
Research Article
Austin J Cancer Clin Res 2015;2(6): 1050.
Determination of Combined Effect of pH and Withania somnifera on Human Breast Cancer Cell Line MDAMB- 231
Khan MA¹, Ahmad R², Trivedi A³ and Srivastava AN²*
¹Era’s Lucknow Medical College & Hospital, India
²Department of Pathology, Era’s Lucknow Medical College & Hospital, India
³Department of Biochemistry, Era’s Lucknow Medical College & Hospital, India
*Corresponding author: Srivastava AN, Department of Pathology, Era’s Lucknow Medical College & Hospital, Sarfarazganj, Hardoi Road, Lucknow-226003, UP, India
Received: April 17, 2015; Accepted: July 25, 2015; Published: August 17, 2015
Abstract
Background: The intratumor microenvironment is intrinsically acidic due to accumulation of lactic acid as a result of increased aerobic and anaerobic glycolysis by the tumor cells. In general, the extracellular pH (pHe) in human tumors is below 7.0, whereas the intracellular pH (pHi) is maintained at neutral range, i.e., >7.0, by powerful pHi control mechanisms. The low pHe and the significant gradients between pHe and pHi affect markedly the response of tumors to various treatments such as chemotherapy, radiotherapy and hyperthermia.
Methods: In the present study, the effect of alkaline pH on human breast cancer cell line MDA-MB231 was investigated in presence of methanolic extract of Ashwagandha (Withania somnifera) in 10% DMSO at the rate of 50 μg/ml.
Results: It was found that alkaline pH caused an increase in the cytotoxic effect of Withania somnifera on MDA-MB-231 cells by approximately ten folds.
No. of Live Cells
1C2
(pH=7.4)
E1
(pH 7.4+ Drug @ 50µg)
% Cytotoxicity
2C2
(pH=8.3)
E2
(pH 8.3+ Drug @ 50µg)
% Cytotoxicity
3C2
(pH=8.6)
E3
(pH 8.6+ Drug @ 50µg)
% Cytotoxicity
1.09x106±22.12
1.48x105±10.30
86.4±8.90
1.44x 106±18.25
4.15x104±12.56
97±15.42
1.31x106±16.32
3.10x104±26.10
97.6±10.25
Table 1: Effect of alkaline pH and W. somnifera dose on MDA-MB-231cells in vitro.
Conclusion: The alkaline pHe caused an increase in the cellular uptake of Withania extract and thus increased its overall cytotoxicity. Better understanding of the control mechanisms of pHe and pHi in tumors may lead to device effective treatment strategy of human tumors.
Keywords: pH; Cancer therapy; in-vitro; MDA-MB-231; Breast cancer; Withania somnifera
Introduction
The extracellular pH (pHe) of malignant solid tumors is acidic, in the range of 6.5 to 6.9, whereas the pHe of normal tissues is significantly more alkaline, 7.2 to 7.5 [1-3]. Mathematical models of the tumorhost interface [4] and in vivo measurements have shown that solid tumors export acid into the surrounding parenchyma [5,6]. Previous in vitro studies have shown that tumor cell invasion can be stimulated by acidic conditions and that this may involve lysosomal proteases [7–9]. These observations have led to the “acid-mediated invasion hypothesis,” wherein tumor-derived acid facilitates tumor invasion by promoting normal cell death and extracellular matrix degradation of the parenchyma surrounding growing tumors. Furthermore, pretreatment of tumor cells with acid before injection leads to increased experimental metastases [10,11] and these observations suggest that low pH up-regulates pro-invasive and survival pathways. It has been argued that metastatic cancers are selected for their ability to export acid [12]. Acid is a by-product of glucose metabolism and notably, elevated consumption of fluorodeoxyglucose by more aggressive cancers have been observed with fluorodeoxyglucose positron emission tomography [13]. It has been reported previously by our group [14] that alkaline pH of medium inhibits the growth of cancer cells in vitro. The present study attempts to investigate whether alkalinity increment of the medium can improve the effect of cytotoxic drugs (W. somnifera) on cancer cells in vitro. Cytotoxic effect of methanolic extract of stem of W. somnifera, a medicinal plant commonly used in herbal pharmaceuticals and nutraceuticals, was evaluated in presence of varying alkaline pH using cytometry and cytotoxic assays against MDA-MB-231 cell line.
Materials and Methods
Collection of plant material
Fresh stems of Withania somnifera Dunal were collected and dried under the shade and then blended into fine powder with a grinder.
