
Research Article
Austin J Cancer Clin Res. 2023; 10(1): 1110.
Periodontitis and Breast Cancer: A Bidirectional Two-Sample Mendelian Randomization Study
Feng KX; Xing ZY; Ren F; Shang QY; Wang X*; Wang X*
Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China
*Corresponding author: Xin Wang & Xiang Wang Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China. Email: xinwang@vip.126.com; xiangw@vip.sina.com
Received: August 29, 2023 Accepted: September 23, 2023 Published: September 30, 2023
Abstract
Background: Observational studies linking periodontitis and breast cancer may be unreliable due to potential reverse causation and confounders.
Methods: A two-sample bidirectional Mendelian Randomization (MR) analysis was conducted. Techniques such as Inverse-Variance Weighted (IVW) analysis, weighted median, weighted mode, simple mode, and MR-Egger regression were utilized. Sensitivity analyses were also executed.
Results: The IVW results indicated no significant genetic link between periodontitis and increased breast cancer risk (OR=1.01, 95% CI: 0.90-1.13, P=0.92). Four other MR methods concurred. Reverse MR analysis also revealed no association. The IVW findings (OR=1.01, 95% CI: 0.90-1.13, P=0.92) were further validated by four additional MR techniques. As a validation step, periodontitis Genome-Aide Association Study (GWAS) data from the FinnGen consortium and breast cancer GWAS data from UKB were used.
Conclusions: The MR study confirms no bidirectional genetic causality between periodontitis and breast cancer. This aids in understanding the relationship between the two conditions and emphasizes further exploration into how periodontitis might relate to other systemic diseases.
Keywords: Breast cancer; Periodontitis; Mendelian randomization
Abbreviations: GWAS: Genome-Wide Association Study; IVW: Inverse Variance Weighted; MR: Mendelian Randomization; UKB: UK Biobank; SNPs: Single-Nucleotide Polymorphisms; IVs: Instrumental Variables; CI: Confidence Interval; OR: Odds Ratio; R2: Coefficient of Determination; RCT: Randomized Controlled Trial; WM: Weighted Median; EUR-UKB: European Ancestry-UK Biobank.
Introduction
As a public health burden and one of the primary causes of tooth loss, periodontitis is a chronic inflammatory condition with a complex multifactorial etiology [1]. In epidemiological investigations that have previously been performed, periodontal disease has been demonstrated to be causally related to an assortment of systemic illnesses in addition to its local effects, such as cardiovascular disease (Zhou, Dong, Zha, & Liao, 2021), rheumatoid arthritis (Bae & Lee, 2020), Parkinson's Disease (Botelho, Machado, Mendes, & Mascarenhas, 2021), depression (Nolde et al., 2022) and diabetes mellitus (Shah, Schooling, & Borrell, 2021). The investigation of the relationship between periodontal disease and the risk of suffering from cancer has become extremely popular. According to published studies, colorectal, lung, and pancreatic cancers have been associated with the pathogenesis of periodontitis [2-4].
Globally, the risk of contracting breast cancer is the highest among cancers and the second highest in terms of mortality [5]. Numerous risk variables, including endogenous hormone levels, immunological factors, genetic predisposition, and lifestyle choices, have been associated with this cancer according to the data that was accessible. A risk factor for breast cancer has also been associated with periodontal health. Studies that have been published on the possible association between periodontitis and breast cancer have resulted in varying results, with some arguing for a strong association and others arguing against it.
A meta-analysis involving eight studies, 168111 individuals confirmed periodontitis did increase susceptibility to breast cancer (RR=1.18, 95%CI: 1.11-1.26, I2=17.6%) [6]. Freudenheim et al. [7] and Sfreddo et al. [8] reported the same conclusion, while Mai et al. [9], Jia et al. [10], and Han et al. [11] reported different observations. Likewise, breast cancer treatment such as radiotherapy, chemotherapy, and endocrine therapy has an impact on periodontal health [12]. These observational studies are generally limited by residual confounders and reverse causality bias. Therefore, studying the possible causal relationship between periodontitis and breast cancer is a valuable tool to improve the treatment of both diseases.
Mendelian Randomization (MR) is a method based on whole genome sequencing data, similar to randomized controlled trial (RCT) studies, and has developed into a multifaceted approach to assessing causal relationships in epidemiology [13,14]. Using genetic variation closely related to exposure as Instrumental Variables (IVs) to evaluate causality and limit bias due to confounders, MR provides reliable insights into the effects of modifiable exposures on traits of interest, transforming phenotype-to-phenotype causality studies into genotypic studies, compared to traditional observational studies [15,16].
The present study is based on the hypothesis that there may be a bidirectional causal relationship between periodontitis and breast cancer. Therefore, two-sample summary data MR analysis was implemented in the study to investigate the possible bidirectional causal relationship between periodontitis and breast cancer.
