Research Article
Austin J Cancer Clin Res 2014;1(3): 1016.
Immunohistochemical Panel for Differentiating Renal Cell Carcinoma with Clear and Papillary Features
Hanan Al-Saeid Al-shenawy*
Department of Pathology, Tanta University, Egypt
*Corresponding author: Hanan Al-Saeid Al-Shenawy, Department of pathology, Tanta University, 25 Hamdy Gado street, Tanta, Egypt
Received: July 16, 2014; Accepted: August 08, 2014; Published: August 13, 2014
Abstract
Aim: Renal cell carcinoma (RCC) in which clear cells with papillary architecture is present is a difficult diagnostic challenge. The most common type, clear cell RCC, only rarely has papillary architecture. The second most common one, papillary RCC, only rarely contains clear cells. However, two recently described less-common types; clear cell papillary and Xp11 translocation RCC characteristically feature both papillary architecture and cells with clear cytoplasm. Accurate diagnosis has both prognostic and therapeutic implications. This study aims to highlight the helpful cytomorphologic and immunohistochemical features of each of these entities to enable reproducible classification.
Materials and Methods: Sixty RCC cases with clear cells and papillary architecture were selected and classified according to The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia and graded according to The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma then stained for CK7, carbonic anhydrase IX (CA IX), α-methylacyl-CoA-racemase (AMACR) and TFE-3.
Results: The characteristic immunoprofile of Clear RCC is CK7-, AMACR-, CA IX+ and TFE3-, papillary RCC is CK7+, AMACR+, CAIX- and TFE3-, while for clear cell papillary RCC it is CK7+, AMACR-, CAIX+ and TFE3- and lastly Xp11translocation RCC is CK7-, AMACR+, CAIX- and TFE3+.
Conclusion: Immunohistochemical staining for CA IX, CK7, AMACR and TFE3 comprises a concise panel for distinguishing RCC with papillary and clear pattern.
Keywords: Renal cell carcinoma; CK7; Carbonic anhydrase IX; α-methylacyl- CoA-racemase; TFE-3
Introduction
Renal epithelial tumors are renal neoplasms arising from renal tubules and can be classified into many major categories based on morphology [1]. Different tumor type appears to have different outcome. With increased understanding of pathogenesis of each type of tumors, new target therapy may be developed [2].
Primary renal cell carcinomas (RCCs) with both papillary architecture and cells with clear cytoplasm may be a difficult diagnostic challenge. The most common RCC, clear renal cell carcinoma, CRCC, which represent about 75% of the cases, may sometimes have papillary architecture. The second most common RCC, papillary renal cell carcinoma PRCC, which represent about 15%, May also, contains clear cells [3]. However, 2 recently described but less-common RCCs, clear cell papillary renal cell carcinoma, CPRCC and Xp11 translocation RCC, characteristically feature both papillary architecture and cells with clear cytoplasm. Accurate diagnosis of these distinct entities has prognostic and therapeutic implications [4]. Immunohistochemical markers may be needed to establish the correct diagnosis [5].
CPRCC is a recently recognized renal neoplasm, composed of an admixture of cystic, glandular, and papillary components, all lined by cells with clear cytoplasm, usually of low nuclear grade. The nuclei are characteristically located away from the basement membrane to show a “piano-key-like” pattern [3]. The outcome data are limited; however, the available data suggest that this type of tumor usually have a good prognosis. Originally they were discovered in a background of end-stage renal disease and acquired cystic kidney disease [6].
Xp11 translocation RCC initially described in children and young adults. Recently, the term “MiTF/TFE family translocation– associated carcinoma” has been proposed for tumors that have translocations involving TFE3. TFE3 is transcription factor that belong to the same family of transcription factors that will overexpress nuclear TFE3. These immunohistochemical findings are important given the occurrence of these tumors in the adult population, as they morphologically overlap with CCRCC and PRCC. In the literature, these tumors do not appear to respond to immunotherapy [7]. Outcome data of this entity are still premature and good long-term follow-up data are necessary. Published outcome series in adults show a poor prognosis [4].
