Department of Radiology, Royal Marsden Hospital, UK
*Corresponding author: Manish Chand, Department of Radiology, Royal Marsden Hospital, Downs Road, Sutton, SM2 5PT, UK
Received: August 11, 2014; Accepted: November 25, 2014; Published: December 01, 2014
Citation: Chand M, Wale A and Brown G. Evaluation of Mesorectal Lymph Nodes with High-Resolution MRI. Austin J Cancer Clin Res 2014;1(4): 1021. ISSN 2381-909X
Adverse tumour characteristics demonstrated by staging imaging investigations commonly influence pre-operative treatment decisions in rectal cancer. These include involvement of the circumferential resection margin, increasing tumour penetration through the bowel wall and Mesorectal nodal involvement. Local nodal disease remains chal-lenging to detect regardless of imaging technique. High-resolution MRI is the most accurate imaging modality to identify malignant lymph nodes however this is dependent on strict adherence to optimal technique and criteria. Commonly, nodal size is incorrectly used as a measure of malignancy despite there being a lack of evidence to sup-port this. Whilst the need to irradiate early, low-risk tumours with local nodal involvement continues to be routine practice in many units, it is important to correctly stage these patients to avoid unnecessary morbidity.
Most patients with rectal cancer present with locally advanced disease and an increasing number of patients are being considered for pre-operative or neoadjuvant therapy. The treatment algorithms vary and the rationale for neoadjuvant therapy depends on local guidelines. However, much of the staging information which influences these decisions is obtained from imaging studies and Magnetic Resonance Imaging (MRI) is central to this process. Whilst there may be disagreement on the prognostic value of specific tumour features, increased tumour depth of penetration and tumour involvement of the Circumferential Resection Margin (CRM) are routinely offered chemo radiotherapy. The prognostic importance of local Nodal disease (N1) if optimal total mesorectal excision is performed for rectal cancer is not as clear as previously thought [1] and therefore a more selective approach is taken by some with regards to neoadjuvant radiotherapy. If the plane of surgery is not compromised and the circumferential resection margin is free of tumour, the local recurrence rate is less than 4% even with N1 disease. Yet many units continue to irradiate tumours on the basis of N1 disease alone, which makes accurate identification of mesorectal lymph node metastases important to prevent unnecessary morbidity – up to 20% of patients with T1 or T2 disease have nodal involvement. The “gold-standard” for detecting lymph node metastasis remains histopathological assessment but this information can only influence adjuvant treatment whereas radiotherapy, in particular is given pre-operatively [2-4].
The role MRI and endoanal ultrasound (EAUS) has been investigated for the local staging of rectal cancer.
Whilst EAUS is able to accurately stage T1 tumours, overall MRI is superior for local staging with imaging of the entire mesorectum, the identification of poor prognostic factors and assessment of the circumferential resection margin. As such MRI is recommended for the local staging of rectal cancer (8).
MRI is the most accurate of the commonly used imaging techniques in identifying local nodal disease in rectal can-cer but this is dependent on technique and the criteria used to determine whether a node is malignant or benign [5-7]. Using features of nodal border, contour and differing signal characteristics the sensitivity and specificity increases to 85% and 97%, respectively (8). EAUS does not predict lymph node involvement any better. Indeed sensitivity and specificity for detection of cancerous lymph nodes in rectal cancer is 73.2% and 75.8%, respectively although more likely to be accurate in the more proximal parts of the rectum. Swollen reactive nodes, small blood vessels and even local structures such as the seminal vesicles may mimic malignant nodes whilst lymph nodes less than 5mm will not be identified by EAUS (8).
Using incorrect criteria to assess mesorectal nodes may result in under- or over-staging of nodal disease and subsequent poor correlation with histopathology. This is the most likely explanation for the low specificity and sensitivity shown in published reports. Further, the consequences of inaccurate nodal staging can lead to unnecessary treatment and potential morbidity.
