
Research Article
Austin J Cancer Clin Res 2015;2(7): 1055.
Establishment and Characterization of Six Primary Pancreatic Cancer Cell Lines
Rückert F¹*#, Werner K¹#, Aust D², Hering S³, Saeger H-D¹, Grützmann R¹ and Pilarsky C¹
¹Department of Visceral, Thoracic and Vascular Surgery, Dresden University of Technology, Germany
²Institute of Pathology, Dresden University of Technology, Germany
³Institute of Legal Medicine, Dresden University of Technology, Germany #Both Authors Contributed Equally
*Corresponding author: Felix Rückert, Department of Visceral, Thoracic and Vascular Surgery, University Hospital Carl Gustav Carus, Dresden University of Technology, Fetscherstr, 74, 01307 Dresden, Germany
Received: May 18, 2015; Accepted: August 08, 2015; Published: August 11, 2015
Abstract
Background: Pancreatic ductal adenocarcinoma is an aggressive tumor; treatment remains a challenge because of the lack of effective therapeutic strategies. Basic research in this field is dependent on the availability of model systems. New pancreatic cancer cell lines are therefore important for the study of its biology. In the present study, we report the establishment and characterization of six new pancreatic cancer cell lines (PaCaDD-141, -159, -161, -165, -183, -188).
Material and Methods: All cell lines were derived from pancreatic ductal adenocarcinomas by the Dresden outgrowth protocol. The six cell lines originated from different sample locations. We characterized the cell lines by examining their morphology and their cytostructural and functional profiles.
Results: All cell lines were cultured in optimized Dresden-Medium. The doubling time ranged from 20 to 43 hours. KRAS mutations were detected in four of the six cell lines. Immunohistochemical staining showed cytoplasmic expression of CK8/18, mostly membrane and partially cytoplasmic expression of E-cadherin and strong expression of ezrin in all cell lines. Three cell lines showed nuclear p53 accumulation and heterogeneous expression of vimentin. SMAD4 was heterogeneously expressed in the cell lines.
Conclusions: We were able to establish six new primary pancreatic carcinoma cell lines. As applicable tools for basic research, these cell lines might contribute to a better understanding and treatment of this aggressive tumor.
Keywords: Cell lines; Pancreatic cancer; Outgrowth method
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is by far the most common type of tumor in the exocrine pancreas [1]. Biological models are needed for the study of pancreatic cancer, as better knowledge about the pathophysiology, molecular biology, and functional characteristics might help to better treat this neoplasm. Without cell lines of human pancreatic cancer, investigation of the biological and especially the functional properties of this tumor is not possible. As well, there can be no statement about possible therapeutic responses of this tumor towards new therapeutic agents without new cell lines. In recent years, we and others have reported the isolation of different human pancreatic cancer cell lines [2]. To obtain a greater phenotypic heterogeneity of the disposable cell lines, and to circumvent the use of “old” cell lines, it is recommended for research labs that focus on pancreatic cancer to establish their own primary carcinoma cell lines. Therefore, we have attempted to establish additional carcinoma cell lines from surgical specimens of pancreatic adenocarcinomas.
In the present study we report the successful isolation of six new primary human pancreatic carcinoma cell lines, designated as PaCaDD-141, PaCaDD-159, PaCaDD-161, PaCaDD-165, PaCaDD-183 and PaCaDD-188, and will describe the phenotypes of the cell lines, including the histopathology and the in vitro growth characteristics.
Material and Methods
Culture procedure and patient cohort
Primary tumor tissues were taken from primary pancreatic tumors, malignant ascites/pleural effusions, metastatic liver tumors, or metastases to lymph nodes, all of which were obtained surgically from patients with ductal adenocarcinomas of the pancreas. All patients were treated at the Department of Visceral, Thoracic and Vascular Surgery, University Hospital Dresden, Germany and gave informed consent prior to operation. After the surrounding connective tissues and hemorrhagic regions were removed, the tumor tissues were minced finely using scalpels into cubes of approximately 1 mm3. Neither enzymatic nor mechanical dissociation of the tumor cells was performed. For a detailed report of our technique see Rückert et al., 2011 [3]. For the primary culture, we used Dresden-medium, consisting of CP medium and KSF medium at a ratio of 2:1 as described previously [3]. All media were supplemented with penicillin (100 U/ mL) and gentamicin (2.5 mg/mL) (Invitrogen, Karlsruhe, Germany). The cells were maintained at 37oC in a humidified atmosphere of 5% CO2 in air, and the medium was replaced every 3 days. All cell lines showed an absence of mycoplasma. Cell lines were named Pancreatic Cancer Dresden (PaCaDD). All experiments for this study were performed on cell lines between the 4th and 9th passage.