The treatment paradigm for renal tumors is changing, and these changes are in part driven by tumor classification. Traditionally, RCC has been considered a surgical disease. In some cases, surgery with its associated complications and negative impact on long-term renal function may be very harmful, so follow up after chemo radiotherapy may be used in low grade small tumours [8].
Cytokeratins are a family of intermediate filaments that characterize epithelial differentiation, There have been conflicting results on the expression of CK7 in renal epithelial tumors in the literature as some authors recognized its role in the differentiation of “non-clear cell” RCC from CRCC [9].
The most useful positive immunohistochemical stain in supporting a diagnosis of PRCC is α-methylacyl-coenzyme A racemase (AMACR). It is now recognized that AMACR can show positivity in tumors from many different organs and in several different types of renal tumors. But AMACR staining has conflicting results in CPRCC as it is often negative [10] but in other studies it is focally or, rarely, diffusely positive [6].
Carbonic anhydrase IX (CA IX) protein is thought to play a role in the regulation of cell proliferation and may be involved in oncogenesis and tumor progression. Previous immunobiochemical studies revealed that CA IX expression may be a useful diagnostic biomarker in RCC subclassification. Clinical tumor targeting studies with a monoclonal antibody to CA IX have shown that CA IX shows promise as a marker for selecting patients with advanced disease who would benefit from certain specific systemic agents, specifically interleukin-2 (IL-2) [7,11].
This work aims to highlight the helpful cytomorphologic and immunohistochemical features of each of these entities to enable reproducible classification. We examined the expression of 4 markers in a series of the 4 major renal cell tumors with clear and papillary architecture. In this study, we evaluated the expression of carbonic anhydrase IX (CA IX), α-methylacyl-CoA-racemase (AMACR), CK7 and TFE-3 for differential diagnosis and subclassification.
Materials and Methods
Case selection and histopathological study
A retrospective study was performed on RCC cases selected from January 1, 2010 to April 30, 2014. A total of 250 cases of RCC were removed by nephrectomy either partial or radical and brought to the Department of Pathology, University of Tanta. Representative tissue sections from the surgical specimens were fixed in 10% buffered formalin and embedded in paraffin. For routine microscopy, 4µ-thick sections were stained with Hematoxylin and Eosin (H&E). The clinical sheets for all cases were reviewed. The cases were classified according to The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia [12]. Tumors that fulfill the morphological criteria of clear and papillary renal cell neoplasms were selected. They were 60 cases. Only the selected cases were assessed for size, laterality, multifocality, presence of associated end-stage renal disease (ESRD). Then they assessed histologically for the presence of branched tubular structures, subnuclear vacuoles, acini, thin walled sinusoid-like vessels, ‘secretory’ cells with nuclei aligned at the apical end of the cells, cystic components, character of the stromal compartment, presence of tumor pseudocapsule, and calcification. The selected carcinomas were later reevaluated for morphologic characteristics of those tumors that qualify them in either one of the following categories; CRCC, PRCC, CCPRCC or Xp11 translocation RCC. Specifically, the criteria used for classification of a tumor as a CCPRCC included the following: (1) diffuse cytoplasmic clarity; (2) papillary, tubular or cystic architecture; and (3) characteristic linear arrangement of the nuclei away from the basement membrane [10]. Xp11 translocation RCC cases were confirmed by TFE3 immunostaining positivity. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma grading system was applied to access the nucleolar grades [13]. The tumors were staged according to the 2010 UICC/AJCC consensus guidelines [14].
Immunohistochemical study and evaluation
Immunohistochemical staining was performed using the following antibodies: CK7 (OV-TL 12/30, 1:100, DAKO, Glostrup, Denmark), CA IX (dilution 1:200, mouse monoclonal, Leica), AMACR (13H4, 1:100; DAKO, Glostrup, Denmark) and TFE3 (1:1500, Santa Cruz Biotechnology Inv., Santa Cruz, CA, USA).
