Research Article
Austin J Cancer Clin Res 2014;1(2): 1010.
Palifermin for Prevention of Oral Mucositis in Hematological Malignancies: Present Position and Future Perspectives
Shaiquel Jilani1#, ZiadKanaan1#, Rishi Agarwal2, Nishant Tageja3 and Muneer H Abidi4*
1Department of Internal Medicine, Wayne State University, USA
2University of Cincinnati, USA
3National Cancer Institute, USA
4Department of Bone Marrow Transplantation, Karmanos Cancer Institute, USA
*Corresponding author: Muneer H Abidi, Department of Bone Marrow Transplantation, Karmanos Cancer Institute, 4100 John R, 4 HWCRC, Rm: 4257, Detroit, MI, 48201, USA
Received: April 14, 2014; Accepted: May 12, 2014; Published: May 14, 2014
Abstract
Background: Oral Mucositis (OM) is an inevitable side effect of cytotoxic chemotherapy and⁄or radiotherapy used for haematopoietic stem cell transplantation (HSCT) in hematologic malignancies. Paliferminis the only available effective preventive strategy for management of OM in the setting of HSCT but is frequently overlooked in the treatment algorithms.
Aims: The objectives of our study were to 1) review the present evidence for the use of palifermin in prevention of gastrointestinal mucositis. 2) Explore the implications of palifermin use on the management of hematological malignancies, particularly in the setting of autologous transplants.
Methods: A comprehensive systematic review of the literature was performed. There were no language restrictions and the following medical subject heading terms were used: “melphalan,” “multiple,” “myeloma,” “oral,” “mucositis,” “doseescalation,” “autologous,” “stem–cell transplantation”. The quality of studies, whether randomized controlled trials (RCTs) or retrospective studies was measured using Jadad or new–castle Ottawa scale respectively.
Results: A total of 10 out of 24 studies met the inclusion criteria. These were2 randomized controlled–trials, 3 case–series, and 5 prospective studies. In 4 out of 10 studies included, no specific data was available regarding the outcome of palifermin use in OM in multiple myeloma (MM) patients following ASCT. Majority of the studies had insufficient power and⁄or lacked sufficient sample size but palifermin use was found toper form reasonably well in preventing OM in MM patients. In 3 RCTs, palifermin was noted to enable safe administration of higher doses of melphalan.
Conclusion: Palifermin is an effective cytoprotective agent that aids in prevention of OM in MM patients.
Keywords: Solid pancreatic tumor; Multi–detector computed tomography
Introduction
During the administration of cancer therapies, chemotherapeutic agents and⁄or radiotherapy have the potential to cause injury to the mucosal barrier of the upper and lower gastrointestinal tract [1]. This resulting mucosal barrier injury (MBI) often manifests as oral or intestinal mucositis [2–4]. Oral Mucositis (OM) is an inevitable side effect of the conditioning regimens used for hematopoietic stem cell transplantation (HSCT) [5] and is viewed as one of the most debilitating side effects of cancer treatment both clinically and economically [3]. The severity of OM often leads to dose reductions, drug interruptions or premature discontinuation of life–saving chemotherapeutic agents [1].
The pathophysiology of OM is a complex biologic process and not very well understood. The development of OM has been hypothetically divided into four phases: 1) an inflammatory or vascular phase, 2) an epithelial phase, 3) an ulcerative or bacteriological phase, and 4) a healing phase. The process involves multiple mechanisms including cytokine medicated tissue damage, direct effect of medications and status of patients’ bone marrow [2,4]. The ulcerative phase is mostsevere [2,4] where bacterial super infection can cause life–threatening bacteremia and sepsis in neutropenic patients [6].
Presently, the management of OM relies on supportive care and palliation of symptoms [5]. Palifermin, a recombinant keratinocyte growth factor (rHu–KGF1) originally derived from embryonic lung fibroblasts line, has shown to cause epithelial cell proliferation and eventually showed significant merit as a cytoprotective agent [7– 9]. Paliferminis currently known to be the only effective preventive strategy of OM in the HSCT setting [5].