Materials and Methods
Data Sources and Selection of Genetic Variants
For the validity of each IV, the Single-Nucleotide Polymorphisms (SNPs) used in MR analyses must fulfil three key assumptions [4]:
(1) Relevance assumption: there is robust association between the instrument and the exposure; (2) Independence assumption: the SNP cannot be related to any confounders of the exposure-outcome association; (3) Exclusion restriction assumption: the SNP has to affect the outcome only through the exposure of interest and not through any other pathway. Figure 1 shows a schematic of the Mendelian randomization study of periodontitis and breast cancer.
We performed the two sample MR analysis on the basis of aggregated statistics from the largest available Genome-Wide Association Study (GWAS) on periodontitis, which included 17353 periodontitis cases and 28210 controls [17]. For breast cancer, the data was from the GWAS including 133384 breast cancer cases and 113789 controls [18]. Both GWAS were performed among people of White European descent. As a validation set, the second data of periodontitis were obtained from the FinnGen consortium R8 release data (3837 cases and 242518 controls) [19,20]. The phenotype “periodontitis” was adopted in the current study. The data of breast cancer was from the UKB (EUR-UKB), including 13879 cases and 198523 controls [21]. The summary of the GWAS included in this Mendelian randomization study was in Table 1.
Exposures/Outcomes
Consortium
Ethnicity
Sample Sizes
Year
Training set
Periodontitis
GLIDE consortium and UKB
European
17,353 cases;
2019
28,210 controls
Breast cancer
BCAC
European
133,384 cases;
2020
113,789 controls
Validation set
Periodontitis
FinnGen R8
European
3837 cases;
2022
242518 controls
Breast cancer
UKB
European
13,879 cases;
2021
198523 controls
GWAS: Genome-Wide Association Studies; SNPs: Single Nucleotide Polymorphisms; IVs: Instrumental Variables; GLIDE: Gene-Lifestyle Interactions in Dental Endpoints; UKB: UK Biobank; BCAC: Breast Cancer Association Consortium
Table 1: Summary of the GWAS included in this Mendelian randomization study.
Selection of Genetic Instrumental Variables
Generally, the SNPs were selected at a genome-wide significance of P < 5×10-8 to satisfy the first assumption. Considering that few SNPs were associated with periodontitis at the level of P < 5×10-8, the genetic instruments with P<5×10-6 were selected. Eight SNPs were proposed as being strongly related with periodontitis based on a significant P-value (< 5×10−6 threshold) and were used as IVs [17]. To investigate the causal effect of breast cancer on periodontitis, 12 index SNPs reported significantly associated with breast cancer in a meta-analysis of GWAS on breast cancer (P<5×10-10) were included as candidate genetic instruments [18].
To exclude SNPs that were in strong Linkage Disequilibrium (LD), we performed the clumping procedure with R²<0.001 and a window size = 10,000 kb with the European ancestral individuals from the 1000 Genomes Project to ensure the independence of the instruments [22,23].
Finally, we harmonized the exposure and result datasets to eliminate ambiguous SNPs with non-concordant alleles and SNPs with intermediate allele frequencies in order to effectively ensure that the effect alleles correspond to the same allele. These meticulously selected SNPs served as the final genetic IVs for the ensuing MR analysis.