Evaluation of the immunohistochemical staining was performed by light microscopy using a 10× objective lens with the selective use of a 20–40× objective lens for confirmation. The interpretation of immunoreactivity was performed in a semiquantitative manner by analyzing the extent of the staining positivity of the tumor cells. Immunostaining of greater than 10% of tumor cells was required for scoring as a positive case. The interpretation score was as follows: 0 or negative =10% tumor cell positivity; +1 or weak = 11–25% tumor cell positivity; +2 or moderate = 26–50% tumor cell positivity; and +3 or strong >50% tumor cell positivity [6]. Cytoplasmic and/or membranous expression of CK7 and AMACR were considered positive. Only distinct membranous staining for CA IX and distinct nuclear staining for TFE3 were considered positive [15].
Statistical analysis
The clinicopathological and immunohistochemical features were tested for their association with the histological subtype using Student’s t-test for continuous variables and the chi-square test (or Fisher’s exact test) for qualitative variables. All statistical analyses were performed using the SPSS 13.0 software. Statistical significance was considered when P value ≤ 0.05.
Results
Clinicopathologic findings of the selected cases
The clinicopathologic findings are listed in Table 1. The selected cases were comprised of 60 cases; 40 men and 20 women (men to women ratio, 2:1) with a mean age of 54 years (range, 40–72 years). A definite positive history of hemodialysis was found in 14 cases end stage renal disease with a duration ranging from 1 to 24 years. The International Society of Urological Pathology (ISUP) grading system was as follows: 24 were grade I, 21 were grade II and 15 were grade III. The tumors pathologic stage was as follows; 38 cases were stage I, 15 were stage II, 7 were stage III and no cases were stage IV.
Clinicopathological variable
Number of cases
Size
Less than 7 cm
45
More than 7 cm
15
Laterality
Right
30
Left
24
Bilateral
6
Multifocality
Yes
8
No
52
End stage renal disease
Yes
14
No
46
Grade
Grade I
24
Grade II
21
Grade III
15
Stage
Stage I
38
Stage II
15
Stage III
7
Stage IV
0
Histological types
CRCC
28
PRCC
15
CPRCC
8
Xp11 translocation RCC
9
Table 1: The clinicopathological variables of the selected cases.
Overall, of the 60 cases, 28 (47%) were CRCC, 15 (25%) PRCC, 8 (13%) CPRCC and 9 (15%) Xp11 translocation RCC was identified. The cases were classified according to the published data into these categories according to some variables summarized in Table 2.
Histological features
CRCC
PRCC
CPRCC
Xp11 translocation
Clear cells
Yes
Yes
Yes
Yes
‘secretory’ cells with nuclei aligned at the apical end of the cells
No
No
Yes
No
Subnuclear vacuoles
No
No
Yes
No
True papillae
No
Yes
Yes
Yes
Tubules
No
No
Yes
Yes
Cysts
Yes
Yes
Yes
Yes
Acini
Yes
No
Yes
Yes
Biologic aggressiveness
Yes
Yes
No
Yes
Capsule
No
Yes
Yes
No
Psammoma bodies
No
Yes
No
No
Leiomyomatous stromal components
Yes
No
Yes
Yes
Thin walled sinusoid-like vessels
Yes
No
No
No
Table 2: Microscopic finding in the selected cases.
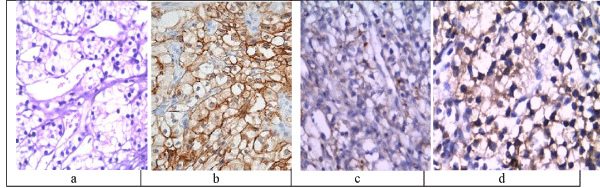
CRCC are usually characterized by optically clear cytoplasm and a very well-defined cytoplasmic membrane. The cells are arranged in nests, alveolar architectures, strands and papillae, or cysts. The stroma contains a very typical and prominent capillary network. Hemorrhage and necrosis are frequent (Figure 1a).
Figure 1: A case of CRCC grade I showing clear cells with faint papillary structures (H&E x100, a) showing positive membranous staining for CA IX (x400, b) and week positivity for both CK7 (x200, c) and AMACR (x400, d).