Traditionally radiologists have resorted to nodal measurements of varying sizes as a method of determining malignant status but none with any supporting histopathological evidence. This practise is continuing in many units and it is not unusual to find current research articles using size-criteria to determine nodal involvement thus reducing the strength of the findings. A study which matched nodes from in vivo and specimen MRIs with pathology specimens showed that there was no useful size cut-off for predicting nodal status [8]. Further, a histological survey of over 12,000 lymph nodes in rectal cancer showed considerable size overlap between normal or reactive nodes and those containing metastases [9]. A perceived limitation of MRI is the lack of accuracy and ability to detect nodes smaller than 3mm. Yet this may not be as clinically relevant as first appears. Only 2% of nodes which are malignant were of this size [8].
Lymph node architecture is not commonly assessed using MRI as most radiologists prefer to measure the diameter of nodes instead [10]. As a consequence, the sensitivity and specificity is less than ideal; Bipat and colleagues per-formed a meta-analysis and reported sensitivity of 66% and specificity of 76% [11]. This paper included studies which gave little information on technique and criteria. Considering the technology and equipment available at the time of this review none of the studies which employed the use of body coils would be considered high resolution – Table 1. Schnall et al [12] and Drew et al [13] were able to achieve high resolution voxel sizes of 0.54-1.32 and 1.38 respectively using endorectal coils. This lack of consistency makes the conclusion questionable in the context of high resolution imaging.
Modern MRI scanners employ multichannel, multiarray pelvic coils which enable imaging with a high resolution voxel size of 1.1mm3, this is superior to traditional body coils and means it is unnecessary to use endorectal coils. So when a high resolution MRI technique is used, it is easier to evaluate lymph node architecture and this has enabled new criteria for lymph node involvement to be developed. Tumour infiltration into lymph nodes leads to characteristics radiological features which can be readily identified on MRI. Tumour leads to capsular disruption causing the nodal border to become irregular as opposed to the more rounded border of benign nodes. A very small number of lymph nodes with a smooth bordered contour (<6%) have been shown to be malignant whilst those demonstrating irregular outline are malignant in over 90% of cases [8]. Mixed signal intensity occurs due to the heterogeneity of the tumour and necrosis within the node. When using the signal characteristics and border outline together, the sensitivity is much improved.
Figure 1a/b – Benign and malignant nodes on MRI showing differences in signal characteristics and border outline.
Technique is the most important factor when examining for the malignant nodes. The importance of field alignment has been demonstrated previously, with consequences of under- or over-staging tumour depth and height if the field is not perpendicular to the long axis of the rectum. Specific to mesorectal nodes, it is therefore important to not only use thin slices but also small Field Of View (FOV) for accurate identification. Many MRI scanners and resultant images are produced with adequate slices of 3mm but with a FOV of 22cm by 22cm and a low matrix resolution. Suspicious lymph nodes can be detected however this is not as accurate as a high matrix resolution of 0.6mm by 0.6mm. Figures 3a and 3b show examples whereby the same patient has been scanned using different fields of view and different slice thicknesses. These figures demonstrate the accuracy of the smaller field and how this could potentially upgrade the staging incorrectly. Using correct FOV also affects the voxel size. If the voxel size is increased, resolution is lost and morphological characteristics become less obvious.
Figure 2a/b – Difference in imaging quality using different fields of view.
Neoadjuvant treatment is mandatory for those patients with a high risk of local recurrence. As mentioned the most important risk factor for local recurrence is involvement of the circumferential resection margin [14]. Radiotherapy is well known to cause down-staging of disease including nodal involvement [15,16]. The optimal methods of measuring treatment response to CRT remain contentious and therefore some units prefer not to re-stage the tumour. Of the current common imaging modalities, MRI is the most accurate in re-staging disease and has been shown to correlate highly with histopathology using tumour regression grading and down-staging of specific tumour characteristics. Using the same criteria as above with T2-weighted MRI, lymph nodes can be accurately identified following neoadjuvant treatment. Koh et al prospectively evaluated the MR staging of lymph nodes before and after chemo radiotherapy and compared this with histopathological analysis to demonstrate significant correlation between post-treatment MR assessment and histopathology of nodal disease [17].
The advent of DWI technology initially held promise as a potentially reliable method of distinguishing between tumour containing versus benign nodes. However, the studies to date have not found the technique to improve accuracy compared with high resolution T2 criteria after CRT [18]. The likely reason is the technique is not specific to tumour but water to cellular interfaces which are likely to be present in benign reactive nodes.