Furthermore, the F statistics for each SNP have been solely and cumulatively calculated with the following equation: F=R²*(N-2)/(1-R²). R² denotes the variance of exposure explained by each IV. IVs with F statistics of less than ten were considered weak instruments and would be excluded for MR analysis [13,24]. An illustration of the Mendelian randomization study of periodontitis and breast cancer is shown in Figure 1.
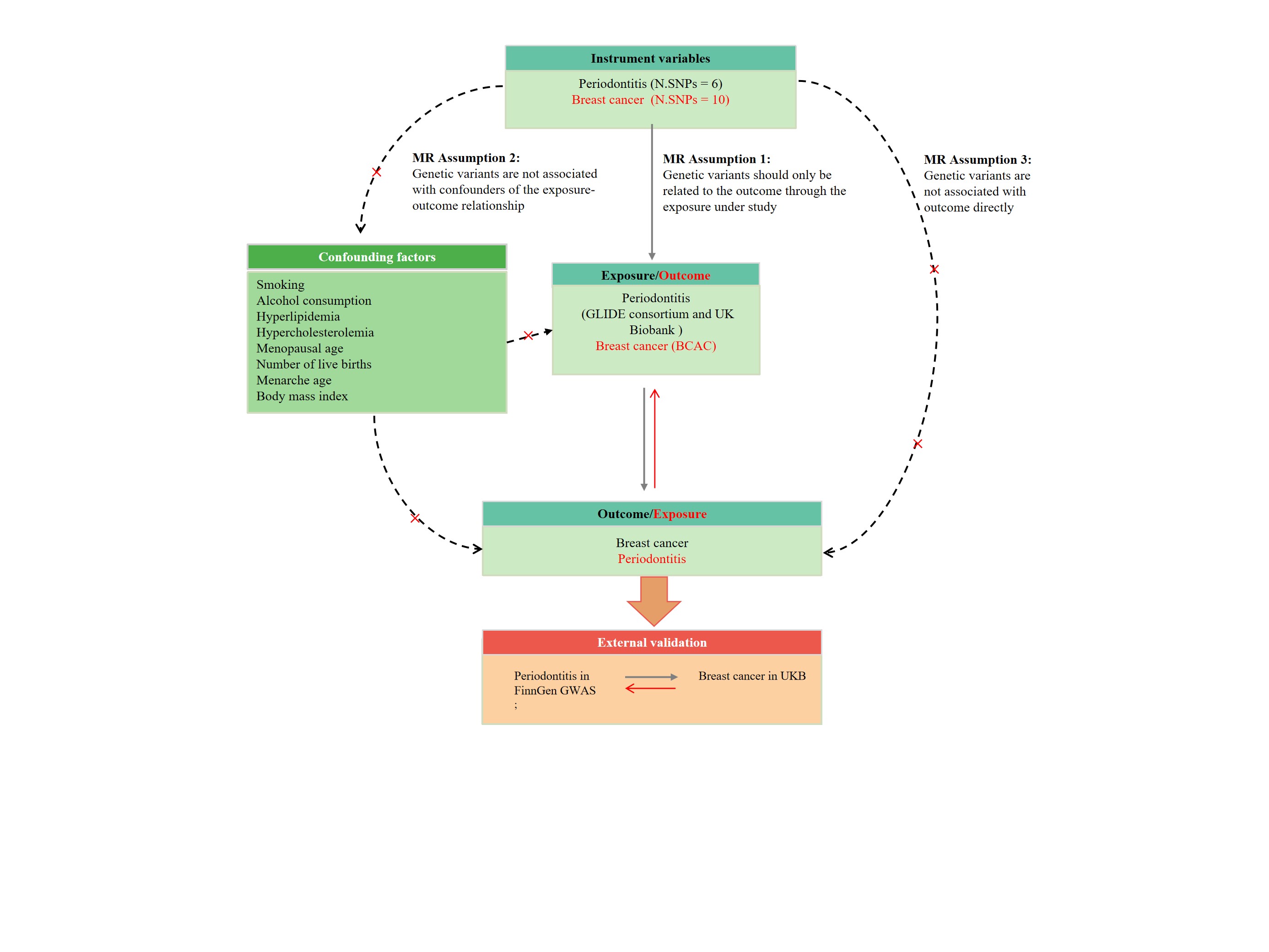
Figure 1: The flowchart of Mendelian randomization analysis.
IVW: Inverse Variance Weighted; MR: Mendelian Randomization; SNPs: Single Nucleotide Polymorphisms; IVs: Instrumental Variables; GLIDE: Gene-Lifestyle Interactions in Dental Endpoints; BCAC: Breast Cancer Association Consortium; UKB: UK Biobank
Statistical Analyses
Multiple statistical methods of MR for causal effect investigation of breast cancer and periodontitis were employed, including the Inverse Variance Weighted (IVW) [25], the Weighted Median (WM) [26], Simple Mode, Weighted Mode, and the MR-Egger [27] methods. Due to its robust causal estimates, IVW is the main method commonly used for two-sample MR studies [25]. WM is the median of the distribution function obtained by sorting all individual SNP effect values according to the weight. The WM can be estimated robustly when at least 50% of the information comes from valid instrumental variables [26]. MR-Egger is a multivariate MR method modified from IVW, assessing potential asymmetry for bias from the pleiotropic effect of the multiple genetic variants and estimating the causal effect [28].
Pleiotropy and Sensitivity Analysis
To assess the effect of risk of bias on the pooled results, we performed the pleiotropy and sensitivity analysis in which we excluded studies with a high risk of bias and assessed whether this changed the results appreciably, using the pleiotropy test, heterogeneity test, and leave-one-out sensitivity test. The intercept term of the MR-Egger regression and the asymmetry of the funnel plot can be utilized for calculating the IVs' average horizontal pleiotropy [29]. MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) analysis was conducted as well to determine whether pleiotropy was present, correct for horizontal pleiotropy by eliminating the outlier, and determine whether there are any substantial disparities between the causal effects prior to and following outlier removal [28]. We used the IVW and MR-Egger methods to detect heterogeneity, and a significant P-value (<0.05) indicates the presence of heterogeneity. In addition, we utilized the leave-one-out analysis to detect the robustness and consistency of the results.
All results are presented as an estimate or Odds Ratio (OR) with a 95% Confidence Interval (CI) of the outcomes OR or per predicted increase/decrease. All statistical tests were two-sided, and the evidence of association was cutoff at a prespecified P-value below 0.05. The open-source statistical software R (version: 4.2.0) was used for all analyses. The TwoSampleMR (version: 0.5.6) [28], MR-PRESSO (version: 1.0), and were mainly used to perform all analyses.
