is aggressive with frequent local invasion, recurrence, and metastasis. Complete surgical resection is the key to good prognosis in the absence of metastatic disease. Chemotherapy and radiotherapy are recommended for recurrent, unresectable, or metastatic cases, but there has been no standard regimen so far [9].
Adult pancreatoblastoma is an extremely rare neoplasm and accounts for 0.5% of all pancreatic exocrine tumors. There are a total of only 24 cases that have been reported in the literature Adult pancreatoblastomas are slow growing and large tumors and the majority of tumors are larger than 8 cm at the time of diagnosis. They typically present with symptoms and signs related to mass effects which are predominantly abdominal pain, weight loss, palpable mass and jaundice. Metastasis is seen in 26% of adults and usually involves the liver and then the lymph nodes [10].
Pancreatoblastoma has a predilection for male patients (1.3–2.7 times more common in males than in females) and Asian individuals (>50% of cases). The serum a-fetoprotein level is elevated in 25%–33% of cases. Pancreatoblastoma is generally manifests as an asymptomatic large mass (mean, 10 cm; range, 1.5–20 cm). Symptoms (when present) relate to mass effect from the tumor and include abdominal pain, early satiety, vomiting, and constipation [3].
Metastases may occur to the liver (the most common location), lymph nodes, lung, bone, posterior mediastinum, peritoneum, and omentum. Despite its size, the tumor rarely causes biliary or duodenal obstruction, since it has a soft, gelatinous consistency. Arterial encasement and venous invasion have been observed. At CT, Pancreatoblastoma generally manifests as a multiloculatedinhomogeneous mass with enhancing septa. Calcifications (when present) have a rim–like or clustered configuration [3].
Acinar cell carcinoma
Acinar cell carcinoma represents about 1% of all exocrine pancreatic tumors. In 10% of cases, the tumor produces excess pancreatic enzyme notably lipase resulting in lipase hypersecretion syndrome, which is characterized by subcutaneous fat necrosis, bone infarcts, and polyarthritis [3].
At imaging: The tumor appears both more notable and more voluminous compared to ductal adenocarcinoma. Despite the conspicuous dimensions of the tumor, the Wirsung duct can appear normal upstream. Marked hypodensity after CM administration, compared to the normal pancreatic tissue, is usually reported for this tumor, relating to its poor vascularization. In personal experience and in that of other authors, acinar carcinoma of the head has been documented as markedly hyperdense after CM administration [4].
The prognosis is midway between that of ductal adenocarcinoma and that of the endocrine tumors; however, only 6% have a 5–year survival. Favorable prognostic factors are: less than 60 years of age, resectability and absence of metastases at intervention [4].
Pancreatic lymphoma
Extra–nodal involvement of non–Hodgkin’s lymphoma occurs in more than 50% of patients. The most frequent site is the gastrointestinal tract, especially the stomach and small intestine. In contrast, pancreatic lymphoma is uncommon: less than 0.5% of pancreatic tumors are of lymphomatous origin, and only 0.2–2% of patients with non–Hodgkin’s lymphoma has pancreatic involvement at presentation. Most cases of pancreatic lymphoma are of the diffuse large B–cell type. The diagnosis of pancreatic lymphoma may be difficult, as symptoms, laboratory studies and imaging are often nonspecific. LDH can be elevated in 50% of cases and tumor marker CA 19–9 may occasionally be elevated. The most common presentation of pancreatic lymphoma is abdominal pain [83% of cases)], as well as weight loss, nausea and vomiting. Typical B–symptoms of lymphoma, such as fever and night sweats, are uncommon [11].
Several features of lymphoma may help distinguish it from adenocarcinoma. 1. A bulky localized tumor in the pancreatic head without significant MPD dilatation. The MPD is typically mildly ilated in lymphoma but grossly dilated in adenocarcinoma. CBD dilatation is more common than MPD dilatation in lymphoma. 2. Enlarged lymph nodes below the level of the renal vein. 3. Invasive tumor growth that does not respect anatomic boundaries and that infiltrates retroperitoneal or upper abdominal organs and the gastrointestinal tract. Vascular invasion is less common in lymphoma than in adenocarcinoma. Intratumoral calcification or necrosis is not a feature of lymphoma and may be helpful in its exclusion. Biopsy is recommended for establishing the diagnosis. Pancreatic lymphoma carries a beter prognosis than adenocarcinoma because first–line treatment with chemotherapy is generally effective in producing long–term disease regression or remission. Surgery is not required in most cases [3].
Metastases to the pancreas
Unlike primary pancreatic carcinoma, metastatic lesions of the pancreas are uncommon and account for approximately 2% of pancreatic malignancies. The most common primary tumours to give rise to pancreatic metastases are lung cancer, breast cancer, renal cell carcinoma, malignant melanoma, carcinoma of gastrointestinal origin and prostate cancer. Less commonly metastases from osteosarcoma, leiomyosarcoma, and Chondrosarcoma have been reported. Although many patients have widespread disease, isolated metastases can be found. Surgical management seems to be associated with improved survival in these cases [12].
