
Case Report
Austin J Cancer Clin Res 2015;2(2): 1029.
Primary Cutaneous Alk Positive Anaplastic Large Cell Lymphoma in a Melanoma Patient
Paolino G*1, Didona D1, Gianno F2, Garelli V1, Soda G2, Cantisani C1, Calvieri S1, and Richetta AG1
1Dermatology Clinic, La Sapienza University of Rome, Italy
2Pathology, Department of Sperimental medicine, La Sapienza University of Rome, Italy
*Corresponding author: Giovanni Paolino, Dermatology Clinic, La Sapienza University of Rome, Viale del Policlinico 15, 00186 Rome, Italy.
Received: February 27, 2015; Accepted: March 28, 2015; Published: April 01, 2015
Abstract
Primary cutaneous anaplastic large cell lymphoma (PCALCL) is a rare subset of CD30+ lymphoproliferative disorder, characterized by the presence of large anaplastic cells, which express CD30, CD2, CD3, CD4, and CD5. A 62 year-old male patient presented to our Institute with a history of a fastgrowing and pinkish-brown asymptomatic cutaneous nodule. His medical past history was positive for a malignant melanoma (MM) of the abdomen (0.7 mm Breslow thickness; pT1a). Histologically the lesion showed a diffuse infiltrate consisting in cohesive sheets of large cells with anaplastic morphology with a kidney-shaped nucleus, also known as hallmark cells. Immunohistochemical studies revealed a CD30 expression, and a positivity to perforin and anaplastic lymphoma kinase (ALK). The laboratory and instrumental investigations were all normal and a final diagnosis of PCALCL was made. The patient showed a good response to radiotherapy. Usually PCALCL shares with systemic anaplastic large cell lymphoma (ALCL) the presence of neoplastic CD30+ large T cells, but lack ALK translocations and protein expression. However, the detection of ALK expression in PCALCL should be considered highly suspicious of a cutaneous manifestation of an underlying systemic disease (not detected in our patient). We recommend to exclude a systemic involvement in this kind of disease, with a strict follow-up, especially in patients with double malignancies.
Keywords: Primary cutaneous anaplastic large cell lymphoma; Melanoma; Radiotherapy; Osteomedullary biopsy; Anaplastic lymphoma kinase; Systemic anaplastic large cell lymphoma
Case Presentation
A62 year-old Caucasian male patient presented to our Institute with a 15 days history of a fast-growing and pinkish-brown asymptomatic nodule (2cm x 3cm) in the left pectoral area. The lesion was not painful, not malodorous, firm on palpation and produced a serous secretion. The surrounding skin was erythematous (Figure 1).
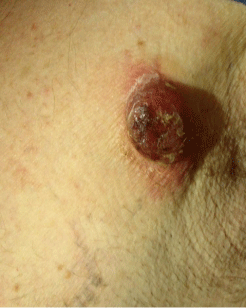
Figure 1: Asymptomatic nodule (2cm x3cm) in the left pectoral area. The
lesion was not painful, not malodorous and firm on palpation. The surrounding
skin was erythematous.
His familiar history was negative for malignancies and other diseases; while his medical past history was positive for a malignant melanoma (MM) of the abdomen, 0.7 mm Breslow thickness, not ulcerated, (pT1a N0 M0; Stage IA), which was removed 5 years before.
For the nodular lesion, the patients’ general practitioner prescribed an oral antibiotic therapy (amoxicillin + clavunalate 1gr daily for 5 days), without any improvement.
In this regard, according to the appearance of the cutaneous lesion and to the personal medical history, a wide excision was performed.
Histologically the lesion showed a diffuse infiltrate consisting in cohesive sheets of cells with anaplastic morphology and variable sizes (from small to large). The neoplastic elements showed round, oval and/or irregularly-shaped nuclei with prominent eosinophilic nucleoli. The most distinctive appearance was the presence of large cells with a kidney-shaped nucleus, also known as hallmark cells. The cytoplasm was typically abundant, eccentrically placed, with prominent nucleoli (Figure 2A; 2B). Immunohistochemical studies revealed a CD30 expression in the majority of the neoplastic cells (Figure 3A), and a strong positivity to perforin and anaplastic lymphoma kinase (ALK) were also detected (Figure 3B).
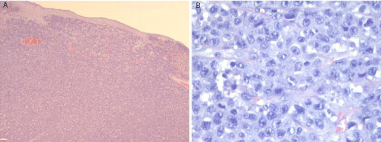
Figure 2 b: Alarge cell population with kidney-shaped nucleus, with abundant
cytoplasm, eccentrically placed. (X 40, Hematoxilin and Eosin).