Morphology and infiltration assay
Growth patterns and cell morphologies were determined in vitro using a Zeiss phase-contrast microscope.
Cell doubling time
Cell doubling time was determined by counting the number of viable cells derived from freshly trypsinized monolayers in duplicate. Seven 6-well plates (9.6 cm²/well) with 5 x 105 cells plated per well were used. Cells were counted at 24 h intervals for 7 days. The culture medium was changed every 3 days. The doubling time of the cell population was calculated from the logarithmic growth curve by the following formula:
υ= lgN - lgN0/ lg2 (t- t0), with doubling time= 1/ υ.
STR-Assay
Cell line purity and clonality were verified by microsatellite analyses using the commercially available multiplex PCR kits Mentype® NonaplexQS (Biotype AG, Germany) and genRES® MPX-2 (Serac, Germany). Amplicons were detected by capillary electrophoresis in the denaturing polymer POP4 in an ABI 310 sequencer (Perkin-Elmer, USA) according to the manufacturer’s instructions.
Immunohistochemistry
For immunohistochemical analysis, cells from each cell line were centrifuged into a cell pellet, embedded in paraffin and then cut for immunohistochemical staining. Additionally, a representative slide of the corresponding primary tumors was used for comparison. All cell lines and sections were examined by one observer (D.A.) blinded to both clinical and pathological data. Table 1 shows the details of the antibodies used. Immunohistochemical staining for CK8/18, vimentin, ezrin, E-cadherin and p53 was done using a Lab-Vision 480- 2D immunostainer (Thermo Fisher, Fremont, CA, USA); staining for SMAD4 was done by hand. All reactions were visualized with DAB as a chromogen. Positive and negative controls were included in each run for all the antibodies used. Isotype controls for all antibodies were negative.
Antibody
Dilution
Pretreatment
Antibody Supplier
Vimentin (clone VIM3B4)
1:1.500
Heat in citrate buffer (pH6.0) for 45 minutes
Progen Biotechnik GmbH, Heidelberg, Germany
p53 (clone DO1)
1:2.000
Heat in citrate buffer (pH6.0) for 45 minutes
Calbiochem, Merck Chemicals Ltd., Nottingham, UK
Ezrin (clone 3C12)
1:10.000
Heat in citrate buffer (pH6.0) for 45 minutes
Sigma, St. Louis, Missouri, USA
E-cadherin (clone NCH-38)
1:25
Heat in citrate buffer (pH6.0) for 45 minutes
Thermo Scientific, Fremont, CA, USA
Cytokeratin 8/18
1:50
Pronase for 15 minutes
Novocastra Laboratories Ltd. Newcastle upon Tyne, UK
SMAD4
1:100
Heat in citrate buffer (pH6.0) for 45 minutes
Santa Cruz Biotechnology Inc., Heidelberg, Germany
Table 1: Antibody description.
KRAS-2 Mutation Analysis
Genomic DNA was isolated from the cell lines using the QIAamp DNA Micro kit (Qiagen, Hildesheim, Germany) as recommended by the manufacturer. The DNA was then checked for quality and concentration. Mutational analysis of codons 12 and 13 in exon 2 of the KRAS-gene was done using the Pyromark® KRAS Kit according to the manufacturer’s protocol. Wild type and mutant controls were included in each pyrosequencing run. The pyrograms were analyzed using Pyromark® analysis software.
Statistical analysis
For statistical analysis, we used Excel 2003 (Microsoft) and SPSS 13.0 for Windows.
Results
Clinical summary of patients
All specimens were acquired from patients treated in our department (Table 2). PaCaDD-159 and -188 were obtained from resectable primary tumors. PaCaDD-165 and -183 were obtained from the malignant ascites in patients with advanced disease. PaCaDD-141 was obtained from a lymph node metastasis in a patient who intraoperatively showed irresectable disease. The same holds true for PaCaDD-161 that was isolated from a liver metastasis. Histopathologically, all tumors were classified as adenocarcinomas of pancreatic ductal origin. To ensure the correct identity of the cell lines used for the experimental procedures, a STR-assay was performed using first passage cells. Each cell line used for the present study was compared with this first passage sample to double-check its origin (S1).