Sample preparation
In separate experiments, 25g of powder was extracted with 50% methanol (1:8). After 24 hrs, the upper layer of solvent was collected in a beaker and the procedure was repeated thrice in the interval of 24 hrs continuously till the color of solvent disappeared. All extracts from a single source were pooled together and filtered using Whatman No.1 filter paper (125 mm). The filtered extracts were concentrated at 100oC in water bath. The semi solid paste formed was transferred to a Petri plate and kept in hot air oven till it attained a powdered form. The total weight of powder was measured and stored in air tight container for further use. For biological studies, 20 mg of methanolic extract was dissolved in 10% DMSO (Calbiochem) at a concentration of 20 mg/ml. The extracts were passed through 0.22 μm sterile Millipore syringe filter units (Fisher Scientific) prior to being used in cell culture studies.
Biological evaluation
Cell line
MDA-MB-231 (human breast carcinoma, ER-, tumorigenic and invasive) was obtained from the National Centre for Cell Science (NCCS), Pune, India, and as such, was maintained by sub-culturing and passaging as monolayers in 25 and 75 cm2 cell culture flasks (Nest, Tarsons) at 37oC in Tissue and Cell Culture Lab, Era’s Medical College, Lucknow, in an incubator gassed with an atmosphere of 5% CO2 at 95% humidity, in advanced Dulbecco’s Minimum Essential Medium (DMEM) containing phenol red as a pH indicator and supplemented with 5% FBS. The medium, prior to be used in cell culture experiments was vacuum filtered using a Corning filtration system. The medium requires an atmosphere of 5% CO2 to produce HCO3 buffering capacity to maintain pH at 7.4 for normal cell growth.
Cell Culture
For experiments, cells were trypsinized and cultured in 6-flatbottomed well plates (Linbro, MP Biomedical) at a density of 0.5 x 105 cells/well initially for 24 h, so as to allow the cells to attach. After 24h, two sets of controls were taken. One set (1C1, 2C1, 3C1) each contained 50,000 human breast cancer cells/ml maintained in medium having pH 7.4, 8.3 and 8.6 respectively. Second set (1C2, 2C2, 3C2) each contained 0.5 x 105 breast cancer cells/well maintained in medium having pH 7.4, 8.3 and 8.6 respectively and each containing drug vehicle (10% DMSO) only. The experimental set (E1, E2 and E3) each contained 50,000 human breast cancer cells/ well maintained in medium having pH 7.4, 8.3 and 8.6 respectively and each containing methanolic extract of Ashwagandha (Withania somnifera) in 10% DMSO at the rate of 50 μg/ml. The plates were kept in 5% CO2 (incubator) and observed at an interval of 24, 48 and 72 and 96 hours. After 96 hrs, cells from each well were trypsinized, passaged and counted in a cytometer to determine cytotoxicity and in-vitro anticancer activity. Results were interpreted as cell viability versus time period graph.
Morphological Study
For morphological analysis, cells in 6-well plate were observed under phase contrast microscope & photographed (Nikon Eclipse Ti, Japan).
Cytotoxicity assays
Trypan blue dye exclusion assay
A cell suspension was made at a suitable dilution (1.0 x105 cells/ ml) in PBS. 50 μl of cell suspension was taken and mixed with an equal volume of 0.4% trypan blue. The solution was mixed thoroughly and allowed to stand for 5 min at room temperature. 50 μl of the solution was transferred to a hemocytometer and viable cells were counted as clear cells and dead cells as blue ones. The number of live cells per ml was calculated using the following formula: % viability = (live cell count/total cell count)*100.
(Methyl tetrazolium-MTT assay)
Determination of optimal cell number for assay: In order to determine optimal cell number required for the assay, serial dilutions of MDA-MB-231 (2,000, 4,000, 6,000, 8,000, 12,000, 14,000, 16,000 and 18,000 cells/100 l) were made in cell culture media and seeded in 96 well microtiter tissue culture plates (Linbro, MP Biomedicals). Cells were cultured, in a humidified 5% CO2 incubator at 37oC for 24 h. At the end of the incubation period, 20 μl of MTT solution (Stock concentration, 5.0 mg/ml in PBS) was added to each well and incubated for 4 hours under the same conditions. Thereafter, medium containing MTT was gently replaced by 200 μl DMSO to dissolve formazan crystals and the absorbance values were read in an ELISA plate reader at (Biorad PW41) at 550 nm with a reference wavelength of 630 nm. A graph was plotted with the number of cells in X-axis and absorbance at 570/630 nm in Y-axis. Optimal cell density of cell line corresponding to absorbance values of 0.9 to 1.0 in the assay was selected for MDA MB 231 to facilitate measurement of both stimulation and inhibition of cell proliferation within the linear range.