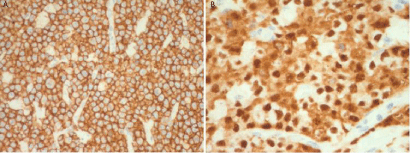
Figure 3b: Diffuse ALK positivity(X 40).
According to the histopathological and clinical features a diagnosis of cutaneous anaplastic large cells lymphoma (CALCL) CD30 positive and ALK positive was made. Consequently, an osteomedullary biopsy (OMB) was performed, showing a hematopoietic bone-marrow cellularity of 25% (CD20 + B lymphocytes 5% and CD3+ T lymphocytes 7% with interstitial random distribution) in the absence of localization of lymphoma. The total body CT and the laboratory investigations were also negative.
According to the absence of systemic involvement, a final diagnosis of primary of cutaneous anaplastic large cells lymphoma (PCALCL), ALK+ was made. Our patient was staged as T2a N0 M0, according to the International Society for Cutaneous Lymphoma (ISCL) and Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC) [1].
Two weeks after the primary surgical excision of the cutaneous lesion, the patient returned at our Department with a further nodular lesion in correspondence of the surgical scar. Therefore, a second wide excision was performed and the histological examination confirmed the same diagnosis. Hence, the patient started a course of local radiotherapy with 40 Gy (2 Gy in 20 daily fractions).
Currently, after a follow-up of 1 year, the patient is disease free and he performs periodical clinical and instrumental controls.
Discussion
Primary cutaneous T-cell lymphoma (PCTCL) is a wide group of different non-Hodgkin lymphomas, characterized by clonal expansion of skin-homing malignant T lymphocytes [1,2]. Primary cutaneous CD30+ disorders accounts for 30% of cutaneous T-cell lymphomas and are currently classified in primary cutaneous anaplastic large cell lymphoma (PCALCL), lymphomatoid papulosis (LyP) and borderline subset [1-3]. Diagnosis of PCALCL is possible when the following three criteria are fulfilled: (a) >75% presence of CD30+ anaplastic large cells in skin biopsy, (b) negative clinical past history for other cutaneous lymphomas, (c) absence of extracutaneous localization [2,3].
PCALCL is histologically characterized by the presence of large anaplastic cells, which express CD30, CD2, CD3, CD4, and CD5 (T-helper phenotype); although, to date, in literature, several histopathological variants (basing on the type of cell that predominate in the infiltrate and on the size of the malignant lymphocytes) are also reported [4-6].
Our patient fulfilled all the diagnostic criteria and therefore was correctly diagnosed as PCALCL. Besides, according to the literature, PCALCL can be also classified as T-(CD3+), B-(CD20+) or null- (CD3-, CD20-) cell immunophenotype [4,7,8]. Our case was CD3- and CD20- and was classified as a null-cell type. Finally, a cytotoxic T-cell phenotype (CD8+) PCALCL has been also rarely reported in literature [9].
Clinically, PCALCL is often a solitary, asymptomatic, reddish to lilaceous nodule, with erythematous peripheral borders. It is frequently localized on the trunk and extremities and affects mostly male and elderly patients, with a median age of 60 years, as reported in our case.
The etiopathogenesis of the disease is currently unknown; most likely the clonal expansion of CD30+ cells is influenced by host immune response [1,4]. In this regard and in the general spectrum of anaplastic large cell lymphomas, previous reports found also a close relationship among pro-inflammatory mediators such as HMBG- 1, MMP-9, PAR-2 and IL-8 chemokine in the development of the lymphoma cell invasion and metastasization [4,10,11]. However, to date, the causes of the onset of the disease are still to elucidate.
ALK is frequently observed in systemic ALCL, mostly in childhood or adolescence, but only rarely in primary cutaneous cases [4]. Translocations involving ALK gene detected by immunohistochemistry as positive staining with antibodies against ALK is observed in 50 to 80% of systemic ALCL cases, but is rarely detected in PCALCL. Besides, PCALCL remains confined to the skin, virtually never disseminates beyond local lymph nodes, and shows an excellent prognosis after surgical resection without systemic therapy [12]. However, in this regard, recently published recommendations state that immunohistochemical detection of ALK expression should be considered highly suspicious of a cutaneous manifestation of an underlying systemic ALCL [12,13].
For this reason, after the histological diagnosis, we decided to perform an osteo-medullar-biopsy (OMB) and a total-body CT. Besides, although up to 25% of patients with PCALCL show a regression of the lesion [8], in our case the nodule reappeared 15 days after the primary surgical wide excision, showing a locally aggressive course.