Results
Causal Effects of periodontitis on breast cancer
After removing two SNPs, rs2921075, rs4811024, for being palindromic with intermediate allele frequencies, we incorporated 6 independent SNPs from periodontitis GWAS as IVs for breast cancer. Figure 2 depicts the MR estimations obtained using various different approaches. Overall, no causal associations have been identified between genetically predicted periodontitis and breast cancer risk. The predominant findings of IVW revealed that an increase in the risk of developing periodontitis did not correlate significantly with an increased risk of contracting breast cancer (OR=1.01, 95% CI: 0.90-1.13, P=0.92, Table 2). In addition, the MR-Egger, Weighted Median, and Weighted Mode approaches also yield consistent results. Forest plot showing MR results testing for causal relationships between periodontitis and breast cancer (Figure 2). The scatter plot and funnel plots for effect sizes of SNPs for periodontitis and those for breast cancer were shown in Figures 3A and 3B. There was no pleiotropy between periodontitis (MR-Egger regression test, intercept =-0.018, P=0.305; Cochran’s Q report, P>0.05) and breast cancer (Table 2). No high-impact points were found in the leave-one-out analysis (Figures 3C). The F-statistic values were all greater than 10, ranging from 22.257 to 24.303, which indicated that the selection of instrumental variables was effective. The Supplementary Table S1, encompasses detailed information on instrumental variables for periodontitis.
Outcome
Exposure
Method
Beta
SE
P value
OR (95% CI)
Cochran's Q
Horizontal pleiotropy
Q
Q_df
Q_pval
Egger Intercept
SE
P value
Periodontitis
Breast cancer
MR Egger
0.056
0.073
0.482
1.058(0.917-1.220)
3.579
4
0.466
-0.018
0.015
0.305
IVW
0.006
0.059
0.918
1.006(0.896-1.129)
4.963
5
0.42
Weighted median
0.044
0.071
0.542
1.044(0.908-1.201)
Simple mode
0.042
0.108
0.711
1.043(0.845-1.288)
Weighted mode
0.055
0.072
0.481
1.057(0.917-1.218)
Breast cancer
Periodontitis
MR Egger
0.049
0.104
0.649
1.050(0.856-1.289)
10.688
8
0.22
0
0.02
0.984
IVW
0.047
0.034
0.17
1.048(0.980-1.122)
10.688
9
0.298
Weighted median
0.018
0.046
0.69
1.018(0.931-1.114)
Simple mode
0.001
0.071
0.987
1.001(0.871-1.151)
Weighted mode
-0.002
0.058
0.979
0.998(0.892-1.118)
MR: Mendelian Randomization; IVW: Inverse Variance Weighted; df: Degree of Freedom; MR: Mendelian Randomization; Q: Heterogeneity Statistic Q.
Table 2: Mendelian randomization estimates for the relationship between genetically instrumented periodontitis and breast cancer.
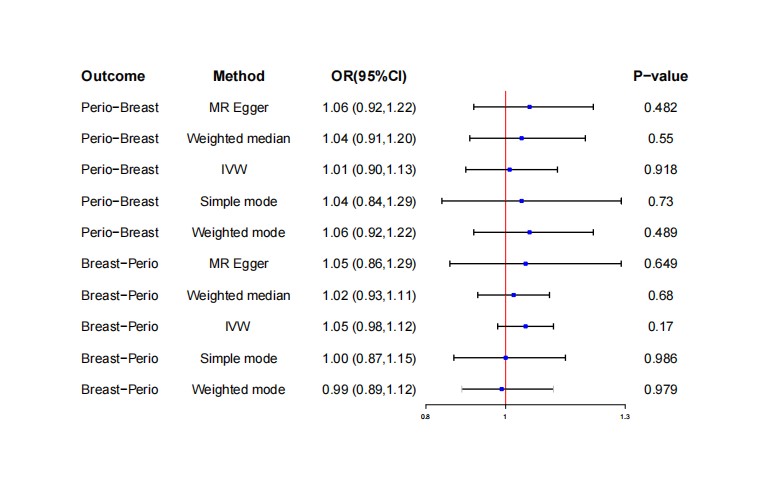
Figure 2: Mendelian randomization estimates for the relationship between genetically instrumented periodontitis and breast cancer, and vice versa.
CI: Confidence Interval; IVW: Inverse Variance-Weighted; OR: Odds Ratio.
