
Review Article
Austin J Cancer Clin Res 2015;2(7): 1058.
The Roles of Ultrasonography in the Management of Axillary Node Metastases in Breast Cancer
Rosso KJ¹, Ko Un Park¹, Shah R¹, Rubino G² and Nathanson SD¹*
¹Department of Surgery, Henry Ford Hospital and Wayne State University School of Medicine, USA
²Wayne State University School of Medicine, USA
*Corresponding author: S David Nathanson, Department of Surgery, Henry Ford Health System, Wayne State University School of Medicine, 2799 W, Grand Boulevard, Detroit, MI 48202, USA
Received: June 22, 2015; Accepted: August 05, 2015; Published: August 08, 2015
Abstract
In patients with early stage breast cancer, surgical management of the axilla has become less invasive. Multiple randomized control trials have demonstrated that in patients with minimal axillary nodal disease, complete axillary lymph node dissection does not offer a survival benefit when compared to sentinel lymph node biopsy alone. Ultrasonography of the axilla and ultrasound guided biopsy of suspicious lymph nodes has become a highly specific test to identify locoregional disease. Nodal metastasis detected by ultrasound guided lymph node biopsy has allowed patients to be treated as “lymph node positive” or N1, receive neoadjuvant chemotherapy and undergo a subsequent definitive axillary operation. In those patients who achieve a pathologic complete response after neoadjuvant chemotherapy, however, removal of axillary nodes that are free of residual cancer may be of no benefit. Targeted axillary dissection is a novel technique that allows limited, image guided removal of the previously biopsied axillary nodes and the sentinel lymph node during definitive axillary dissection following neoadjuvant chemotherapy. This practice relies on the specificity of ultrasound guided axillary lymph node biopsy to detect disease as well as the placement of markers that label the biopsied node. Contemporary research that utilizes ultrasound to differentiate between patients with minimal axillary nodal metastasis from those with extensive axillary nodal disease will contribute substantially to the less invasive surgical management of the axilla.
Keywords: Axillary node; Breast cancer; AUS
Introduction
The sentinel lymph node (SLN) is the first node (or nodes) to receive tumor cells traveling from the primary tumor to the locoregional nodal basin. The surgical sentinel lymph node biopsy (SLNB) relies on orderly lymphatics from the breast to the axilla and the combined use of blue dye and radiolabeled colloid tracers allows for its highly accurate localization. Since its inception and subsequent application, the SLNB has become the validated gold standard to stage the axilla. Clinically, lymph node status remains one of the most important prognostic factors in breast cancer and guides treatment algorithms.
Axillary ultrasound (AUS) with ultrasound guided lymph node biopsy (either by fine needle aspiration or core needle) can identify most patients with extensive axillary nodal disease burden with specificity approaching 100%. Sonographic characteristics suspicious for metastasis in the lymph node include cortical thickening, changes in shape and size and absence of the fatty hilum but the morphology that defines presence of metastasis or indication for ultrasound guided lymph node biopsy (USLNB) is not standardized.
Prior to the Z0011 trial [1] demonstrating that complete axillary dissection offered no survival benefit in patients with limited axillary disease, AUS was used for preoperative locoregional staging. A positive AUS and USLNB allowed the patient to undergo neoadjuvant chemotherapy and one definitive axillary surgery by avoiding the SLNB altogether.
Following Z0011 and the subsequent randomized trials like AMAROS [2] echoing its results, management of the axilla has become less invasive and the role of AUS and USLNB is being redefined. Some centers abandoned preoperative ultrasonographic axillary staging in the clinically negative axilla, others rely on a negative preoperative AUS with USLNB to determine if the patient is indeed a candidate for surgical management by Z0011 criteria, while other institutions consider a positive AUS and USLNB to be N1 disease and treated as such. The clinical application of AUS and USLNB in the post-Z0011 era is highly variable. The National Comprehensive Cancer Network guidelines do not advocate for routine axillary ultrasonography in patients with clinically negative axilla (cN0) [3]. New promising randomized trials like the SOUND study [4] are attempting to identify the most efficient and beneficial utilization of this imaging modality in the management of the axilla breast cancer. These contemporary studies aim to investigate disease free and overall survival in patients with early breast cancer and negative AUS that are randomized to SLNB or no further axillary staging. This article reviews the abnormal morphology of a suspicious lymph node, sensitivity, specificity, predictive values and common indications for AUS and USLNB, techniques to mark the biopsied node, utilization of AUS USLNB following neoadjuvant chemotherapy, and lastly, some promising studies that will help further define and standardized its use.
Identifying a suspicious lymph node by ultrasonography
Ultrasound examination should include the total axilla extending to the anatomic borders from the level of the fourth rib inferiorly to the costoclavicular ligament and axillary vein superiorly, and from the pectoralis muscles anteriorly to the subscapularis and latissimus dorsi muscles posteriorly [5]. There is a high concordance rate of finding the true SLN when the ultrasound is focused in the same anatomic area as used by surgeons during open SLNB [5]. Most SLNs are found intraoperatively close to the lowest axillary hair follicle [5] and more than 80% of sentinel nodes by ultrasound represent the lowest node in the axilla [6].
Distinctions between normal appearing nodes, solitary sonographically abnormal appearing nodes or multiple abnormal nodes should be documented [7], as should the location of the abnormal node or nodes within the axilla. A normal appearing lymph node is characterized by having an elliptical shape, a thin, even hypoechoic cortex measuring <3mm, and a hyperechoic hilum with blood vessels entering the hilum [5,8]. Common sonographic findings of a suspicious or abnormal lymph node include a thickened, lobulated cortex >3mm, loss or effacement of the fatty hilum, and loss of normal lymph node architecture [5,6,9-20] (Table 1), but these morphological characteristics have yet to be standardized. Such variations in the criteria that define an abnormal node and thus the indications for biopsy affect the sensitivity and specificity of AUS and USLNB [5,7,8,12-15,17-20] (Table 2).