Finally there has been interest in the potential role of lymph-node specific MRI contrast agents to aid the detection of lymph node metastases, for example ferumoxtran-10 and Trisodium (Vasovist) [19]. Will et al performed a meta-analysis which found ferumoxtran-10 may improve the diagnostic performance of MRI for the detection of liver metastases [20]. However any potential improvement in nodal detection must be offset by the additional time and intervention required to generate these images and the small number of patients likely to benefit. Patients with TRG 3-5 disease following neoadjuvant therapy will all proceed to further treatment regardless of their nodal status and therefore only patients with TRG 1-2 disease are likely to benefit from the increased diagnosis of lymph node metastases. However this group is small with only 3% of patients with TRG 1-2 developing local recurrence [21].
Figure 3a/b – MRI showing malignant nodes before and after chemo radiotherapy.
Inaccurate assessment of mesorectal nodes can conceivably lead to either under- or over-staging of disease pre-operatively. The current modern management of rectal cancer relies on imaging staging information for risk-stratification and treatment decisions and MRI has been shown to accurately risk-stratify patients [22,23]. The use of pre-operative radiotherapy for Nodal disease (N1) in the absence of other adverse features such as increasing tumour depth or involvement of the circumferential resection margin is contentious, particularly where neo-adjuvant therapy is used more selectively [24]. This means that under-staging may not be as problematic as over-staging where patients experience additional morbidity with no clinical benefit on the basis of inadequate staging. If nodal disease continues to be a determinant for neoadjuvant treatment, pre-operative staging must be highly accurate. The clinical implications for re-staging of nodal involvement following CRT are even less clear particularly if there has been down-staging to N0 disease. One could argue that as these nodes are to be removed with the mesorectal package if oncologically successful surgery is performed, the prognostick importance of ‘treated’ nodes may be redundant. Nevertheless if a consistent criterion is adhered to re-staging may be equally accurate.
In summary, detection of mesorectal lymph nodes remains an important part of the local staging of rectal cancer. MRI is the optimal imaging modality for identifying malignant nodes however its accuracy depends on appropriate technique and the criteria used to distinguish benign from malignant nodes. The use of signal characteristics in conjunction with the nodal border is more sensitive and specific than using nodal diameter. Inaccurate staging of nodes can lead to additional morbidity for patients if the policy is to offer all patients routine radiotherapy on the basis of nodal disease.
This table shows technology and equipment available at the time of this review none of the studies which employed the use of body coils would be considered high resolution.
Author |
Magnet |
No. pts |
Coil |