In many clinical trials examining its role in the HSCT setting, palifermin has been shown to reduce the incidence, duration andseverity of OM induced by high–dose chemotherapy followed by autologous and allogeneic HSCT without negative influence onengraftment [10–15].Current guidelines recommend the use of palifermin in addition to topical analgesia, TPN, narcotic pumps and cryotherapy for oral mucositis management in various settings [16, 17]. In line with previous reports, a recent systematic review by Raber–Durlacher and colleagues examining the role of cytokinesand growth factors in OM prevention in cancer patients, palifermin was recommended for the prevention of OM in patients with hematological malignancies having high–dose chemotherapy and total body irradiation followed by autologous HSCT [18]. However, they noted conflicting and rather scarce evidence on the use of Palifermin in other settings [18]. Few studies, however, have focused on the role of Palifermin in prevention of OM in the setting of autologous transplants or in dose escalation setting of melphalan in multiple myeloma (MM) patients undergoing high–dose chemotherapy and autologous stem cell transplant.
This manuscript aims to systematically review the role of paliferminin prevention of gastrointestinal mucositis and explore its implications on the management of hematological malignancies, specifically in the setting of autologous transplants.
Methods
Study selection
A systematic review was conducted beginning with a search of MEDLINE (January 2004–January 2013), EMBASE, and Cochrane databases using Ovid and PubMed search engines. Full articles, abstracts of cohort studies (whether retrospective or prospective) reporting the frequency and treatment of AMM were included. Case reports, reviews, comments, and letters to the editor were excluded from the search. There were no language restrictions and the following medical subject heading terms were used: “melphalan,” “multiple,” “myeloma,” “oral,” “mucositis,” “dose–escalation,” “autologous,” “stem–cell transplantation”. Boolean operators (“not,” “and,” “or”) were also used in succession to narrow or widen the search. Based on the title of the publication and the abstract, we either downloaded or requested full articles through our library.
Inclusion criteria
All articles initially retrieved were then screened for the presence of prospective and retrospective cohorts. We included thosecontaining clinical data about the use of palifermin in prevention of oral mucositis in multiple myeloma patients and dose–escalation of melphalan in MM patients undergoing ASCT.
Exclusion criteria
Case reports, review articles, letters to the editor, and abstracts with insufficient details to meet the inclusion criteria were excluded from the study.
Quality assessment
For assessment of the quality of retrospective and non–randomized cohort studies, we used the Newcastle–Ottawa scale [19].This scalegives “quality” points based on the selection, exposure⁄outcome, and comparability of the studied cohorts. The maximum attainable pointsare 10 with a score above 6 considered an indicator of “good quality”[19]. As for assessment of prospective or randomized–controlledtrials, we used the Jadad score [20].
Institutional report
Patients seen at our institution for treatment of oral mucositis over the last 10 years were added to this study. Demographic data,cancer type, palifermin use, and OM rates were recorded.
Results
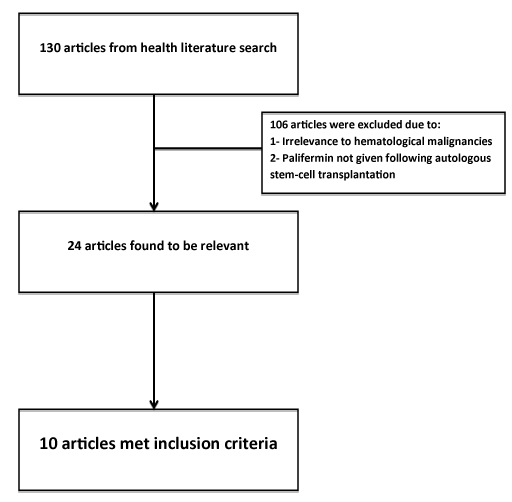
Following health literature search, 130 articles were found relevant as to the use of palifermin in OM prevention. Out of those, 106 articles were excluded due to irrelevance to this study since many of those studies examined palifermin use in tumors or palifermin usewas not examined following ASCT. A total of 10 out of 24 studies met the inclusion criteria and were included in this study (Figure 1).Those studies included: two randomized controlled–trials, 3 caseseries, and 5 prospective studies. In 4 out of 10 studies included, nospecific data was included regarding the outcome of palifermin use in OM in MM patients following ASCT. Further information could notbe obtained from the corresponding authors (Table 1). Mean Jadad score for the RCTs was 4.5 out of 6 maximum attainable points and 7.5 out of 10 maximum attainable points for the retrospective studies.