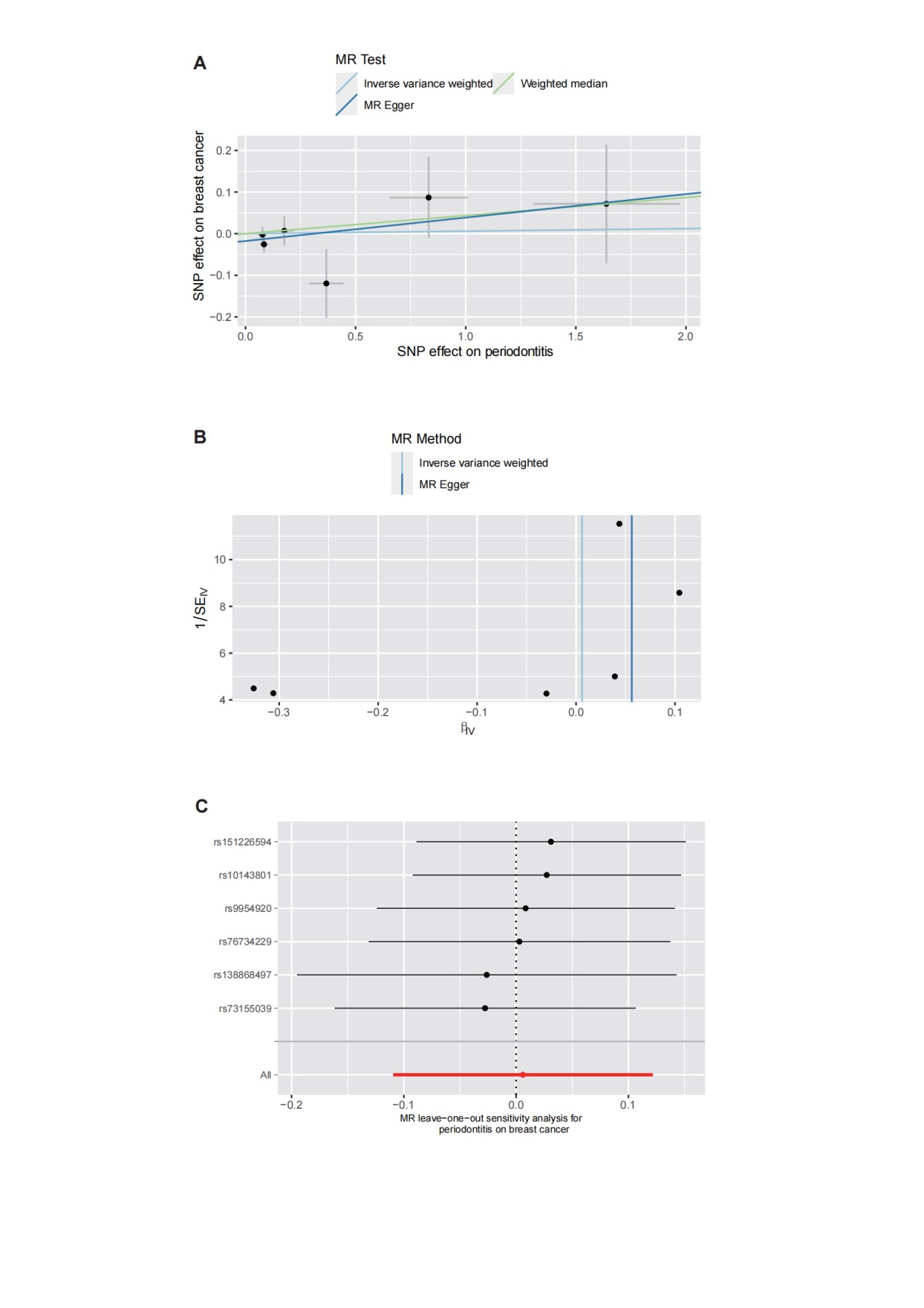
Figure 3: The scatter plot (A), funnel plots (B), and leave-one-out (C) for effect sizes of SNPs for periodontitis and those for breast cancer.
Causal Effects of breast cancer on periodontitis
After removing two SNPs, rs10483813, rs10995181, for being palindromic with intermediate allele frequencies, we incorporated 10 independent SNPs from breast cancer GWAS as IVs for periodontitis. The F-statistics ranged between 49.160-168.415, indicating that the selection of instrumental variables was effective (Supplementary file, Table S1). There was no association between breast cancer and periodontitis (OR=1.01, 95% CI: 0.90-1.13, P=0.92, Table 2), based on the IVW estimate, which was supported by the MR-Egger, the Weighted Median, Simple Mode, and the Weighted Mode methods (Table 2, Figure 2). The scatter plot and funnel plots for effect sizes of SNPs for breast cancer and those for periodontitis were shown in Figure 4A and 4B. The leave-one-out analysis did not reveal any leverage points with high influence (Figures 4C). There was no pleiotropy between breast cancer (MR-Egger regression test, intercept =-0.013, P=0.270; Cochran’s Q report, P>0.05) and periodontitis (Table 2).