Three patterns of metastatic involvement of the pancreas have been described thus far. The first one, reported in 5– 10% of patients, is represented by many small nodules, which can coalesce occasionally into larger masses. The attenuation of the neoplastic nodules may be variable and generally depends on the histological type of the causative primary tumor (Figs. 4.19 & 4.20), Circumscribed lesions (single or multifocal) appear iso–dense or, more often, slightly hypodense on unenhanced CT. Bulging of the gland contour is occasionally seen. After I.V. contrast medium administration, a peripheral rim of enhancement is usually demonstrated and a central area of low attenuation may be clearly visible. Rim enhancement is especially common in lesions larger than 1.5 cm in size, whereas smaller lesions sometimes demonstrate homogeneous enhancement [12].
It should be emphasized that metastases from many primary hypovascular tumours (lung, colonic, gastric cancers) often present as hypoattenuated lesions compared to the normal enhanced pancreas after I.V. injection, while metastases from primary hypervascular tumours (HCC, thyroid, renal cell cancers) also display increased vascularity on contrast–enhanced CT [12].
Miscellaneous neoplastic solid exceedingly rare tumors
Described in small case series and isolated reports in the literature. 1. Epithelial tumors such as giant cell tumor, and colloid carcinoma. 2. Mesenchymal tumors such as granular cell tumor, fibrous histiocytoma, juvenile hemangio–endothelioma, fibroma, inflammatory myoblastic tumor, and sarcoma. 3. Mixed tumors such as squamous cell carcinoma and mixed endocrine–exocrine tumor [3].
Squamous cell carcinoma (SCC) is a rare malignancy of the pancreas and its exact cell of origin is not known. Normally, the pancreas is devoided of squamous cells and multiple theories have been suggested to explain the development of pancreatic SCC. Achieve a tissue diagnosis. However, EUS–FNA has been increasingly used in the diagnosis of pancreatic malignancies with a high sensitivity and specificity. Squamous cell carcinoma of the pancreas usually carries a poor prognosis. In a review of 25 cases, median survival was 7 monthsfor patients who underwent curative resection and 3 months for patients who did not undergo curative resection.
Conclusion
MDCT has the capability to improve selection of patients who may benefit from tumor resection, so that significant perioperative morbidity and mortality of unnecessary laparotomies can be avoided MDCT facilitates the generation of multiplanar reconstructions, such as curved planar reformations, providing the potential to improve the detection and staging of pancreatic tumors The accurate determination of resectability in patients with pancreatic cancer is the most important contribution of preoperative staging; the goal being to reduce needless surgery to a minimum. Recent studies reported a predictive value for the ability of MDCT to detect resectability of 87% and most of the studies reported better results when predicting unresectability, with predictive values ranging from 96% to 100%. Due to the particular anatomical relationship between the pancreas and the surrounding vessels, three–dimensional reconstructions are helpful in presenting additional information about this relationship.
For the surgeon, it is valuable to be able to see the tumor, both by itself and in the context of its surrounding structures, in three dimensions from all sides whereby particular interest is obviously accorded to the presence and extent of contact to the relevant vessels or surrounding organs. In addition, the surgeon can more easily assess the tumor volume in relation to healthy pancreatic parenchyma. Thus, the surgeon is provided with a picture as possible of the field ofoperation even before the operation takes place.
References
- Cho HW, Choi JY, Kim MJ, Park MS, Lim JS, Chung YE, et al. Pancreatic tumors: emphasis on CT findings and pathologic classification. Korean J Radiol. 2011; 12: 731-739.
- Zakharova OP, Karmazanovsky GG, Egorov VI. Pancreatic adenocarcinoma: Outstanding problems. World J Gastrointest Surg. 2012; 4: 104-113.
- Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011; 31: 993-1015.
- Carbognin Giovanni, Lucia Pinali, Carlo Procacci. Pancreatic Neoplasms and Tumor-like Conditions. Gourtsoyiannis NC, Ros PR, editors. In: Radiologic-Pathologic Correlations from Head to Toe, Understanding the Manifestations of Disease, Springer-Verlag Berlin Heidelberg. 2005; 409-446.
- Kumar V, Abul K Abbas, Nelson Fausto. Robbins and Cotran pathologic basis of disease 7th edn. Elsevier Saunders. 2005; 19: 939-951.
- Olpin JD, Shaaban A. Imaging of cystic pancreatic lesions. Journal of Biomedical Graphics and Computing. 2012; 2: 128–148.
- Adkisson CD, Harris AS, Bridges M, Nguyen JH, Asbun HJ, John A. Solid pseudopapillary tumor of the pancreas: Report of five cases. 2012; 2: 9–14.
- Yoon HJ, Lim JH. Solid pseudopapillary tumor of the pancreas with hepatic metastasis: spontaneous regression over 10-year follow-up period. Korean J Radiol. 2012; 13: 648-651.
- Tanaka H, Kitano Y, Takayasu H, Matuda S, Yamada W. Pancreatoblastoma with portal vein involvement in a child: A case report. 2013; 3: 44–49.
- Balasundaram C, Luthra M, Chavalitdhamrong D, Chow J, Khan H, Endres PJ. Pancreatoblastoma: a rare tumor still evolving in clinical presentation and histology. JOP. 2012; 13: 301-303.
- To CA, Quigley MM, Saven A, Nicholson L. Masquerade without a mass: an unusual cause of severe acute pancreatitis. J Gastrointest Oncol. 2013; 4: 114-117.
- Triantopoulou C, Kolliakou E, Karoumpalis I, Yarmenitis S, Dervenis C. Metastatic disease to the pancreas: an imaging challenge. Insights Imaging. 2012; 3: 165-172.