Figure 1: Flowchart showing included and excluded articles for the use of Palifermin in prevention of oral mucositis in multiple myeloma patients.
Authors
Study Design
Cancer
Placebo Group (N)
Palifermin Group (N)
Multiple myeloma patients (N)
Dose of Palifermin
Dose Limiting Toxicity
Days given
Outcomes in Grade 3 & 4 OM in MM patients (p<0.05)
[11]
RCT-PC
HM
105
105
11
60µg/kg/day
N/A
3 days before TBI, then 0,1, and 2$
6.7 days mean decrease
[21]
RCT-PC (Phase III)
HM
106
106
Data not available
60µg/kg/day
N/A
3 days before TBI, then 0,1, and 2$
Data not available
[10]
Prospective trial with a retrospective control
HM
53
53
3
60µg/kg/day
N/A
3 days before & after conditioning regimen
Data not available
[14]
Case series
HM
27
32
10
60µg/kg/day
N/A
3 days before & after conditioning regimen + 3 days after stem cell transplant
Data not available
[23]
Prospective study
HM
20
15
7 (vs.. 3 controls)
60µg/kg/day
N/A
3 days before the conditioning regimen
Data not available
[16]
Prospective study
MM
5
5
10
60µg/kg/day
N/A
3 days before myeloablative therapy and 3 days after stem-cell therapy
4 out of 5 had grade 3 or 4 OM
[25]
Case series
MM
21
67
92
60µg/kg/day
N/A
3-day short course of 60mg/kg Palifermin before and G-CSF after transplantation (-7,-6, & -5 days)
Reduced TPN, pain medications, and transfusion. Reduced the number of days with severe OM
[33]
Phase I prospective study on the use of Palifermin in patients with MM with renal insufficiency**
MM
N/A
19
19
60µg/kg/day
N/A
-5,-4,-3 and day +1,+2,+3 following ASCT
53% (8 of 15) had grade 3 OM. Permitted dose-escalation of melphalan up to 180mg/m2
[40]
Phase I dose-escalation trial of high-dose melphalan with Palifermin
MM
N/A
19
19
60µg/kg/day
N/A
--5,-4,-3 and day +1,+2,+3 following ASCT
44% (8 of 18) had OM grade 3. No TPN.
Table 1: A review of the medical literature on multiple myeloma patients receiving Palifermin for cytoprotection following ASCT.
Discussion
Palifermin use in the prevention of oral mucositis
Palifermin was initially approved by the U.S. Food and Drug Administration (FDA) in 2004 to decrease the incidence and duration of severe OM in patients with hematologic malignancies who receive high doses of chemotherapy and radiation therapy followed by stem cell rescue. This approval was based on a large double–blinded, placebo–controlled, randomized trial by Speilberger and colleagues that enrolled 212 patients with hematological malignancies [non– Hodgkin’s lymphoma (n=72), Hodgkin’s lymphoma (n=21), multiple myeloma (n=11) and leukemia (n=2)] undergoing ASCT (11). In this study, 106 patients were randomized to the palifermin group and 106 patients to the placebo group. These patients received conditioning regimen of fractionated total–body irradiation plus etoposide and cyclophosphamide and supportive care as per institutional guidelines. A significant improvement in the incidence of OM was noted in the palifermin group (63%) as compared to the placebo group (98%) (p<0.05).A significant decrease in duration of OM (9 days in the placebo group vs. 6 days in the palifermin group) and in the incidence of grade 4 mucositis (20% vs. 62%, p<0.001) and its duration (9 days in the placebo vs. 3 days in the Palifermin group) was also noted. In the MM patient cohort, a significant median decrease of 6.7 days in grade 3 and 4 OM was reported.