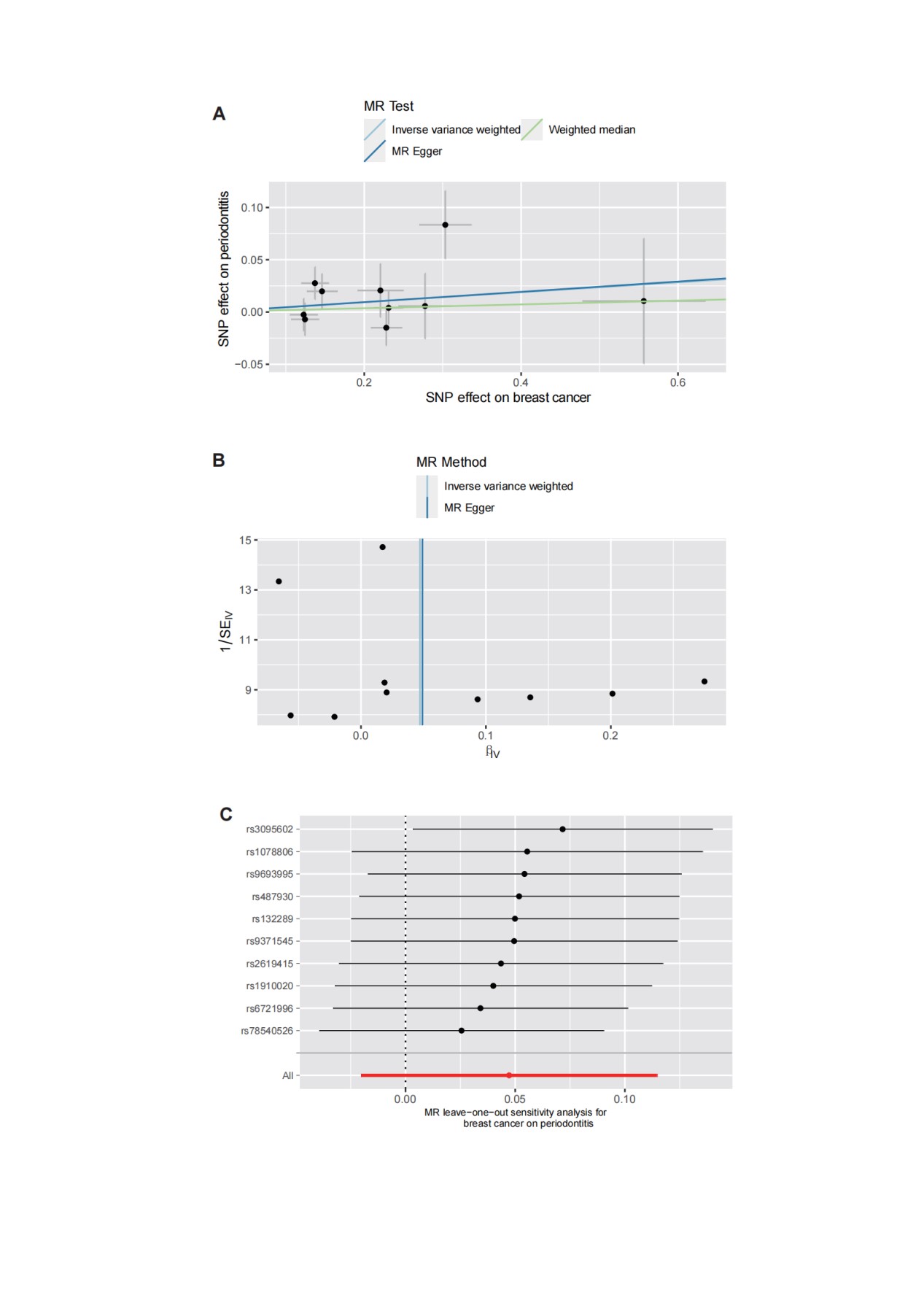
Figure 4: The scatter plot (A), funnel plots (B), and leave-one-out analysis (C) for effect sizes of SNPs for breast cancer and those for periodontitis.
The validation of bidirectional Mendelian Randomization
The validation using 30 non-overlapping SNPs from the FinnGen consortium R8 release data (19, 20) as periodontitis IVs and 28 SNPs from UKB (21) as breast cancer IVs confirmed the aforementioned results (Supplementary Table S2).
Discussion
In the realm of understanding the complex interplay between chronic inflammatory conditions and cancer susceptibilities, our rigorous two-way MR analysis offers a novel perspective on the potential causal relationship between periodontitis and breast cancer. While previous epidemiological studies painted an inconsistent picture of this relationship, our data-driven approach, grounded in large-scale GWAS datasets, provides a compelling narrative.
It's well-established that periodontitis, a chronic inflammatory disease, has the potential to influence systemic immune and inflammatory responses, primarily through the release of inflammatory mediators [30]. Such chronic inflammation can compromise the immune system's equilibrium, which may subsequently impair tumor cell recognition and elimination, thereby fostering tumor progression [31].
Regarding the association between periodontitis and malignancies, existing observational studies have yielded inconsistent findings. While some studies have reported significant associations between periodontitis and malignancies such as colorectal [32,33], lung [4,34], prostate cancers [35], and pancreatic cancers [36], others have failed to confirm such associations. There is evidence that periodontitis may promote the occurrence and development of breast cancer, and even inflammation of the periodontal tissues promotes breast cancer metastases [37]. In turn, the treatment of breast cancer, radiation therapy, chemotherapy, and endocrine therapy, can affect periodontal health [12]. These conflicting results may be attributed to variations in sample size, study design, population characteristics, and definitions of periodontitis and neoplasia.
The underlying mechanisms influencing the relationship between periodontitis and malignancy are multifaceted and encompass chronic inflammation, the role of bacteria and periodontal pathogens, and alterations in the immune system [38,39]. A particularly intriguing facet of this relationship is the role of the oral microbiome. Disruptions in the oral microbiota have been spotlighted as risk factors for a plethora of diseases, including gastrointestinal cancers, lung cancer, and notably, breast cancer [40,41]. Fusobacterium nucleatum, an oral bacterium, has emerged as a noteworthy player, being linked with various tumor types [42]. Its proposed mechanisms span from modulating the tumor microenvironment to intricate interactions with host cells and inducing host inflammation. Although our results, consistent across multiple MR methods, suggest that the genetic underpinnings of periodontitis may not have a direct causal influence on breast cancer risk, the alterations in the oral microbiome remain a tantalizing area warranting deeper exploration.
