
Short Communication
Austin J Cancer Clin Res 2015;2(2): 1031.
Gli1 Expression with Survival in Esophageal Adenocarcinoma
Okereke IC1*, Fan QL2, Pulikal A2, Kohtz P2 and Xie J2
1Division of Thoracic Surgery, Department of Surgery, Warren Alpert Medical School of Brown University, USA
2Departments of Pediatrics, Biochemistry and Molecular Biology and Surgery, Indiana University School of Medicine, USA
*Corresponding author: Ikenna Okereke, Division of Thoracic Surgery, Department of Surgery, Warren Alpert Medical School of Brown University, 2 Dudley St., Suite 470, Providence, RI 02903, USA.
Received: February 16, 2015; Accepted: March 13, 2015; Published: March 26, 2015
Abstract
Esophageal adenocarcinoma (EAC) is the fastest growing cancer over the last decade in the US, but the exact mechanisms responsible for EAC remain elusive. Identification of specific biomarkers for EAC has significant impacts on early detection of EAC and potential novel cancer therapeutics. The sonic hedgehog (Hh) signaling pathway is known to be activated in esophageal squamous cell carcinomas but its specific role in EAC remains unknown. Our goal was to determine the association between Hh signaling activation and outcomes in patients with EAC.
A total of 761 patients underwent esophagectomy at a tertiary care institution from 1993 to 2011. Of this group, 28 patients underwent esophagectomy alone for EAC, without neoadjuvant chemoradiation treatment. Portions of their tumor were freshly frozen and stored for later use. Expression of Hh target genes or ligands was determined in each tumor using real-time polymerase chain reaction (Gli1, Gli2, Shh and Ihh). Demographics, pathologic data and survival were recorded.
Mean patient age was 66. There were no perioperative mortalities. Expression of Gli1 was significantly related to poor overall survival (p = 0.048) but was not related to age (p = 0.53) or gender (p = 0.19). Of all the genes analyzed, expression of Gli1 is significantly correlated to poor survival. Based on our data, we propose to perform novel clinical trials to treat patients with activated Hh signaling in EAC.
Keywords: Esophageal cancer; Hedgehog; Biomarkers; Tumorigenesis
Introduction
Esophageal cancer is the most rapidly growing solid organ malignancy, while the incidence of many other cancer types have had a diminishing prevalence overall. Almost 18,000 people each year in the United States are affected by esophageal cancer [1]. Due to delayed diagnosis and usual advanced stage at presentation, the prognosis of EAC is generally poor. Adenocarcinoma of the esophagus predominates in the United States, whereas squamous cell cancer is more prevalent in the eastern hemisphere [2].
Current treatments of esophageal cancer are dependent upon the stage at diagnosis. Early stage disease is generally treated with surgical resection alone. For locally advanced disease, the treatment option is generally a combination of chemotherapy, radiation treatment and surgery [3]. Stage IV esophageal cancer, on the other hand, is usually treated with chemotherapy.
Initially identified as an important development signaling pathway, activation of Hh signaling has been reported in many solid organ tumors [4,5]. Hh ligands bind to the “patched” receptor, which triggers a signaling cascade leading to expression of Hh targets [6]. Specifically, two of these targets, glioma-associated oncogene 1 and glioma-associated oncogene 2 (Gli1 and Gli2), have been implicated in development of multiple cancers when overexpressed, including esophageal cancer, basal cell cancer, pancreatic cancer [7-9]. Overexpression of Hh targets has been associated with the development of esophageal squamous cell cancer [10]. Furthermore, high levels of Gli1 have been correlated with poor survival in esophageal squamous cell cancer [11]. It remains unclear the exact role of Hh signaling in esophageal adenocarcinoma. In this study, our goal was to examine the association of hedgehog signaling gene expression and outcome in esophageal adenocarcinoma.
Material and Methods
Tumor specimens
From 1993 through 2011, 761 patients underwent esophagectomy at Indiana University School of Medicine. After institutional review board approval, twenty-eight of these patients who underwent esophagectomy alone for esophageal adenocarcinoma had portions of their primary tumor freshly frozen to -80 degrees Celsius and stored for use. None of these patients had chemoradiation treatment prior to surgical resection. Pathologic evaluation of histology and stage was determined by an expert pathologist in all cases. Demographic variables, pathologic results and survival data were analyzed retrospectively. Statistical analysis of survival was performed using Kaplan-Meier, logistic regression, and Cox proportional analyses. Ap value of 0.05 or less was regarded as statistically significant.