T1-weighted spin-echo |
Voxel size/mm3 |
T2 Weighted spin-echo |
Voxel size/mm3 |
T2 weighted fast spin-echo |
Voxel size/mm3 |
Intravenous contrast enhancement |
Voxel size/mm3 |
Brown et al [8] |
1.5T |
42 |
Pelvic coil |
- |
|
- |
|
+ |
1.17 |
No |
- |
Hodgman et al [25] |
0.15T |
27 |
Elliptical coil |
Not stated |
|
Not stated |
|
Not stated |
|
Not stated |
|
Guinet et al[26] |
0.5T |
19 |
Body Coil |
+ |
Not stated |
+ |
Not stated |
No |
- |
No |
- |
de Lange et al [27] |
1.0T |
24 |
Pelvic coil |
+ |
5.72 |
+ |
11.44 |
No |
- |
No |
- |
1.0T |
3 |
Body coil |
+ |
16.15
|
+ |
32.30 |
No |
- |
No |
- |
|
1.0T |
2 |
Pelvic coil + body coil |
+ |
16.15
|
+ |
32.30 |
No |
- |
No |
- |
|
Lange et al [27] |
1.0T |
16 |
Pelvic Coil |
+ |
4.76 |
+ |
11.40 |
No |
- |
No |
- |
1.0T |
6 |
Body Coil |
+ |
13.40 |
+ |
32.30 |
No |
- |
No |
- |
|
Okizuka et al [28] |
1.5T |
33 |
Body coil + rectal balloon |
+ |
7.81 |
+ |
10.42 |
No |
- |
No |
- |
Thaler et al[29] |
Not stated |
34 |
Body Coil |
+ |
Not stated |
+ |
Not stated |
No |
- |
No |
- |
McNicholas et al[30] |
0.5T |
20 |
Body Coil |
+ |
Not stated |
+ |
Not stated |
No |
- |
No |
- |
Schnall et al [12] |
1.5T |
14 |
Endorectal surface coil + endorectal prostate coil |
+ |
0.59-1.32 |
+ |
0.59-1.32 |
+ (plus fat saturation in 3 patients) |
Not stated |
No |
- |
1.5T |
22 |
Prototype endorectal coil + external surface coi |
+ |
0.59-1.32 |
+ |
0.59-1.32 |
+ (plus fat saturation in 3 patients) |
Not stated |
No |
- |
|
Wallengren et al [31] |
0.3T |
12 |
Body coil or Pelvic coil |
+ |
7.00 |
+ |
7.00 |
No |
- |
Yes |
7.00 |
Okizuka et al [32] |
1.5T |
17 |
Body Coil |
+ |
7.81 |
+ |
7.81 |
+ |
7.81 |
Yes +/- fat suppression |
7.81 |
1.5T |
15 |
Pelvic Phased Array |
+ |
7.81 |
+ |
5.16 |
+ |
5.16 |
Yes +/- fat suppression |
7.81 |
|
Zerhouni et al [33] |
1.0 /1.5T |
79 |
Body Coil |
+ |
20.00 |
+ |
20.00 |
No |
- |
No |
- |
Drew et al [13]
|
1.5T |
36 |
Pelvic coil + endorectal coil |
+ |
1.38 |
+ |
1.38 |
+ |
4.27 |
Yes +/- fat suppression |
1.03-1.38 |
Kim et al [34] |
1.5T |
73 |
Endorectal coil |
Not stated |
- |
Not stated |
- |
Not stated |
- |
Not stated |
- |
Gualdi et al [35] |
1T |
26 |
Endorectal coil |
+ |
1.98 |
+ |
1.98 |
No |
- |
|
|
Blomqvist et al [36] |
1.5T |
37 |
Pelvic coil |
+ |
3.13 |
No |
- |
+ |
2.60 |
No |
- |
1.5T |
12 |
Endorectal coil + pelvis phased array coil |
Yes |
3.13 |
No |
- |
Yes |
1.76 |
No |
- |
|
Kim et al [37] |
1.5T |
217 |
Not stated |
+ |
Not stated |
No |
- |
+ |
Not stated |
Yes +/- fat suppression |
Not stated |
Gagliardi et al [38] |
1.5T |
28 |
Body coil + air insufflation into rectum |
+ |
Not stated |
No |
- |
Yes |
Not stated |
No |
- |
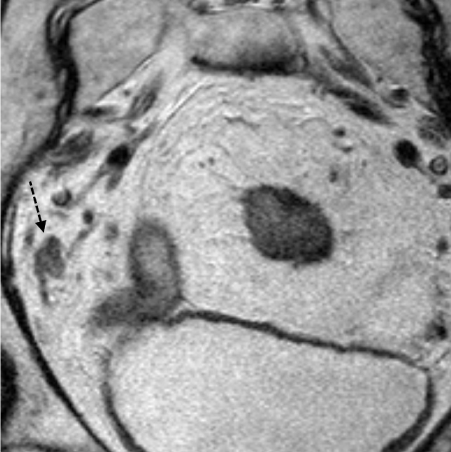
T2 Axial High Resolution MRI of a T4 rectal cancer with a benign lymph node seen in the mesorectum (white arrow). The lymph node is homogeneous with a smooth border.
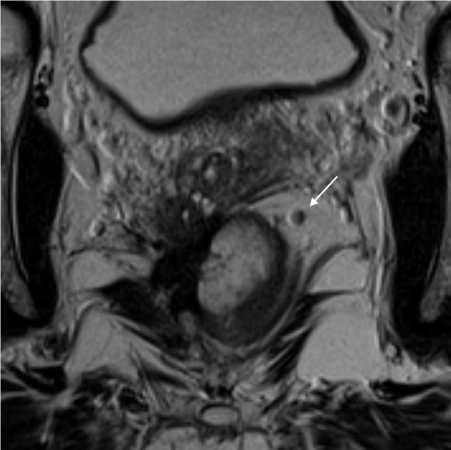
T2 Coronal High Resolution MRI of a T4N2M1 rectal cancer with a malignant lymph node in left obturator fossa (white arrow). The lymph node is enlarged and heterogeneous with an irregular border.