Stiff and colleagues reported results of a Phase III randomized trial for the use of palifermin and OM prevention in patients with hematological malignancies but did not include the type of hematological malignancies included in the study [21]. Nasilowska– Adamska assessed the role of palifermin in the prevention of OM and acute GvHD (aGvHD) during autologous or allogenic HSCT. Fifty–three patients with hematological diseases (which included 3 MM patients) received palifermin and were compared to 53 matched retrospective controls. Significant reduction was noted in the incidence of WHO grade 1–4 OM (58% in the palifermin group vs. 94% in placebo group, p<0.001), grade 3–4 OM (13 vs. 43%, p<0.001) and the median duration of OM (4vs.9 days, p<0.001). Useof analgesics and total parenteral nutrition was also significantly reduced [10]. However, the outcomes in the MM group were not reported.
Few case series reported small number of MM patients and the role of palifermin in OM prevention [14,22]. Keefe and colleagues reported a case–series of 5 patients with hematologic malignancieswho received palifermin for OM mucositis prevention with only one MM patient included with a reported 12 days of grade 4 OM [22].Similarly, Horsley and colleagues reported a series of 59 patients (10 of which were MM patients) where 32 patients receiving palifermin were compared with 27 patients receiving standard treatment. Overall, a significant reduction in the incidence of severe OM (13 vs. 48%, p=0.003), dysphagia (p=0.044), nutritional problems (4.9 vs. 6.0, p=0.003), and prolonged length of inpatient stay (14 vs. 18 days, p=0.026) was noted in the palifermin group compared to standard care. A subgroup analysis of outcome in MM patients was not performed. Tsirgotis reported beneficial use of palifermin in the prevention of intestinal mucositis in their patients (n=35, of which 7 MM patients were included) undergoing auto HSCT [23] but anotherreport by Herbers al did not show any clinically relevant impacton intestinal mucositis or incidence of oral mucositis in BEAM (carmustine, etoposide, cytarabine and melphalan)–treated HSCT recipients, but incidence of grade II or above OM was lower in the palifermin group (47% in palifermin group vs. 100 % in controls [24].
The largest single report of palifermin use for OM prevention in MM came from a retrospective analysis by Kobbe that included 92 patients with MM. Sixty seven patients received palifermin (Group A) as compared with 21 patients who received pegfilgrastim (Group B) and to 21 patients who did not receive any growth factors (Group C).In patients with normal renal function, the incidence of OM in Group was 16 % (p< 0.002), and 64% in patients with abnormal renal function at the time of HDCT. The patients with severe mucositis spent more days in hospital (median 19 days, range 16–49 versus median 17 days, range 13–39, p< 0.05) and needed more supportive care in terms of parenteral narcotics (median 5 days, range 0–14 versus median 0days, range 0–17, p<0.0001), intravenous antibiotics (median 8 days, range 0–37 versus median 5 days, range 0–24, p< 0.01) and parenteral nutrition (median 9 days, range 0–29 versus median 0 days, range 0–20, p< 0.01). Compared to the other groups, patients in group A experienced a reduced number of days in hospital (p<0.002), and a reduced need of parenteral narcotics (p< 0.01), parenteral nutrition(p< 0.01) and blood transfusions (p<0.01). No improvements were noted in patients with abnormal renal function [25].
Role of Palifermin in dose–escalation of Melphalan in patients with Multiple Myeloma for HDC and ASCT
Dose–escalation of melphalan with the aim of improving response rates in patients of MM has been an area of interest amongst transplant researchers over the past three decades [26]. Unfortunately, these attempts in the past have encountered dose–limiting toxicities with severe oral and gastrointestinal mucositis [27–29].Administration of melphalan at 220 mg⁄m2 was attempted by Moreau but significantOM, delayed platelet engraftment, and cardiac arrhythmias were observed [26]. Tandem transplantation is an innovative approach but considered experimental and has failed to catch up because of reviously negative results [28, 29]. OM has often the dose–limiting side effect of melphalan and has hindered the attempts to raise the dose greater than 200mg⁄m2 in patients with normal renal function and 140 mg⁄m2 in patients with abnormal renal function [30]. Effective cytoprotection could be a feasible method to increase the dose of melphalan. Philips attempted increasing the dose of melphalan by combining it with amifostine [31] and were successful in increasingthe dose of melphalan to 280 mg⁄m2 in patients with normal renal function [31].Cardiac toxicity with a trial fibrillation was seen in 3 of 6 patients treated with melphalan doses >280 mg⁄m2 and was fatal in 1 patient who received melphalan 300 mg⁄m2. Infusional toxicities such as hypotension and nausea were also seen with amifostine and hence, this agent is currently not recommended for prevention of OM in the setting of hematologic malignancies [31].