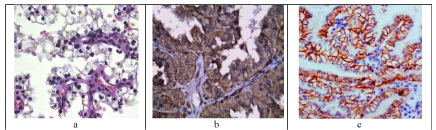
Papillary architecture is the characteristic histology of renal tumors in PRCC. The Delicate fibrovascular stalks lined by small cells with low grade nuclei and scant cytoplasms (Figure 2a). Psammoma bodies and clear cells are common.
Figure 2: A case of PRCC grade II showing prominent papillae with occasional clear cells (H7a Ex100, a). It expresses strong membranous positivity for CK7 (x200, b). AMACR demonstrates cytoplasmic granular positivity (x200, c).
CPRCC were well circumscribed with a well-defined, thin, fibrous capsule and were composed histologically of a mixture of cysts and papillae. Tubular/acinar features were also common. Even in the predominantly cystic areas, the lining cells frequently showed papillary infoldings. The tumors were composed of cuboidal or columnar cells with clear cytoplasm, small hyperchromatic, round or oval nuclei, and inconspicuous nucleoli. A conspicuous nuclear positioning away from the basement membrane with subnuclear vacuoles was noted in all cases examined (Figure 3a). The cysts, tubules, and acini contained pink secretion. Renal capsular and vascular invasion, mitoses, tumor necrosis, and stromal aggregates of macrophages were not observed. The stromal component formed variably thick bands of fibrous tissue admixed with strands of leiomyomatous tissue inside the tumors.
Figure 3: A case of CPRCC grade I show clear cells with low grade nuclei show characteristic linear polarization away from the basement membrane (H&Ex200, a), it demonstrates positive cytoplasmic staining for CK7 (x200, b), CA-IX diffusely expressed in a membranous distribution in cup-shaped distribution (x400, c).
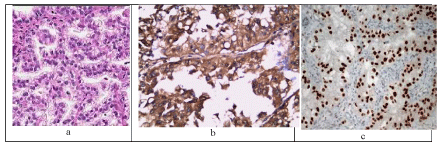
Renal carcinomas with Xp11.2 translocation have a characteristic morphology; they are composed of very large clear cells that form nests, alveoli, and papillae (Figure 4a) accompanied by an abundance of psammoma bodies. The cells are not very cohesive, which causes alveolar images and pseudo papillae.
Figure 4: A case of Xp11translocation grade II shows clear cells and papillary architecture with high grade nuclei (H&Ex200, a), strongly expressing AMACR in the cytoplasm (x200, b), and characteristically show nuclear positivity for TFE3 (x200, C).
Correlation study between the studied cases and clinicopathological variables
In correlation between the different pathological types of RCC and the clinicopathological variables, it was found that there is a significant difference between the histological type of RCC and the tumour size as most of the cases of PRCC, Xp11.2 translocation RCC (12/15 and 7/9 respectively) and all the cases of CPRCC were of smaller size (≤7cm) while most of the large sized tumours were CRCC. As regards the laterality, PRCC was the most common bilateral RCC (5/15) as 5 cases out of the 6 bilateral cases were PRCC with statistical difference. Also the multifocality was only seen in PRCC (8/15) as all the multifocal cases were PRCC with strong statistical difference. End stage renal disease was associated with most of CPRCC (6/8) and some CRCC (6/28) with statistical significance.
The grade was correlated significantly with RCC types as it was low in all the cases of CPRCC (either grade I or II). On the other hands, more than half of the Xp11 translocation RCC cases were of high grade (grade III). The tumour stage was also low in most of the cases of CRCC, PRCC and all the cases of CPRCC with strong significant difference. These data were summarized in Table 3.
Histological type
Size
Laterality
Multifocality
ESRD
Grade
Stage
≤7cm n=45
>7cm n=15
Rt n=30
Lt n=24
Bilateral n=6
Yes n=8
No n=52
Yes n=14
No n=46
I n=24
II n=21
III n=15
I n=38
II n=15
III n=7
IV n=0
CRCC
n=28
18
10
15
13
0
0
28
6
22
10
15
3
12
12
4
0
PRCC
n=15
12
3
6
4
5
8
7
1
14
6
2
7
13
1
1
0
CPRCC
n=8
8
0
3
4
1
0
8
6
2
5
3
0
8
0
0
0
Xp11.2 translocation RCC n=9
7
2
6
3
0
0
9
1
8
3
1
5
5
2
2
0
P value
0.01
0.04
0.003
0.05
0.04
0.008
Table 3: Correlation study between the studied cases and clinicopathological variables.