Real-time PCR analyses
To measure the expression of Gli1 and Gli2, RNA was extracted from each specimen and reverse-transcribed to cDNA. Thereafter, real-time PCR was performed using tagman primers/probes to analyze the relative expression of Gli1, Gli2, Shh and Ihh compared to GAPDH expression. Several specimens had matched esophageal tissue surrounding the tumor, and were included in the analysis. Expression levels of these genes were then correlated to pathologic and survival data.
Results
Analyses of patient information
A list of patient demographics is shown in Table 1. Eighty-six percent of patients (24/28) were male. Median age was 67 (43-83) yrs. No patient underwent neoadjuvant chemoradiation treatment. Patients who had a T stage of 3 or greater or nodal metastases received adjuvant chemoradiation treatment. In total, fifty-seven percent of patients (16/28) received adjuvant chemoradiation treatment. Median follow-up was 21.6 months. There were no perioperative mortalities.
N
28
Age (median)
66
Male
86% (24/28)
T1/T2
57% (16/28)
T3/T4
43% (12/28)
Stage I
36% (10/28)
Stage II
25% (7/28)
Stage III
39%(11/28)
Node positive
57% (16/28)
Number of lymph nodes harvested (median)
18
Median follow-up (months)
21.6
Table 1: Patient demographics.
Association of Gli expression and tumor stage
As shown in Figure 1, twelve of 28 patients (43%) had a tumor stage of T3 or T4. Patients who were stage T3 or greater had a trend toward higher expression of Gli1 but the association was not significant (p = 0.14). Expression of Gli2 was not statistically related to stage T3 or higher (p = 0.828).
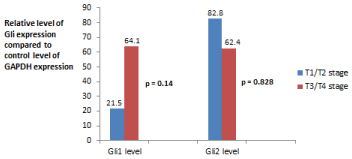
Figure 1: Comparison of mean level of Gli mRNA expression vs. tumor stage.
Gli1 expression mean levels: T1/T2—(μ = 21.5 ± 15.8) vs. T3/T4 (μ = 64.1
± 40.9).
Gli2 expression mean levels: T1/T2—(μ = 82.8 ± 66.2) vs. T3/T4 (μ = 62.4
± 35.8).
Association of Gli expression and nodal status
Sixteen of 28 patients (57%) had lymph node metastases detected during surgery. Levels of Gli expression vs. nodal status is displayed in Figure 2. There was a strong trend toward lymph node positivity and increased levels of expression of Gli1 (p = 0.06). Expression of Gli2 was not statistically related to N status (p = 0.272).
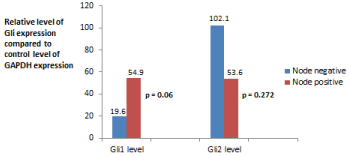
Figure 2: Comparison of mean level of Gli mRNA expression vs. nodal status.
Gli1 expression mean levels: T1/T2—(μ = 19.6 ± 10.6) vs. T3/T4 (μ = 54.9
± 40.2).
Gli2 expression mean levels: T1/T2—(μ = 102.1 ± 56.3) vs. T3/T4 (μ = 53.6
± 40.3).
Number of positive lymph nodes
Increased number of positive lymph nodes was associated with significantly higher expression of Gli1 (p = 0.02, Figure 3). Though there was a strong trend, increased number of positive lymph nodes was not statistically associated with Gli2 expression (p = 0.064).
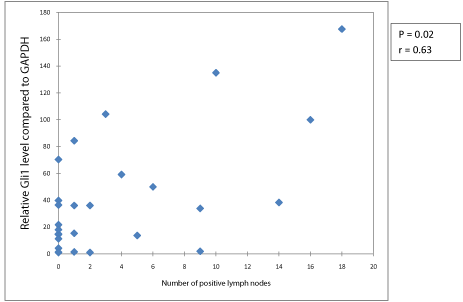
Figure 3: Level of Gli1 expression vs. number of positive nodes.
Survival
Five year survival was 44% overall. Decreased survival was associated with significantly higher expression of Gli1 (p = 0.048). Furthermore, when patients with tumors with a level of expression greater than 50 were compared to patients with tumors with a level of expression less than 50, there was a significant difference in survival (p <0.001, Figure 4). Levels of Gli2 expression were not associated with survival (p = 0.936).