A randomized–controlled trial by Blijlevens examined the efficiency of palifermin in a chemotherapy–only, high–dose melphalan (HDM) transplant setting, to reduce OM and its sequelae measured by patient–reported outcomes (PRO) and medical resource use in MM patients [32]. Palifermin, relative to placebo was given either pre–⁄ post–HDM or pre–HDM in patients with MM undergoing autologous stem–cell transplantation at 39 European centers and palifermin was found not to reduce OM or OM–related patient’s burden in MM transplant patients [32]. However, pre–HDM had lower incidence of Grade 3–4 OM compared with placebo (24% vs.37%) where pre–⁄post– DM showed no reduction in grade 3–4 OM compared to placebo (38% vs. 37%)(30). There were other numerical differences in favor of palifermin in pre–HDM compared with placebo for the primary and some secondary benefits including mean duration of severe OM (1.9 vs.2.4 days), incidence of ulcerative OM (51% vs.58%), as well as mean duration of ulcerative OM (4.8 days pre–HDM vs. 5.0 days with placebo). Suboptimal timing of the post–dose may impair healing and exaggerate oral toxicity [32].
Though the findings of recent clinical studies carried out in patients undergoing hematopoietic stem cell transplantation and⁄or high dose melphalan showed non–significant effects of palifermin in ral mucositis prevention, the effect of palifermin yet remains to be validated in larger randomized trials due to lack of sample size and effective randomization [22].
Our group designed a dose–escalating trial using palifermin to explore if higher dose of melphalan could be tolerated in patients with MM undergoing ASCT [33]. The study was 3+3 phase 1 design divided into two arms, one with the normal renal function and the other with abnormal renal function. Level 1 began at dose 200 mg⁄m2 in patients with normal renal function and 140mg⁄m2 in patients with abnormal renal function with subsequent dose–escalations of melphalan were done at 20 mg⁄m2 increments in both the arms while the dose of palifermin remained constant. Palifermin was given as anintravenous bolus on days –5, –4, –3 and then on day +1, +2 and +3 (PBSCs were infused on day 0).
The overall incidence of OM > grade 3 in normal renal function arm was 44% (8⁄18) with a median duration of severe OM was 10 days (range 4 –20 days). Eleven patients (61%) required opioid analgesics and none of the patients received parenteral feeding. A trial fibrillation developed in one of six patients treated with melphalan 280 mg⁄m2. In the abnormal renal function arm, 15 patients were evaluable. The overall incidence of OM ≥ grade 3 was 53% (8 of 15) and a medianduration of ≥ grade 3 OM was 6.5 days (range, 3–42 days).
Dosing of palifermin
The dosing of palifermin in prevention of OM has not been uniformly established. Current studies in the medical literature reported the use of palifermin doses of 60 mcg⁄kg⁄day. Vadhan–Raj and colleagues described 1–day dosing of palifermin before auto–SCT as patient–friendly and cost–effective in properly selected patients [34]. Doses up to 180 mcg⁄kg have been tested safe in healthy volunteers and one–day dosing of palifermin of 180 mcg⁄kg, 3 days before cycled doxorubicin–based chemotherapy had reported efficacy in reducing severe OM in sarcoma patients from 51 to 13% [34]. Trials examiningdifferent palifermin doses need to be conducted while taking into consideration the timing of palifermin administration.
Cost of palifermin
Palifermin is currently priced at 4700 US dollars per 5 mg vial. In a retrospective analysis of phase 3 trials, the impact of palifermin on the cost of hospitalization was studied [35]. The hospital costs of patients on Palifermin were compared to those on placebo. A non–significant mean savings of $3,595 per patient (95% confidence interval: $2,090– $5,103) was observed in patients who were treated with palifermin [35]. A prospective study with a larger cohort of patients may firmly establish palifermin to be an economically favorable option.