Immunohistochemical findings in the studied cases
The immunohistochemical findings are listed in Table 4.
Studied marker
CRCC
N=28
PRCC
N=15
CPRCC
N=8
Xp11 translocation
N=9
CK7
Negative
26
0
0
8
+
2
0
0
1
++
0
0
0
0
+++
0
15
8
0
AMACR
Negative
23
0
8
0
+
5
0
0
0
++
0
0
0
3
+++
0
15
0
6
CA IX
Negative
0
13
0
7
+
0
2
0
2
++
10
0
2
0
+++
18
0
6
0
TFE3
Negative
28
15
8
0
+
0
0
0
0
++
0
0
0
0
+++
0
0
0
9
Table 4: Immunohistochemical profile of the studied cases.
The immunohistochemical profile of CRCC was that all the cases show positive membranous staining for CA IX (18/28 and 10/28 were strongly positive (Figure 1b) and moderately positive consequently), while they were negative for both CK7 and AMACR, although both may be weekly positive (2/28 (Figure 1c) and 5/28 were (Figure 1d) weak positive respectively), especially in higher-grade tumors. TFE3, marker of Xp11 translocation RCC, is consistently negative.
PRCC cases show strong membranous positivity for CK7 (Figure 2b). Staining with AMACR demonstrates cytoplasmic granular positivity in all the cases (Figure 2c). The CA IX staining is either completely negative (13/15) or may be weak positive near areas of necrosis (2/15). TFE3 is consistently negative.
CPRCC displays a unique IHC profile overlapping with CRCC and PRCC. CPRCC show positive cytoplasmic staining for CK7 in all the cases (Figure 3b) similar to PRCC. AMACR, TFE3 were negative in all the cases examined. The tumor cells also expressed CA-IX diffusely in a membranous distribution (6/8 and 2/8 were strongly and moderately positive consequently); the absence of staining along the luminal borders of the tumor cells was quite characteristic (cup-shaped distribution) (Figure 3c). TFE3 is consistently negative.
Renal carcinomas with Xp11.2 translocation do not express, or only very weakly express CK7 and CA IX (8/9 and 7/9 were negative for CK7 and CA IX respectively) but they express AMACR (6/9 (Figure 4b) and 3/9 were strongly and moderately positive respectively). In addition, this group of carcinomas is characterized by nuclear positivity with the TFE3 transcription factor (Figure 4c).
Discussion
The World Health Organization classification of renal tumors synthesizes morphological, immunohistochemical, molecular, and clinical data to define distinct entities that are biologically and clinically relevant. Although most epithelial renal tumors can be diagnosed by morphology alone, the diagnosis can be difficult due to the overlap of histological features. In these cases, differentially expressed immunohistochemical markers can be of help [2]. In this study, we performed a 4-antibody panel on RCC cases that show both papillary and clear cell architecture.
The present study included 60 cases consist of CRCC, PRCC, CPRCC and Xp11.2 translocation RCC. Most of PRCC, Xp11.2 translocation RCC and all CPRCC cases were of smaller size (≤7cm). PRCC was found to be the most common bilateral and multifocal RCC. End stage renal disease was associated with most of CPRCC but also we observed that CPRCC can occur in otherwise normal kidneys and this was observed in Michelle et al. study [10].
CRCC was usually of aggressive architecture as it was characterized by tumor necrosis, mitoses, vascular invasion, and a characteristic network of small, thin-walled sinusoid-like blood vessels, so it was usually presented with higher stage and this was seen in Brannon et al. study [16]. PRCC usually has cystic change and papillary architecture. Clear cell change can become quite extensive in some tumors causing morphologic confusion. The cytoplasmic clearing is typically seen in association with tumor necrosis and this was similar to Aydin et al. study [17].