Our revelations seem to diverge from some observational studies that have asserted a causal interplay between periodontitis and breast cancer [6,8,10,43-45]. Confounding and reverse causation bias are two fundamental drawbacks of observational research that ought to be acknowledged. In particular, age, lifestyle choices, and underlying genetic predisposition constitute common risk factors between periodontitis and breast cancer, which may obscure relationships discovered in observational research. In observational studies, where the existence of breast cancer may influence changes in oral health and the emergence of periodontitis, reverse causality can also be an issue that might arise. Contrarily, confounding and reverse causation biases, which can be prevalent in observational research, have been effectively eliminated by the MR analysis employed in this research. MR analysis is able to produce far more precise causal estimates for investigating the potential causal relationship between periodontitis and breast cancer through the utilization of genetic variation as a proxy for exposure. Nevertheless, it is crucial to reiterate that although our results utilize a robust causal inference technique, it is not sufficient to completely rule out the possibility of an association between periodontitis and breast cancer.
Despite the beneficial effects of MR analysis in resolving confounding variables and reverse causation, it is contingent on certain presumptions that could result in potential constraints to our investigation. As such, it is contingent upon the availability of genetic tools, for instance, and may not be able to adequately account for all genetic heterogeneity. Although we conducted sensitivity analyses and employed MR-Egger and MR-PRESSO tests to detect genetic heterogeneity effects, the possibility of unmeasured genetic heterogeneity cannot be entirely ruled out. Additionally, the GWAS data utilized in our analysis predominantly represented European Caucasians, and the genetic structure and underlying biological mechanisms may vary across different populations. Therefore, the generalizability of our findings to other populations with distinct genetic backgrounds necessitates further investigation.
Although our two-way MR analysis did not provide evidence supporting a causal relationship between periodontitis and breast cancer, the insights gained from this investigation shed light on common risk factors and potential biological pathways shared between these two conditions. Understanding the interactions between periodontitis and breast cancer could inform public health interventions and personalized treatment strategies. Future studies should continue to explore the underlying biological mechanisms involving genetic factors, inflammation, and cancer risk, while also encompassing diverse populations to attain a more comprehensive understanding of the association between periodontitis and breast cancer.
Conclusions
Our bidirectional two-sample MR analysis doesn't buttress a direct causal link between periodontitis and breast cancer. These findings punctuate the ongoing dialogue surrounding periodontitis and breast cancer, emphasizing the intricate choreography between oral health, the microbiome, and systemic maladies like cancer. This underscores the imperative for a more integrative healthcare paradigm, recognizing the symbiosis of seemingly discrete physiological systems.
Author Statements
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 82072097), the CAMS Innovation Fund for Medical Sciences (GIFMS, 2021-I2M-1-014), the Breast Cancer Single Disease Diagnosis and Treatment Capacity Enhancement Project (RXDBZ-2022-11).
Data Availability Statement
The datasets analyzed during the current study are available from https://data.bris.ac.uk/data/dataset/2j2rqgzedxlq02oqbb4vmycnc2 for periodontitis GWAS, https://bcac.ccge.medschl.cam.ac.uk/bcacdata/ for breast cancer GWAS, gs://finngen-public-data-r8/summary_stats/finngen_R8_K11_PERIODON_CHRON.gz for validation of periodontitis GWAS and https://gwas.mrcieu.ac.uk/datasets/ieu-b-4810/ for validation of breast cancer GWAS.
Acknowledgements
We would like to extend our appreciation to Yifan Zhao for her valuable contributions to the language revision. We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
References
- Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet. 2019; 394: 249-60.
- Heikkilä P, But A, Sorsa T, Haukka J. Periodontitis and cancer mortality: register-based cohort study of 68,273 adults in 10-year follow-up. Int J Cancer. 2018; 142: 2244-53.
- Corlin L, Ruan M, Tsilidis KK, Bouras E, Yu YH, Stolzenberg-Solomon R, et al. Two-Sample Mendelian randomization analysis of associations between periodontal disease and risk of cancer. JNCI Cancer Spectr. 2021; 5: pkab037.
- Zeng XT, Xia LY, Zhang YG, Li S, Leng WD, Kwong JS. Periodontal disease and incident lung cancer risk: A meta-analysis of cohort studies. J Periodontol. 2016; 87: 1158-64.
- Cancer today WHO; 2020. (Available from: http://gco.iarc.fr/today/fact-sheets-populations.
- Shi T, Min M, Sun C, Zhang Y, Liang M, Sun Y. Periodontal disease and susceptibility to breast cancer: A meta-analysis of observational studies. J Clin Periodontol. 2018; 45: 1025-33.
- Freudenheim JL, Genco RJ, LaMonte MJ, Millen AE, Hovey KM, Mai X, et al. Periodontal disease and breast cancer: prospective cohort study of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2016; 25: 43-50.