Another cost analysis of palifermin in patients undergoing auto HSCT following non–TBI–based conditioning regimens at a single center reported that mediantotal transplant charges were significantly higher in the palifermin–treated group compared to patients not treated with palifermin (myeloma: $167,820 versus $143,200, P < .001; lymphoma: $168,570 versus $148,590, P < .001) [36]. In this study, the duration of patient controlled anesthesia was less in palifermingroup but this did not convert into a significant decrease in length of hospital stay or overall survival in myeloma or lymphoma group [36]. The cost effectiveness of palifermin is not well established and needs further exploration.
Safety of palifermin
Palifermin has the physiologic tendency to increase epithelial cell growth which is responsible for common side effects seen in patients taking this medication. Side effects that commonly observed include white coating of the tongue, rash, pedal edema, itching, elevated amylase and lipase [37]. Also, there have been reports of Acanthosis nigricans secondary to palifermin [38,39].Such adverse effects were previously noted by our group in both studied arms taking Palifermin [33,40]. In the group with normal renal function, common adverse events related to palifermin included asymptomatic elevation of amylase (10 events, 4 were grade 3–asymptomatic), lipase (5 events, 2 were grade 3 –asymptomatic), rash (18 events, no = grade 3 events), and edema (11 events, no ≥grade 3 events). In the groupwith renal insufficiency, thirteen patients developed skin rash (No grade 3or greater). Nine patients showed amylase elevation (grade ≥3 in 3 patients). Elevated lipase was seen in 2 patients (No grade 3 or greater). One patient in the group with renal insufficiency had a lichenoid growth on the tongue and the biopsy was consistent with Pyogenic Granuloma, a possible manifestation of Palifermin activity.
As palifermin is an epithelial growth factor, concerns have been raised that it can lead to secondary malignancies. As keratinocyte growth factor (KGF) receptors are not expressed in hematological cancers, such risk is less likely in case of hematological malignancies. In an analysis conducted by Speilberger rates of secondary malignancies were similar in patients receiving palifermin compared to patients receiving placebo [11]. The median follow up time was approximately50 months (49.8 months for Palifermin group and 49.5 months for the placebo group [41].To the best of our knowledge, there have been no reports of any secondary malignancy related to Palifermin.
Side effects with palifermin are relatively well tolerated and most often do not lead to discontinuation of the drug [11,12,15,25]. Higher incidence of toxicity mostly skin reactions leading to discontinuation of the drug were seen when patients received more than six doses of the palifermin [12]. The safety of palifermin may be dependent on the conditioning regimen used due to variable drug interactions although clear data suggesting this does not exist.
In allogeneic HSCT transplant setting, multiple studies have shown benefits of palifermin in preventing oral mucositis but the results are variable depending on the conditioning regimen [10,12,15,18,42]. In a retrospective analysis, palifermin reduced total parenteral nutrition and patient controlled analgesia use in patients receiving TBI–based allogeneic HSCT but not in chemotherapy based allogeneic HSCT recipients [42] which was consistent with results of previously published reports of use of palifermin in allogeneic transplant patients [12,15].
Additionally, palifermin administration has been studied with multi–agent conditioning regimens (for e.g. BEAM and FEAM regimens for lymphoma and other Fludarabine (F) combinations) [24]. BEAM and FEAM regimens are potentially more toxic than single–agent melphalan used for myeloma. BEAM is mainly used as conditioning regimen in lymphoma patients with different risk profile for developing mucositis.
Conclusion
Palifermin has manageable toxicity profile and is an effective cytoprotective agent found to aid in prevention of OM in MM patients. Though, prospective RCTs properly examining its use and benefits are scarce. Palifermin enables administration of higher doses of melphalan especially in patients with abnormal renal function. Although, dialysis patients were excluded from the study performed at our institution. This combination might lead to overall improved survival in MM patients. Such benefits need to be further elucidated in larger cohorts.
Conflicts of Interest
Senior author received research funding from Biovitrum (Sobi) pharmaceuticals and member of their speaker’s bureau.