CPRCC were well circumscribed with a well-defined fibrous capsule, and were composed of a mixture of cystic, papillary, tubular, and acinar components. The nuclei show characteristic polarization in a linear array away from the basement membrane and of low grade. The stromal component formed variably thick bands of fibroleiomyomatous tissue inside the tumors among the epithelial component. These features were observed in previous studies [10,18].
Xp11.2 translocation RCC has a distinctive pattern which is the presence of both clear cells and papillary architecture. Nuclei tend to be high grade and the cytoplasm may be clear to granular and eosinophilic. Numerous psammomatous calcifications and stromal hyaline nodules are common. Most of our cases were of higher grades and this was similar to Rajen et al., [7].
The distinction between CRCC, PRCC, CPRCC and Xp11.2 translocation RCC is critical because of different behavior, prognosis and treatment [6].
In the present study, the grade was low in all the cases of CPRCC, and the stage was also low in most of the cases of CRCC, PRCC and all the cases of CPRCC with strong significant difference. On the basis of our results, it appears that CPRCC represents a distinct form of low grade RCC, the recognition of which may be important for prognosis and clinical management no case of CPRCC has behaved aggressively as in previous studies [19]. So immunohistochemical study to differentiate, difficult cases may be needed to classify them.
CK7 is a commonly used marker for RCC. Our results showed diffuse positivity for this marker in a membranous pattern, consistent with previous reports [20], confirming its usefulness in differentiating both PRCC and CPRCC from CRCC and Xp11translocation RCC which is consistently negative in both.
AMACR is a mitochondrial enzyme, which mediates the oxidation of branched-chain lipids. In this study, it has been shown that this marker is usually positive in PRCC and Xp11translocation RCC with a diffuse strong cytoplasmic staining pattern. Our study showed that AMACR was usually negative or weekly positive in CRCC while CPRCC was negative for this marker, consistent with prior reports [2,3].
CA IX is a hypoxia-induced protein and is predominantly reported to be positive in CRCC [2,21]. In this study, CPRCC is largely positive for this marker, a result that concurs with previous observations [11]. CA IX is useful for the differentiation of CRCC and CPRCC from PRCC with clear cell changes. CRCC and CPRCC are usually positive for this marker, whereas the PRCC is either completely negative or may be weekly positive near areas of necrosis. However, the pattern of expression of CA IX in CPRCC differs from that seen in CRCC in that most cells in clear-cell papillary RCC lack labeling on the luminal aspect. This pattern of staining was not observed in any of the CRCCs evaluated in this study and this was in agree with Stephen et al., [15] and Tickoo and Reuter [22]. Xp11 translocation RCC diagnosis is supported by TFE3 positivity.
In summary, The characteristic immunoprofile of CRCC is CK7-, AMACR-, CA IX+ and TFE3-, for PRCC it is CK7+, AMACR+, CA IX- and TFE3-, while for CPRCC it is CK7+, AMACR-, CA IX+ and TFE3- and lastly Xp11translocation RCC is CK7-, AMACR+, CA IX-and TFE3+.
To conclude, immunohistochemical staining for CA IX, CK7, AMACR and TFE3 comprises a concise panel for distinguishing RCC with papillary and clear pattern. Our marker panel is a clear advancement in terms of immunohistochemistry application for RCC subtype differentiation when papillary and clear cells are the predominant architecture as this is very crucial for further prognosis and targeted therapy because of the different behavior of each type.
References
- Deng FM, Melamed J. Histologic variants of renal cell carcinoma: does tumor type influence outcome? Urol Clin North Am. 2012; 39: 119-132.
- Zhanyong B, Priti L, Song L. Role of carbonic anhydrase IX, a-methylacyl coenzyme a racemase, cytokeratin 7, and galectin-3 in the evaluation of renal neoplasms: a tissue microarray immunohistochemical study. Annals of Diagnostic Pathology. 2013; 17: 58–62.
- Williamson SR, Eble JN, Cheng L, Grignon DJ. Clear cell papillary renal cell carcinoma: differential diagnosis and extended immunohistochemical profile. Mod Pathol. 2013; 26: 697-708.