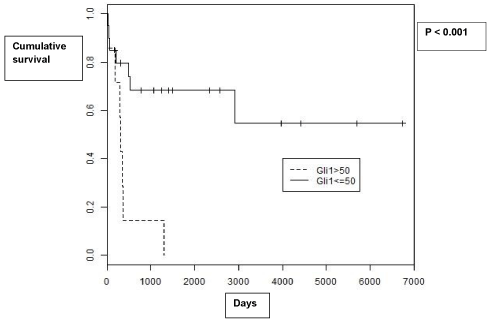
Figure 4: Kaplan-Meier Survival curves for patients with Gli1 expression less
than 50 vs. patients with Gli1 expression greater than 50.
Discussion
Esophageal cancer has an overall poor prognosis, due to the usual advanced stage at presentation and difficulty in staging the tumor accurately. Recent changes in the staging system have addressed the need to predict prognosis better, but five year survival even for Stage I adenocarcinoma of the esophagus is approximately 70-80%. As such, development of a method or test to predict outcome more precisely could affect treatment plans, such as the need for adjuvant treatment with chemotherapy, and possibly improve overall survival.
The hedgehog family regulates normal cell proliferation and differentiation [12]. When overexpressed, however, the hedgehog family has been described in association with multiple cancers, including esophageal cancer [13]. While other reports have focused on the association of the hedgehog family with esophageal squamous cancer, our report differs in that it demonstrates a relationship between hedgehog family overexpression and esophageal adenocarcinoma.
The hedgehog family has been identified as the canonical pathway which upregulates Gli1 and Gli2 [14]. Whereas hedgehog family overexpression has been associated broadly in esophageal cancer, our study shows that a quantitative analysis of the expression of these transcription factors may relate to the severity and aggressiveness of the tumor. Indeed, our study did show that as the level of expression increased, the tumor behaved more aggressively. In our study, tumors had a strong trend toward increased depth of invasion and a significant association toward poorer survival as the level of expression of Gli1 increased. In particular, it appeared that levels of Gli1 expression were predictive of survival, as opposed to levels of expression of Gli2. This stronger association of Gli1 with malignant degeneration has also been seen in other studies [15-16]. We did not find specific association of Shh and Ihh expression with tumor stage, lymph node metastases or overall survival (data not shown).
Currently, tumor depth of invasion and lymph node metastases are the criteria used to determine need for adjuvant chemoradiation treatment and frequency of surveillance imaging postoperatively. These two criteria are fairly crude, however. Development of another variable which can predict outcome in patients with esophageal adenocarcinoma could provide a useful manner to treat high-risk patients more appropriately. If a subset of early stage patients with esophageal adenocarcinoma could be identified to have an increased rate of recurrence based on Gli1 levels, there would be a potential to decrease recurrence rates by administering chemoradiation postoperatively, whereas now they would not receive additional treatment.
Our study evaluated the association between mRNA levels in cancer specimens and outcome without evaluating corresponding protein levels. We encountered difficulty in identifying specific antibodies which recognize endogenous Gli1. In fact, we have tried 4 commercial Gli1 antibodies but none of them are specific to Gli1 because all of these commercial antibodies stained positively in Gli1 knockout MEF cells (data not shown). Another issue is the small size of available biopsies to extract sufficient proteins for Western blotting analysis. Nevertheless, our goal in this study was to find a potential diagnostic test which could predict tumor behavior and clinical outcome. Other published studies have taken this same approach of evaluating mRNA levels vs. clinical behavior in other tumors [17-19]. Further studies evaluating the association of mRNA levels and protein levels of Gli1 (particularly specific antibodies for immunohistochemistry) may certainly be a major focus in the future, and may yield a simple assay for testing Gli1 expression in small specimens.
Our study had some limitations. The clearest limitations are that the sample size in our study was relatively small and collected over a relatively long time period. The overall survival after esophageal cancer surgery has improved significantly in that time and that trend may bias results in such a small cohort. As well, the lymph node dissection may have changed in that time period, potentially biasing the associations with lymph node positivity. The results, however, do indicate that survival and lymph node positivity may be related to hedgehog family expression.