Cell line
Patient
Age (y)
Histology
Site
Classification
Grading
Sample location
PaCaDD-141
Female, Caucasian
82
Dedifferentiated pancreatic Adenocarcinoma
-
Advanced disease
G4
Lymph node
PaCaDD159
Male, Caucasian
78
Ductal Adenocarcinoma
Tail
pT3N1M0
G2
Primary
PaCaDD-161
Female, Caucasian
63
Ductal Adenocarcinoma
Head
Advanced disease
G3
Liver MET
PaCaDD-165
Male, Caucasian
54
Ductal Adenocarcinoma
-
Advanced disease
G3
Ascites
PaCaDD-183
Female, Caucasian
49
Ductal Adenocarcinoma
Head
Advanced disease
G2
Ascites
PaCaDD-188
Female, Caucasian
68
Ductal Adenocarcinoma*
Head
pT3N1M0
G3
Primary
(* Pancreatic cancer from which PaCaDD-188 was obtained developed from a PaNiN lesion.)
Table 2: Clinicopathological characteristics of the six patients with pancreatic carcinoma from whom cell lines were established.
Morphology and growth characteristics in vitro
PaCaDD-141 cells grow in epithelial monolayers with disorganized patterns. Slightly elongated epithelial cells tend to form swirls around smooth, well-demarcated boundaries of large polygonal cells. Clusters of very small, proliferating cells can be observed. PaCaDD-141 cells occasionally contained multiple prominent nuclei (Figure 1A). The doubling time was 33.7 hours. After four passages, most of the cultures contained predominately large cells. These cultures then became static and could not be passaged further. In subsequent studies from frozen stocks of early passage PaCaDD-141 cells, we have consistently observed this effect
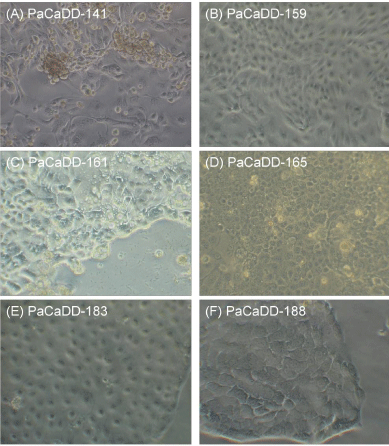
Figure 1: Phase contrast microscopy of the cell lines PaCaDD-141 (A),
PaCaDD-159 (B), PaCaDD-161 (C), PaCaDD-165 (D), PaCaDD-183 (E) and
PaCaDD-188 (F) (40x).
PaCaDD-159 grows in an epithelial monolayer with an organized pattern. Cells are ovoid or round and are characterized by the presence of a relatively small cell nucleus (Figure 1B). The doubling time of PaCaDD-159 cells is 41.3 hours. PaCaDD-159 cells were cultured up to passage 14 and showed no signs of senescence then.
PaCaDD-161 cells grow in an epithelial monolayer with an disorganized pattern. Small and larger cells are both present in the culture. Cells have small nuclei and some cells show production of mucus (Figure 1C). The doubling time of PaCaDD-161 cells is 20.6 hours. PaCaDD-161 cells were cultured up to passage 15 and showed no signs of senescence then.
PaCaDD-165 cells grow in an epithelial monolayer as homogenous, small polygonal cells with a cobblestone pattern. Cells have a prominent nucleus (Figure 1D). The doubling time of PaCaDD-165 cells is 26.2 hours. PaCaDD-165 cells seem to continue to divide indefinitely.
PaCaDD-183 exhibit epithelial-like morphological characteristics and grow in a homogenous cobblestone pattern. Cells have a homogenous size and are mostly rounded. Cells have a medium-sized nucleus (Figure 1E). The doubling time of PaCaDD-183 cells is 43.3 hours. PaCaDD-183 cells were cultured up to passage 15 and showed no signs of senescence then.
PaCaDD-188 also grows in an epithelial monolayer. They show characteristic homogenous, round or polygonal cells. Cells are medium sized with an prominent nucleus (Figure 1E). The doubling time of PaCaDD-188 cells is 30.8 hours. PaCaDD-188 cells were cultured in their 15th passage at submission of the manuscript and showed no signs of senescence then. For a graphical display of the doubling times, see Figure 2.
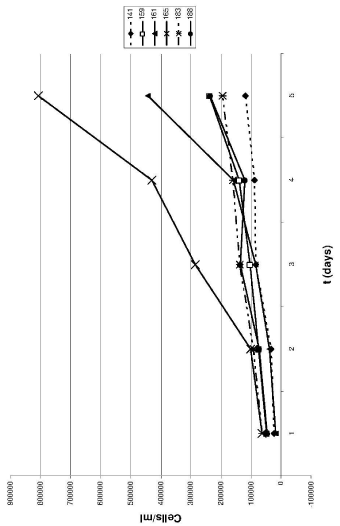
Figure 2: The growth curves of the six PDAC cell lines. Each point represents
the mean of triplicates. As illustrated, PaCaDD-165 and -161 cells had the
shortest doubling times and are characterized by faster growth as compared
with the remaining cell lines.