It is widely reported in literature an association between cutaneous melanoma and non-Hodgkin’s lymphoma, as well as an increased risk of non-Hodgkin’s lymphoma following cutaneous melanoma and vice versa [14]. This intriguing possible association is of interest in terms of understanding their etiology, which is likely to be multifactorial [14]. In this regard, for both the tumors, UV exposure, chronic immunosuppression and genetic predisposition were assessed as possible precipitating factors [14,15]. Further researches are necessary to elucidate the role of different common aetiological factors responsible for this association.
Regarding the therapy, PCACL is a chemosensitive and radiosensitive lymph proliferative disorder; treatments include surgical excision, CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) or local radiotherapy (as well as a combination of these treatments) [16]. Up to date, the optimal dose for treatment of CALCL remains an open issue, although complete responses with dosages between 34 and 44 Gy have proved to be highly effective [16]. Our patient performed two local surgical excisions, followed by only radiotherapy and currently is disease free.
According to our experience, we recommend for PCACL (ALK+) baseline MOB and total body CT, in order to exclude a systemic involvement and a strict follow-up, especially in patients with double malignancies, as reported in our case.
References
- Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007; 110: 479-484.
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005; 105: 3768-3785.
- Rosen ST, Querfeld C. Primary cutaneous T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2006.
- Chao-Lo MP, King-Ismael D, Lopez RA. Primary cutaneous CD30+ anaplastic large cell lymphoma: report of a rare case. J Dermatol Case Rep. 2008; 2: 31-34.
- Xu H, Qian J, Wei J, Zhao Y, Zhou C, Chen D, et al. CD8-positive primary cutaneous anaplastic large cell lymphoma presenting as multiple scrotal nodules and plaques. Eur J Dermatol. 2011; 21: 609-610.
- Kempf W, Kazakov DV, Paredes BE, Laeng HR, Palmedo G, Kutzner H, et al. Primary cutaneous anaplastic large cell lymphoma with angioinvasive features and cytotoxic phenotype: a rare lymphoma variant within the spectrum of CD30+ lymphoproliferative disorders. Dermatology. 2013; 227: 346-352.
- Gould JW, Eppes RB, Gilliam AC, Goldstein JA, Mikkola DL, Zaim MT, et al. Solitary primary cutaneous CD30+ large cell lymphoma of natural killer cell phenotype bearing the t(2;5)(p23;q35) translocation and presenting in a child. Am J Dermatopathol. 2000; 22: 422-428.
- Liu HL, Hoppe RT, Kohler S, Harvell JD, Reddy S, Kim YH, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003; 49: 1049-1058.
- Plaza JA, Ortega P, Lynott J, Mullane M, Kroft S, Olteanu H, et al. CD8-positive primary cutaneous anaplastic large T-cell lymphoma (PCALCL): case report and review of this unusual variant of PCALCL. Am J Dermatopathol. 2010; 32: 489-491.
- Willemze R, Meijer CJ. Primary cutaneous CD30-positive lymphoproliferative disorders. Hematol Oncol Clin North Am. 2003; 17: 1319-1332, vii-viii.
- Dejean E, Foisseau M, Lagarrigue F, Lamant L, Prade N, Marfak A, et al. ALK+ALCLs induce cutaneous, HMGB-1-dependent IL-8/CXCL8 production by keratinocytes through NF-κB activation. Blood. 2012; 119: 4698-4707.
- Oschlies I, Lisfeld J, Lamant L, Nakazawa A, d'Amore ES, Hansson U, et al. ALK-positive anaplastic large cell lymphoma limited to the skin: clinical, histopathological and molecular analysis of 6 pediatric cases. A report from the ALCL99 study. Haematologica. 2013; 98: 50-56.
- Kempf W, Pfaltz K, Vermeer MH, Cozzio A, Ortiz-Romero PL, Bagot M, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoidpapulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011; 118: 4024-4035.
- Lens MB, Newton-Bishop JA. An association between cutaneous melanoma and non-Hodgkin's lymphoma: pooled analysis of published data with a review. Ann Oncol. 2005; 16: 460-465.
- Lam CJK, Curtis RE, Dores G, Engels EA, Caporaso N, Polliacket A, et al. Risk factors for melanoma among survivors of non-Hodgkin lymphoma in the U.S. elderly population. J ClinOncol. 2014; 32: 5s.
- Yu JB, McNiff JM, Lund MW, Wilson LD. Treatment of primary cutaneous CD30+ anaplastic large-cell lymphoma with radiation therapy. Int J Radiat Oncol Biol Phys. 2008; 70: 1542-1545.