- Sfreddo CS, Maier J, De David SC, Susin C, Moreira CHC. Periodontitis and breast cancer: A case-control study. Community Dent Oral Epidemiol. 2017; 45: 545-51.
- Mai X, LaMonte MJ, Hovey KM, Freudenheim JL, Andrews CA, Genco RJ, et al. Periodontal disease severity and cancer risk in postmenopausal women: the Buffalo OsteoPerio Study. Cancer Causes Control. 2016; 27: 217-28.
- Jia M, Wu Z, Vogtmann E, O’Brien KM, Weinberg CR, Sandler DP, et al. The association between periodontal disease and breast cancer in a prospective cohort study. Cancer Prev Res (Phila). 2020; 13: 1007-16.
- Han MA. Oral Health status and behavior among cancer survivors in Korea using nationwide survey. Int J Environ Res Public Health. 2017; 15: 14.
- Zhang Y, Ren X, Hu T, Cheng R, Bhowmick NA. The relationship between periodontal disease and breast cancer: from basic mechanism to clinical management and prevention. Oral Health Prev Dent. 2023; 21: 49-60.
- Burgess S, Foley CN, Zuber V. Inferring causal relationships between risk factors and outcomes from genome-wide association study data. Annu Rev Genomics Hum Genet. 2018; 19: 303-27.
- Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2019; 4: 186.
- Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. 2019; 10: 486-96.
- Sekula P, Del Greco M F, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016; 27: 3253-65.
- Shungin D, Haworth S, Divaris K, Agler CS, Kamatani Y, Keun Lee M, et al. Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nat Commun. 2019; 10: 2773.
- Zhang H, Ahearn TU, Lecarpentier J, Barnes D, Beesley J, Qi G, et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat Genet. 2020; 52: 572-81.
- Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: unique genetic insights from combining isolated population and national health register data. medRxiv. 2022.03.03.22271360.
- FinnGen R8 release. 2022. Available from: https://risteys.finngen.fi/endpoints/K11_PERIODON_CHRON.
- Kimberley Burrows PH. Genome-wide association study of cancer risk in UK Biobank; 2021. (Available from: https://doi.org/10.5523/bris.aed0u12w0ede20olb0m77p4b9.
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81: 559-75.
- 1000 Genomes Project Consortium, Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM et al. A map of human genome variation from population-scale sequencing. Nature. 2010; 467: 1061-73.
- Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011; 40: 755-64.
- Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015; 181: 251-60.
- Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016; 40: 304-14.
- Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017; 32: 377-89.
- Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018; 50: 693-8.
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018; 7: e34408.
- Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021; 21: 426-40.
- Cardoso EM, Reis C, Manzanares-Céspedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018; 130: 98-104.
- Di Spirito F, Toti P, Pilone V, Carinci F, Lauritano D, Sbordone L. The association between periodontitis and human colorectal cancer: genetic and pathogenic linkage. Life (Basel). 2020; 10: 211.
- Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019; 42: 27-35.
- Wang J, Yang X, Zou X, Zhang Y, Wang J, Wang Y. Relationship between periodontal disease and lung cancer: A systematic review and meta-analysis. J Periodont Res. 2020; 55: 581-93.
- Guo Z, Gu C, Li S, Gan S, Li Y, Xiang S, et al. Periodontal disease and the risk of prostate cancer: a meta-analysis of cohort studies. Int Braz J Urol. 2021; 47: 1120-30.
- Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018; 67: 120-7.
- Cheng R, Billet S, Liu C, Haldar S, Choudhury D, Tripathi M, et al. Periodontal inflammation recruits distant metastatic breast cancer cells by increasing myeloid-derived suppressor cells. Oncogene. 2020; 39: 1543-56.
- Nwizu N, Wactawski-Wende J, Genco RJ. Periodontal disease and cancer: epidemiologic studies and possible mechanisms. Periodontol 2000. 2020; 83: 213-33.
- Michaud DS, Fu Z, Shi J, Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017; 39: 49-58.
- Van der Merwe M, Van Niekerk G, Botha A, Engelbrecht AM. The onco-immunological implications of Fusobacterium nucleatum in breast cancer. Immunol Lett. 2021; 232: 60-6.
- Parhi L, Alon-Maimon T, Sol A, Nejman D, Shhadeh A, Fainsod-Levi T, et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 2020; 11: 3259.
- Alon-Maimon T, Mandelboim O, Bachrach G. Fusobacterium nucleatum and cancer. Periodontol 2000. 2022; 89: 166-80.
- Wu Z, Li F, Sang J, Gierach GL, Vogtmann E. The association between poor oral health and risk of breast cancer in the UK Biobank. Cancer Causes Control. 2023; 34: 491-4.
- Chen CC, Ho WL, Lin CH, Chen HH. Stratified analysis of the association between periodontitis and female breast cancer based on age, comorbidities and level of urbanization: A population-based nested case-control study. PLOS ONE. 2022; 17: e0271948.
- Corbella S, Veronesi P, Galimberti V, Weinstein R, Del Fabbro M, Francetti L. Is periodontitis a risk indicator for cancer? A meta-analysis. PLOS ONE. 2018; 13: e0195683.