References
- Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr. 2001; 29: 7-15.
- Blijlevens NM, Donnelly JP, De Pauw BE. Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: an overview. Bone marrow transplantation. 2000; 25: 1269-1278.
- Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol. 2001; 19: 2201-2205.
- Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral oncology. 1998; 34: 39-43.
- Nasilowska-Adamska B, Szydlo R, Rzepecki P, Czyz A, Tomaszewska A, Markiewicz M, et al. Palifermin does not influence the incidence and severity of GvHD nor long-term survival of patients with hematological diseases undergoing HSCT. Ann Transplant. 2011; 16: 47-54.
- Elting LS, Bodey GP, Keefe BH. Septicemia and shock syndrome due to viridans streptococci: a case-control study of predisposing factors. Clin Infect Dis. 1992; 14(6):1201-1207.
- Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1989; 86: 802-806.
- Blijlevens N, Sonis S. Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann Oncol. 2007; 18: 817-826.
- Meropol NJ, Somer RA, Gutheil J, Pelley RJ, Modiano MR, Rowinsky EK, et al. Randomized phase I trial of recombinant human keratinocyte growth factor plus chemotherapy: potential role as mucosal protectant. J Clin Oncol. 2003; 21: 1452-1458.
- Nasilowska-Adamska B, Rzepecki P, Manko J, Czyz A, Markiewicz M, Federowicz I, et al. The influence of palifermin (Kepivance) on oral mucositis and acute graft versus host disease in patients with hematological diseases undergoing hematopoietic stem cell transplant. Bone marrow transplantation. 2007; 40: 983-988.
- Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. The New England journal of medicine. 2004; 351: 2590-2598.
- Blazar BR, Weisdorf DJ, Defor T, Goldman A, Braun T, Silver S, et al. Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Blood. 2006; 108: 3216-3222.
- Radtke ML, Kolesar JM. Palifermin (Kepivance) for the treatment of oral mucositis in patients with hematologic malignancies requiring hematopoietic stem cell support. J Oncol Pharm Pract. 2005; 11: 121-125.
- P, Bauer JD, Mazkowiack R, Gardner R, Bashford J. Palifermin improves severe mucositis, swallowing problems, nutrition impact symptoms, and length of stay in patients undergoing hematopoietic stem cell transplantation. Supportive care in cancer. 2007; 15: 105-109.
- Langner S, Staber P, Schub N, Gramatzki M, Grothe W, Behre G, et al. Palifermin reduces incidence and severity of oral mucositis in allogeneic stem-cell transplant recipients. Bone Marrow Transplant. 2008; 42: 275-279.
- Peterson DE, Bensadoun RJ, Roila F, ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO clinical recommendations. Ann Oncol. 2009; 20: 174-177.
- Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004; 100: 2026-2046.
- Raber-Durlacher JE, von Bultzingslowen I, Logan RM, Bowen J, Al-Azri AR, Everaus H, et al. Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients. Support Care Cancer. 2013; 21: 343-355.
- Wells GA, Shea B, D O'Connell, Peterson J, Welch V, Losos M, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials. 1996; 17: 1-12.
- Stiff PJ, Emmanouilides C, Bensinger WI, Gentile T, Blazar B, Shea TC, et al. Palifermin reduces patient-reported mouth and throat soreness and improves patient functioning in the hematopoietic stem-cell transplantation setting. J Clin Oncol. 2006; 24: 5186-5193.
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006; 14: 580-582.
- Tsirigotis P, Triantafyllou K, Girkas K, Giannopoulou V, Ioannidou E, Chondropoulos S, et al. Keratinocyte growth factor is effective in the prevention of intestinal mucositis in patients with hematological malignancies treated with high-dose chemotherapy and autologous hematopoietic SCT: a video-capsule endoscopy study. Bone Marrow Transplant. 2008; 42: 337-343.
- Herbers AH, van der Velden WJ, de Haan AF, Donnelly JP, Blijlevens NM. Impact of palifermin on intestinal mucositis of HSCT recipients after BEAM. Bone Marrow Transplant. 2014; 49: 8-10.