Evaluation of cytotoxicity and cell viability: Briefly, MDAMB- 231 cells were trypsinized and resuspended in the culture medium to get a defined cell number for MDA MB 231 (16,000/100 l) in a 96-well microtiter tissue culture plate and cultured in a humidified 5% CO2 incubator at 37°C for 24 hours. Defined concentration of methanolic extracts of Withania somnifera in 10% DMSO was freshly prepared culture media by serial dilution to get a final concentration of 50 μg/ml. Serial dilution was carried out in cell culture media in such a way that the final concentration of DMSO in the well did not exceed 0.5% (v/v). Three control wells containing medium alone to serve as blanks were also included. After 24 hours of incubation, cells were treated with the above-mentioned concentration of Withania somnifera extracts in triplicates for 96 hours. Tamoxifen, an anticancer drug was used as a positive control. Equal volume of DMSO was used as a vehicle control. At the end of treatment, 20 μl of MTT (stock made in PSS at 5.0 mg/ml) reagent was added to each well and incubated for further 4 hours. Thereafter, the culture medium was removed and formazan crystals were dissolved in 200μl of DMSO. The plates were read in a 96 well microplate reader (Biotek-ELx-800) at a wavelength of 570 nm with a reference wavelength of 630nm. Percentage cell viability (Y-axis) was calculated from absorbance and plotted against concentration in μg/ml (X-axis). % Cell survival was calculated as = {(AT-AB)/ (AC-AB)} x100 where,
AT= Absorbance of treatment well
AB= Absorbance of blank
AC=Absorbance of control well
% cell inhibition= 100-Cell Survival
Cytometer based analysis
For Cell Viability Analysis, the cells from treated and control wells were trypsinized, resuspended in culture medium. Propidium iodide (20 g/ml) was then added and incubated at RT for 5 min and read in a Tali Image-Based Cytometer, Life Technologies (Invitrogen). The number of live and dead cells in treated and control wells were determined. For comparison purpose, the cytotoxicity of the standard anti-breast cancer drug Tamoxifen (positive control) was also evaluated under the same experimental conditions. The number of live cells in both treated and control wells was used for calculating the percentage cytotoxicity as % Cytotoxicity = Live Cell No. in Treated Wells- Live Cell No. in Control Wells/Live Cell No. in Control Wells x 100).
Statistical analysis
Results were expressed as mean ± SD of experiments done in triplicates.
Results and Discussion
As is visible from Figures 1-4, pH 8.3 and pH 8.6 alone were responsible for considerable cytotoxicity, proving that alkaline pH has an inhibitory effect on cancer cells. However, the presence of methanolic extract of Ashwagandha (W. somnifera), augmented the cytotoxic effect caused due to alkaline pH approximately ten folds (Table 1).
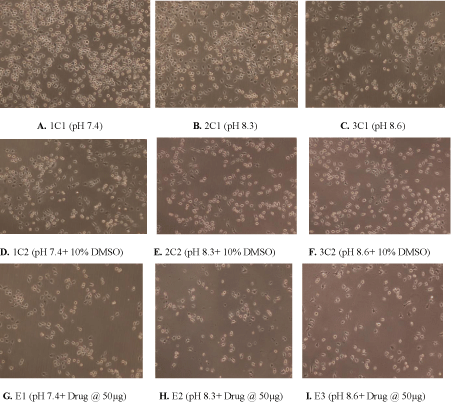
Figure 1: (A) Control showing untreated MDA human breast cancer cells maintained at pH 7.4, (B) pH 8.3 (C) pH 8.6 (D) Control showing untreated MDA human
breast cancer cells in presence of 10% DMSO at pH 7.4 (E) 10% DMSO at pH 8.3 (F) 10% DMSO at pH8.6 (G) Cytotoxic activity of W. somnifera methanolic extract
at 50 μg/ml at pH 7.4 (H) pH 8.3 (I) pH 8.6 after 24 h (Magnification 10X).
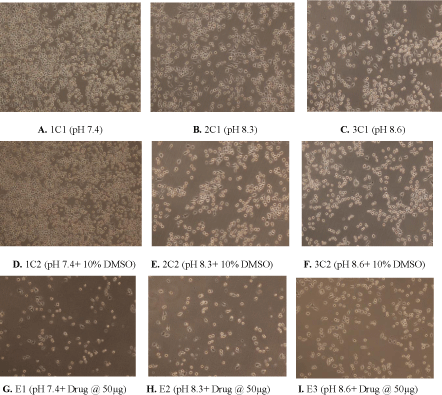
Figure 2: (A) Control showing untreated MDA human breast cancer cells maintained at pH 7.4, (B) pH 8.3 (C) pH 8.6 (D) Control showing untreated MDA human
breast cancer cells in presence of 10% DMSO at pH 7.4 (E) 10% DMSO at pH 8.3 (F) 10% DMSO at pH8.6 (G) Cytotoxic activity of W. somnifera methanolic extract
at 50 μg/ml at pH 7.4 (H) pH 8.3 (I) pH 8.6 after 48 h (Magnification 10X).