Immunocytochemical profile of the cell lines
CK 8/18 and Ezrin were strongly expressed in all of the cell lines. Vimentin expression was observed (to varying extents) in five of the cell lines and negative in the PaCaDD-183 cell line. Nuclear p53 accumulation was seen in PaCaDD-141, PaCaDD-188 and partially in PaCaDD-159 cells. E-Cadherin expression was strong, except in one cell line (PaCaDD-141), that showed a weak expression. SMAD4 expression was generally very low; PaCaDD- 159 and -188 cells did not show any expression. However, PaCaDD- 183 had a strong staining for SMAD4. For semiquantitative evaluation of protein expression see Table 3 and Figure 3.
CK 8/18
CDH
Ezrin
p53
Vim
SMAD4
KRAS
PaCaDD-141
++
+
++
++
++
+
WT / V
PaCaDD-159
++
++
++
+
+
-
G12V
PaCaDD-161
++
++
++
-
+
+
G12V
PaCaDD-165
++
++
++
-
+
+
WT
PaCaDD-183
++
++
++
-
-
++
G12D
PaCaDD-188
++
++
++
++
+
-
G12D
Table 3: Cytogenetic characteristics of the various cell lines and their parent tumors (WT= wild type; V=Valin; CDH= E-cadherin; Vim= Vimentin). “-“ indicates no expression, “+” indicates partial/low expression, “++” indicates strong expression.
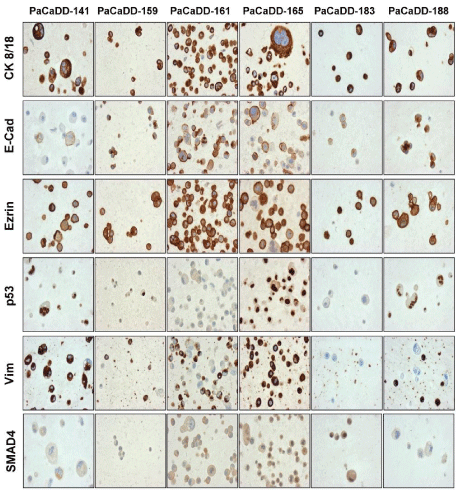
Figure 3: Immunohistochemistry of the PaCaDD cell lines. For
semiquantitative evaluation of protein expression see Table 3 (20x).
KRAS mutational status
\KRAS mutational analysis determined that two cell lines (PaCaDD-141 and -165) were wild-type. However, all of the other cell lines had activating KRAS-mutations (Table 3).
Discussion
The six primary pancreatic cancer cell lines (PaCaDD-141, -159, -161, -165,- 183, - 188) described in the present study were established in explant technique directly from primary biopsy material as described previously [3]. Although the establishment of primary pancreatic cancer cell lines still has an experimental character, we could show that it can be performed relatively easily and economically. From 59 surgical samples, we could establish six primary cell lines. This indicates a success rate of 10.16%. By optimizing the method, it could be a good clinical platform to analyze chemosensitivity of the cancer cells. A response towards chemotherapy is seen infrequently in pancreatic cancer, and the analysis of primary cell lines could help to identify those patients [4]. Another possible clinical use could be to analyze different characteristics of the cell lines, e.g. aggressive growth, cell doubling time or expression of E-cadherins, to identify patients with a high risk of recurrence. Those patients could be screened closely postoperatively. Apart from this, the information on the aggressiveness of the tumor is very important for patients in a social aspect. Least, primary cell lines were often used in animal models to produce and identify tumor markers. Up to date, the only tumor marker that is routinely used is CA19-9 [5]. Possibly a higher number of primary pancreatic cancer cell lines could help to identify newer or better tumor markers in the future. The proof of origin of our cell lines was realized by STR-assay. Immunohistochemistry was used to analyze the expression of typical pancreatic cancer tumor markers (Figure 3). All cell lines had expression of the tumor marker proteins CK 8/18, E-cadherin and ezrin. This is in accordance with previous reports on pancreatic cancer cell lines [2]. p53, SMAD4 and vimentin were expressed heterogeneously within the cell lines. P53 was expressed in three of our cell lines. In endogenous pancreatic cancer, immunohistochemical analysis showed that p53 is mutated in 50-75% of cases, whereas it cannot be detected in samples of chronic pancreatitis [6]. The percentage of cell lines expressing p53 in our study is therefore in accordance with previous reports. We did not find obvious correlation of the expression of p53 with clinicopathological parameters like TNM-stage or survival. Vimentin is an intermediate filament. It is generally absent from normal epithelial cells, but its expression may be upregulated during neoplastic transformation and in vitro culture [7]. Its expression was strongly detected in one cell lines and faintly in four of the cell lines. One cell line did not show