- Kobbe G, Bruns I, Schroeder T, Czibere A, Warnecke J, Hieronimus N, et al. A 3-day short course of palifermin before HDT reduces toxicity and need for supportive care after autologous blood stem-cell transplantation in patients with multiple myeloma. Ann Oncol. 2010; 21: 1898-1904.
- McElwain TJ, Powles RL. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983; 2: 822-824.
- Moreau P, Milpied N, Mahe B, Juge-Morineau N, Rapp MJ, Bataille R, et al. Melphalan 220 mg/m2 followed by peripheral blood stem cell transplantation in 27 patients with advanced multiple myeloma. Bone marrow transplantation. 1999; 23: 1003-1006.
- Barlogie B, Jagannath S, Desikan KR, Mattox S, Vesole D, Siegel D, et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999; 93: 55-65.
- Moreau P, Hullin C, Garban F, Yakoub-Agha I, Benboubker L, Attal M, et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99-04 protocol. Blood. 2006; 107: 397-403.
- Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001; 114: 822-829.
- Phillips GL, Meisenberg B, Reece DE, Adams VR, Badros A, Brunner J, et al. Amifostine and autologous hematopoietic stem cell support of escalating-dose melphalan: a phase I study. Biol Blood Marrow Transplant. 2004; 10: 473-483.
- Blijlevens N, de Chateau M, Krivan G, Rabitsch W, Szomor A, Pytlik R, et al. In a high-dose melphalan setting, palifermin compared with placebo had no effect on oral mucositis or related patient's burden. Bone Marrow Transplant. 2012; 48: 966-971.
- Abidi MH, Agarwal R, Ayash L, Deol A, Al-Kadhimi Z, Abrams J, et al. Melphalan 180 mg/m2 can be safely administered as conditioning regimen before an autologous stem cell transplantation (ASCT) in multiple myeloma patients with creatinine clearance 60 mL/min/1.73 m2 or lower with use of palifermin for cytoprotection: results of a phase I trial. Biol Blood Marrow Transplant. 2012; 18: 1455-1461.
- Vadhan-Raj S, Trent J, Patel S, Zhou X, Johnson MM, Araujo D, et al. Single-dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: a randomized trial. Annals of internal medicine. 2010; 153: 358-367.
- Elting LS, Shih YC, Stiff PJ, Bensinger W, Cantor SB, Cooksley C, et al. Economic impact of palifermin on the costs of hospitalization for autologous hematopoietic stem-cell transplant: analysis of phase 3 trial results. Biol Blood Marrow Transplant. 2007; 13: 806-813.
- Nooka AK, Johnson HR, Kaufman JL, Flowers CR, Langston A, Steuer C, et al. Pharmacoeconomic Analysis of Palifermin to Prevent Mucositis among Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2014; 20: 852-857.
- Pierce GF, Yanagihara D, Klopchin K, Danilenko DM, Hsu E, Kenney WC, et al. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994; 179: 831-840.
- Lee M, Grassi M. Acanthosis nigricans in a patient treated with palifermin. Cutis; cutaneous medicine for the practitioner. 2010; 86: 136-137.
- Lane SW, Manoharan S, Mollee PN. Palifermin-induced acanthosis nigricans. Intern Med J. 2007; 37: 417-418.
- Abidi MH, Agarwal R, Tageja N, Ayash L, Deol A, Al-Kadhimi Z, et al. A phase I dose-escalation trial of high-dose melphalan with palifermin for cytoprotection followed by autologous stem cell transplantation for patients with multiple myeloma with normal renal function. Biol Blood Marrow Transplant. 2013; 19: 56-61.
- Spielberger R, Territo M, Durrant S, Nimer S, McCarty J, Hurd D, et al. No Difference in Survival or Long-Term Disease Outcomes in Palifermin-Treated Patients with Hematologic Malignancies Undergoing Hematopoietic Stem Cell Transplantation. 2007.
- Goldberg JD, Zheng J, Castro-Malaspina H, Jakubowski AA, Heller G, van den Brink MR, et al. Palifermin is efficacious in recipients of TBI-based but not chemotherapy-based allogeneic hematopoietic stem cell transplants. Bone marrow transplantation. 2013; 48: 99-104.