- Ross H, Martignoni G, Argani P. Renal cell carcinoma with clear cell and papillary features. Arch Pathol Lab Med. 2012; 136: 391-399.
- Walter B, Hartmann A, Hofstädter F, Junker K, Moch H, Bertz S, et al. Immunohistochemical marker panel differentiates between the three most common subtypes of renal cell carcinoma independent from histomorphologic criteria. Virchows Arch. 2012; 460: 343-352.
- Borislav A, Cinthia B. Clear cell papillary renal cell carcinoma: Incidence, morphological features, immunohistochemical profile, and biologic behavior: A single institution study. Pathology Research and Practice. 2014; 210: 234–241.
- Rajen G, Elizabeth G, Ximing J. Differential Diagnosis of Renal Tumors with Clear Cytoplasm: Clinical Relevance of Renal Tumor Subclassification in the Era of Targeted Therapies and Personalized Medicine. Archives of Pathology & Laboratory Medicine. 2013; 137: 467-480.
- Park JH, Lee C, Suh JH, Moon KC. Clear cell papillary renal cell carcinoma: a report of 15 cases including three cases of concurrent other-type renal cell carcinomas. Korean J Pathol. 2012; 46: 541-547.
- Saba K, özden T, Sümer B. Diagnostic utility of cytokeratins 7, 10 and 20 in renal cell carcinoma and oncocytoma. Turkish Journal of Pathology. 2008; 24: 140-146.
- Pramick M, Ziober A, Bing Z. Useful immunohistochemical panel for differentiating clear cell papillary renal cell carcinoma from its mimics. Ann Diagn Pathol. 2013; 17: 437-440.
- Genega EM, Ghebremichael M, Najarian R, Fu Y, Wang Y, Argani P, et al. Carbonic anhydrase IX expression in renal neoplasms: correlation with tumor type and grade. Am J Clin Pathol. 2010; 134: 873-879.
- Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol. 2013; 37: 1469-1489.
- Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013; 37: 1490-1504.
- Edge S, Byrd D, Compton C, Editors. AJCC Cancer Staging Manual, 7th ed., Springer, New York, NY. 2010: 479–489.
- Stephen M, Yonghong X, Yupu L. Clear-cell papillary renal cell carcinoma: molecular and immunohistochemical analysis with emphasis on the von Hippel–Lindau gene and hypoxia-inducible factor pathway-related proteins. Modern Pathology. 2011; 24: 1207–1220.
- Brannon AR, Haake SM, Hacker KE, Pruthi RS, Wallen EM, Nielsen ME, et al. Meta-analysis of clear cell renal cell carcinoma gene expression defines a variant subgroup and identifies gender influences on tumor biology. Eur Urol. 2012; 61: 258-268.
- Aydin H, Chen L, Cheng L, Vaziri S, He H, Ganapathi R, et al. Clear cell tubulopapillary renal cell carcinoma: a study of 36 distinctive low-grade epithelial tumors of the kidney. Am J Surg Pathol. 2010; 34: 1608-1621.
- Duan L, Youseff R, Margulis V. Clear cell papillary renal cell carcinoma: clinicopathologic, immunohistochemical, and molecular analysis. Mod Pathol. 2011; 24: 189-193.
- Brunelli M, Menestrina F, Segala D. Renal cell carcinoma with prominent leiomyomatous proliferation appears not to be a variant of clear cell renal cell carcinoma. Mod Pathol. 2009; 22: 160-165.
- Shen SS, Truong LD, Scarpelli M, Lopez-Beltran A. Role of immunohistochemistry in diagnosing renal neoplasms: when is it really useful? Arch Pathol Lab Med. 2012; 136: 410-417.
- Al-Ahmadie HA, Alden D, Fine SW, Gopalan A, Touijer KA, Russo P, et al. Role of immunohistochemistry in the evaluation of needle core biopsies in adult renal cortical tumors: an ex vivo study. Am J Surg Pathol. 2011; 35: 949-961.
- Tickoo SK, Reuter VE. Differential diagnosis of renal tumors with papillary architecture. Adv Anat Pathol. 2011; 18: 120-132.