expression of Vimentin. Again, this corresponds to the numbers reported for native tumor tissue [7]. SMAD4 or DPC4 is a member of the SMAD family of intracellular proteins that mediates the activation of the TGF-β receptor [8]. SMAD4 function is lost in 50% of PDAC, and its expression is decreased in undifferentiated carcinomas compared with well-differentiated carcinomas [9]. We saw a strong expression of SMAD4 in only one cell line. Three cell lines did display a low expression, and two cell lines did not express SMAD4. Expression of SMAD4 therefore correlates with previous reports [9,10]. Again, we could not correlate the expression of SMAD4 with any clinico-pathological parameters. However, one of the tumor markers seemed to correlate to clinical characteristics of the native tumors: E-Cadherin. E-Cadherin is a marker for epithelial to mesenchymal transition and its loss indicates a highly aggressive growth [11]. The one cell line with low expression of E-cadherin, namely PaCaDD-141, was obtained from an undifferentiated pancreatic adenocarcinoma that clinically showed a very aggressive growth. Additional to immunohistochemistry we undertook mutational analysis of KRAS. It is known that over 90% of pancreatic cancers contain mutated KRAS genes [12]. We found mutations in codon 12 of this gene in four of the six cell lines that we generated. This means, that only 66.6% of the cell lines showed a KRAS mutation, and this mutation is therefore underrepresented in our panel of tumor cell lines. This might be a statistical problem, due to the small number of samples.
In conclusion, we showed that the isolated primary pancreatic cancer cell lines have typical immunohistological and molecular properties. We trust that our pancreatic carcinoma cell lines serve as useful tools for the investigation of the biological characteristics of pancreatic cancer.
Acknowledgment
We welcome requests for our cell lines; please contact the authors. Aliquots of the cell lines PaCaDD-159, -161, -165, - 183 and -188 can further be ordered from the DSMZ (“Deutsche Sammlung von Mikroorganismen und Zelllinien”). We would like to thank Beatrix Jahnke, Heike Berthold and Silke Zeugner for technical support. This study was supported by the MeDDrive38 program of the medical faculty of Technische Universität Dresden.
References
- Klöppel G, Lüttges J. The pathology of ductal-type pancreatic carcinomas and pancreatic intraepithelial neoplasia: insights for clinicians. Curr Gastroenterol Rep. 2004; 6: 111-118.
- Rückert F, Pilarsky C, Grützmann R, editors. Establishment of primary cell lines in pancreatic cancer: In Tech. 2011.
- Rückert F, Aust D, Böhme I, Werner K, Brandt A, Diamandis EP, et al. Five primary human pancreatic adenocarcinoma cell lines established by the outgrowth method. J Surg Res. 2012; 172: 29-39.
- Rückert F, Pilarsky C, Saeger H, Grützmann R. Apoptotic Signaling in Pancreatic Cancer – Therapeutic Application. Current Cancer Therapy Reviews. 2009; 5: 122-133.
- Rückert F, Pilarsky C, Grützmann R. Serum tumor markers in pancreatic cancer-recent discoveries. Cancers (Basel). 2010; 2: 1107-1124.
- Talar-Wojnarowska R, Malecka-Panas E. Molecular pathogenesis of pancreatic adenocarcinoma: potential clinical implications. Med Sci Monit. 2006; 12: RA186-193.
- Schüssler MH, Skoudy A, Ramaekers F, Real FX. Intermediate filaments as differentiation markers of normal pancreas and pancreas cancer. Am J Pathol. 1992; 140: 559-568.
- Friess H, Guo XZ, Nan BC, Kleeff J, Büchler MW. Growth factors and cytokines in pancreatic carcinogenesis. Ann N Y Acad Sci. 1999; 880: 110-121.
- Magee CJ, Greenhalf W, Howes N, Ghaneh P, Neoptolemos JP. Molecular pathogenesis of pancreatic ductal adenocarcinoma and clinical implications. Surg Oncol. 2001; 10: 1-23.
- Krautz C, Rückert F, Saeger HD, Pilarsky C, Grützmann R. An update on molecular research of pancreatic adenocarcinoma. Anticancer Agents Med Chem. 2011; 11: 411-417.
- Rückert F, Joensson P, Saeger HD, Grützmann R, Pilarsky C. Functional analysis of LOXL2 in pancreatic carcinoma. Int J Colorectal Dis. 2010; 25: 303-311.
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009; 324: 1457-1461.