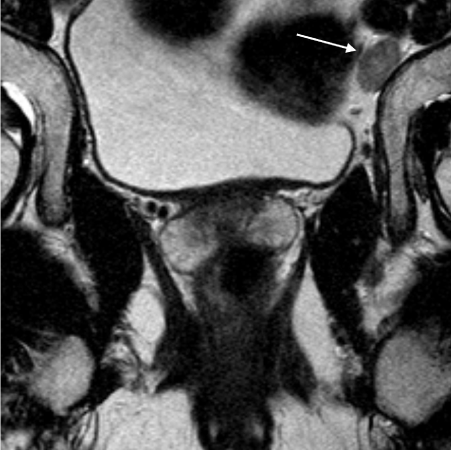
T2 Axial MRIs of the same patient with a T3b N1 rectal cancer. Figure 2a is a high resolution MRI performed with a 16cm FoV and 3mm slice thickness. A malignant lymph node is clearly seen in the left obturator fossa (white arrow), a benign lymph node seen adjacent to it (black arrow).
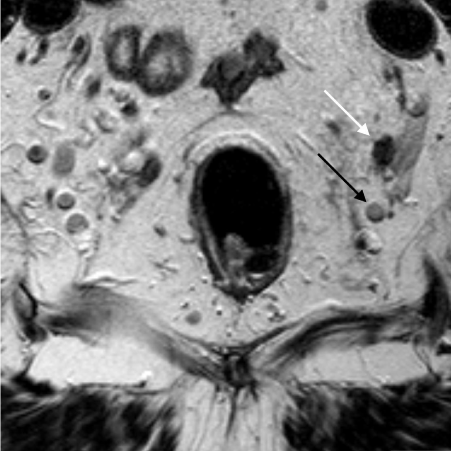
Figure 2b is a low resolution MRI performed with a 20cm FoV and a 3mm slice thickness. The malignant lymph is seen (dashed white arrow) but appears similar to the adjacent benign lymph node (dashed black arrow).
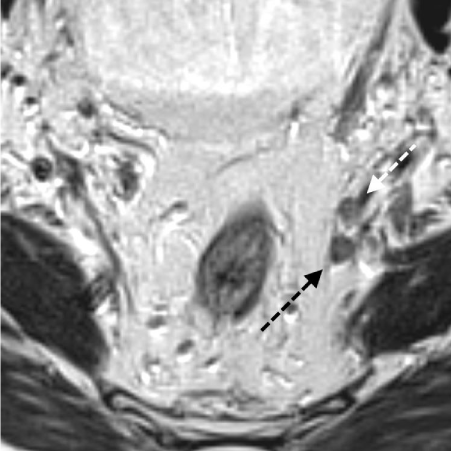
T2 Axial High Resolution MRI of the same patient with a T3d N1/2 rectal cancer. Figure 4a is the pre-chemoradiotherapy MRI which shows a large, heterogeneous irregular lymph node mass in the right obturator fossa (white arrow).
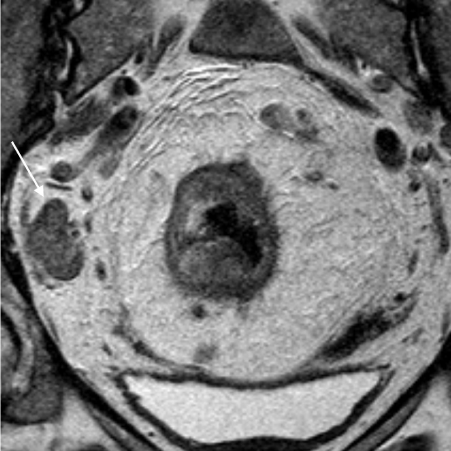
T2 Axial High Resolution MRI of the same patient with a T3d N1/2 rectal cancer. Figure 4b is the post-chemoradiotherapy MRI which shows the nodal mass has decreased in size but is still enlarged, heterogeneous and irregular (dashed black arrow).