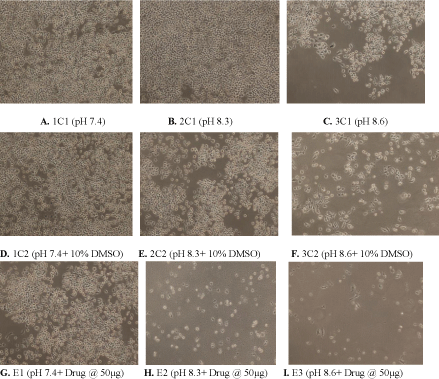
Figure 3: (A) Control showing untreated MDA human breast cancer cells maintained at pH 7.4, (B) pH 8.3 (C) pH 8.6 (D) Control showing untreated MDA human
breast cancer cells in presence of 10% DMSO at pH 7.4 (E) 10% DMSO at pH 8.3 (F) 10% DMSO at pH8.6 (G) Cytotoxic activity of W. somnifera methanolic extract
at 50 μg/ml at pH 7.4 (H) pH 8.3 (I) pH 8.6 after 72 h (Magnification 10X).
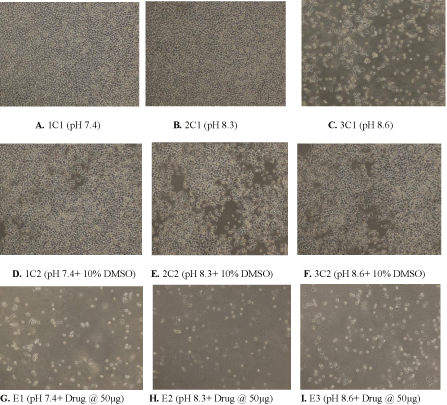
Figure 4: (A) Control showing untreated MDA human breast cancer cells maintained at pH 7.4, (B) pH 8.3 (C) pH 8.6 (D) Control showing untreated MDA human
breast cancer cells in presence of 10% DMSO at pH 7.4 (E) 10% DMSO at pH 8.3 (F) 10% DMSO at pH8.6 (G) Cytotoxic activity of W. somnifera methanolic extract
at 50 μg/ml at pH 7.4 (H) pH 8.3 (I) pH 8.6 after 96 h (Magnification 10X).
The external pH of solid tumors is acidic as a consequence of increased metabolism of glucose and poor perfusion. Acid pH has been shown to stimulate tumor cell invasion and metastasis in vitro and in vivo [15]. The present study investigates whether inhibition of this tumor acidity (by increasing pH) could reduce cancer cell division in vitro. It is clear from the above study that alkaline pH has an inhibitory effect on human breast cancer cells. In future, the mechanism of drug uptake by cancer cells in presence of alkaline pH as well as the effect of ethanolic extract of W. somnifera on cancer cells in presence of alkaline pH would be studied. Medically, oral administration of buffers such as bicarbonate (that would increase the physiological pH) prior to drug administration can be an attractive idea for the treatment of cancer in future.
Conclusion
Alkaline pH significantly augments/increases the cytotoxic effect of methanolic extract of W. somnifera, probably by affecting its uptake by cancer cells.
Acknowledgement
The authors are grateful to Dr. Anil K. Balapure, Sr. Principal Scientist & Deputy Director, Tissue & Cell Culture Unit (TCCU), Division of Biochemistry, CSIR-Central Drug Research Institute, Lucknow, India, for his assistance, support and co-operation in this study.
References
- Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991; 64: 425-427.
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989; 49: 6449-6465.
- Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984; 2: 343-366.
- Gatenby RA, Gawlinski ET. Mathematical models of tumour invasion mediated by transformation-induced alteration of microenvironmental pH. Novartis Found Symp. 2001; 240: 85-96.
- Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006; 66: 5216-5223.
- Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. J Magn Reson Imaging. 2002; 16: 430-450.
- Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia. 2003; 5: 533-545.
- Martínez-Zaguilán R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996; 14: 176-186.
- Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994; 54: 6517-6525.
- Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006; 66: 6699-6707.
- Schlappack OK, Zimmermann A, Hill RP. Glucose starvation and acidosis: effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. Br J Cancer. 1991; 64: 663-670.
- Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008; 8: 56-61.
- Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008; 49 Suppl 2: 24S-42S.
- Khan MAK, Misra A, Trivedi A, Srivastava AN. Effect of alkalinity on cancerous cells at different pH and morphological variations in-vitro. Int J Bioassays. 2014; 3: 3297-3302.
- Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009; 69: 2260-2268.