Several opportunities exist to investigate further the association of the hedgehog family with esophageal adenocarcinoma. A larger, possibly multi-institutional, study examining the association of the hedgehog family with esophageal adenocarcinoma should be performed. A multi-institutional study would accrue a larger sample size more quickly and could eliminate any potential time biases which may exist in a study such as ours with a long time period. And a study examining recurrence rates and survival in patients with Stage I esophageal adenocarcinoma is particularly provocative. Levels of Gli1 expression may ultimately be useful in identifying “high-risk” Stage I patients and determining which Stage I patients would experience improved survival with adjuvant treatment.
A diagnosis of esophageal cancer usually carries a poor prognosis, and determining actual outcome can be difficult. The level of expression of targets of the hedgehog family may be useful to determine the aggressiveness of esophageal cancer, and correspondingly may be able to indicate the need for adjuvant treatment. Future studies of increased size will be helpful to decide the exact role of the hedgehog family in esophageal cancer.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64: 9-29.
- Enestvedt BK, Lugo R, Guarner-Argente C, Shah P, Falk GW, Furth E, et al. Location, location, location: does early cancer in Barrett's esophagus have a preference? Gastrointest Endosc. 2013; 78: 462-467.
- Abrams JA, Buono DL, Strauss J, McBride RB, Hershman DL, Neugut AI, et al. Esophagectomy compared with chemoradiation for early stage esophageal cancer in the elderly. Cancer. 2009; 115: 4924-4933.
- Gupta S, Takebe N, Lorusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2010; 2: 237-250.
- Liu H, Gu D, Xie J. Clinical implications of hedgehog signaling pathway inhibitors. Chin J Cancer. 2011; 30: 13-26.
- Wicking C, Smyth I, Bale A. The hedgehog signalling pathway in tumorigenesis and development. Oncogene. 1999; 18: 7844-7851.
- Ruiz i Altaba A. Gli proteins and Hedgehog signaling: development and cancer. Trends Genet. 1999; 15: 418-425.
- Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013; 14: 416-429.
- Shen MC, Ozacar AT, Osgood M, Boeras C, Pink J, Thomas J, et al. Heat-shock-mediated conditional regulation of hedgehog/gli signaling in zebrafish. Dev Dyn. 2013; 242: 539-549.
- Mori Y, Okumura T, Tsunoda S, Sakai Y, Shimada Y. Gli-1 expression is associated with lymph node metastasis and tumor progression in esophageal squamous cell carcinoma. Oncology. 2006; 70: 378-389.
- Zhu W, You Z, Li T, Yu C, Tao G, Hu M, et al. Correlation of hedgehog signal activation with chemoradiotherapy sensitivity and survival in esophageal squamous cell carcinomas. Jpn J Clin Oncol. 2011; 41: 386-393.
- Plaisant M, Giorgetti-Peraldi S, Gabrielson M, Loubat A, Dani C, Peraldi P, et al. Inhibition of hedgehog signaling decreases proliferation and clonogenicity of human mesenchymal stem cells. PLoS One. 2011; 6: e16798.
- Ma X, Sheng T, Zhang Y, Zhang X, He J, Huang S, et al. Hedgehog signaling is activated in subsets of esophageal cancers. Int J Cancer. 2006; 118: 139-148.
- Aberger F, Kern D, Greil R, Hartmann TN. Canonical and noncanonical Hedgehog/GLI signaling in hematological malignancies. Vitam Horm. 2012; 88: 25-54.
- Carpenter RL, Lo HW. Hedgehog pathway and GLI1 isoforms in human cancer. Discov Med. 2012; 13: 105-113.
- Hsieh A, Ellsworth R, Hsieh D. Hedgehog/GLI1 regulates IGF dependent malignant behaviors in glioma stem cells. J Cell Physiol. 2011; 226: 1118-1127.
- Lo WW, Pinnaduwage D, Gokgoz N, Wunder JS, Andrulis IL. Aberrant hedgehog signaling and clinical outcome in osteosarcoma. Sarcoma. 2014; 2014: 261804.
- Mansour H, Homs S, Desvaux D, Badoual C, Dahan K, Matignon M, et al. Intragraft levels of Foxp3 mRNA predict progression in renal transplants with borderline change. J Am Soc Nephrol. 2008; 19: 2277-2281.
- Zhuang Q, Qian X, Cao Y, Fan M, Xu X, He X. Capn4 mRNA level is correlated with tumour progression and clinical outcome in clear cell renal cell carcinoma. J Int Med Res. 2014; 42: 282-291.