Choy N et al. Annals of surgical oncology 2015 [9]
Generalized or focal thickening of nodal cortex, disparity in size of one or more LNs compared with others, rounded appearance, and effacement of node fatty hilum.
vanWely BJ et al. British Journal of Surgery 2015 [10]
Round, asymmetrical cortex, thicker than 3mm, loss of hyperechoic hilum
Caudle AS et al. JAMA surgery 2015 [11]
Eccentric cortical thickening, hilar compression, displacement, effacement, and irregular nodal margins
Farrell TPJ et al. European radiology 2015. [12]
Cortical thickness >3mm, prominent eccentric lobulation, and a replaced/eccentric hilum
Fung AD et al. Cancer Cytopathology 2014 [13]
Eccentric cortical thickening, diminutive or absent echogenic hilum, or round shape. Areas of the cortex with increased vascularity by color Doppler ultrasound are preferentially targeted for sampling.
Houssami N et al. Cancer biology & medicine 2014 [14]
Thickening of the cortex, cortical thickening may be diffuse or focal; eccentric or irregular; asymmetric; lobulated (uni- or multi-lobulation); absence/loss of central fatty hilum, rounded nodes (ratio of the longitudinal and transverse dimensions).
Cools-Lartigue J et al. Annals of surgical oncology 2013 [15]
Absence of a fatty nodal hilum, eccentric cortical thickening, and a round hypoechoic node, multiple enlarged (>1 cm) nodes
Ibrahim-Zada I et al. Journal of the American College of Surgeons 2013 [16]
Hilar effacement, hilar replacement, node matting, perinodal edema, and unclear node margins and cortical thickening >3 mm, especially if nodular or asymmetric
Valente SA et al. Annals of surgical oncology 2012 [17]
Rounded shape, a long-to-short axis ratio of <2, hypoechoic, compression or disappearance of the fatty hilum, or cortical thickening or asymmetry
Oz A et al. Journal of breast cancer 2012 [18]
Cortical symmetrical and asymmetrical thickening (cortex thickness >3 mm) compared to the lymph nodes on the same or other side, increased size of the lymph nodes, an increase in the sphericity index (short/long diameter =0,5), increased cortex hypoechogenicity (cortex more hypoechoic than the subcutaneous fatty tissue), and non-hilar cortical flow.
Torres Sousa MY et al.Radiología 2011 [19]
Local or diffuse cortical thickness =3mm, absence, obliteration or eccentric location of the hilar fat, and/or non-hilar cortical vascular flow (NHVCF).
Britton P et al. Clinical radiology 2010 [6]
Greater than 5 mm in longitudinal section
Mainiero MB et al. American Journal of Roentgenology 2010 [8]
Benign: even cortex < 3 mm
Indeterminate: even cortex but = 3 mm or < 3 mm but was focally thickened
Suspicious: focally thickened cortex = 3 mm or the fatty hilum was absent.
Nathanson SD et al. Annals of surgical oncology 2007 [5]
Normal lymph nodes: elliptical shape; a thin, hypoechoic cortex; and a hyperechoic hilum with blood vessels entering the hilum.
Abnormal lymph nodes: round shape; uniform or eccentric thickening of the cortex; focal bulging or irregularity of the cortex; displacement or obliteration of the hilum; or total loss of recognizable lymph node architecture.
Krishnamurthy S et al. Cancer 2002 [20]
Increased thickening and/or lobulation of the hypoecholic lymph node cortex compared with other ipsilateral or contralateral lymph nodes, eccentric lobulation of the hypoechoic lymph node cortex with compression of the adjacent hilar fat, and complete disappearance of the hilar fat, which is replaced by hypoechoic cortex.
Table 1: Abnormal lymph node morphology.
Study
Type/Purpose
Sensitivity %
Specificity %
PPV %
NPV %
Farrell TPJ et al. European radiology 2015 [12]
To correlated the number of abnormal LN on AUS with final nodal burden and determine the utility of AUS with sampling in preoperative staging.
AUS alone: 64
AUS with sampling: 86.2
AUS alone: 76.9
AUS with sampling: 100
AUS alone: 63.8
AUS with sampling: 100
AUS alone: 77.1
AUS with sampling: 71.9
Fung AD et al. Cancer Cytopathology 2014 [13]
To characterize the use of rapid onsite evaluation (ROSE) of adequacy by the cytopathologist and/or cytotechnologist during the procedure. A retrospective review of axillary lymph node US guided FNAs.
75
100
100
79
Houssami N et al. Cancer biology & medicine 2014 [14]
A meta-analysis and review to estimate and discuss the utility of UGNB highlighting the role of preoperative UGNB and its consequences on surgical management of the axilla.
79.6
98.3
100
67.4
Cools-Lartigue J et al. Annals of surgical oncology 2013 [15]
A retrospective review to determine the sensitivity, specificity, and accuracy of AUS and FNAB in the identification of axillary nodal metastasis in early breast cancer patients.
All US= 55
Abnormal AUS with FNAB=69%
All US= 88
Abnormal AUS with FNAB= 100
All US= 74
Abnormal AUS with FNAB= 100
All US= 75
Abnormal AUS with FNAB= 54
Valente SA et al. Annals of surgical oncology 2012 [17]
To examine the ability to accurately predict axillary nodal involvement by using physical examination and standard breast imaging studies in combination.
43.5
96.2
79.3
83.3
Oz A et al. Journal of breast cancer 2012 [18]
To detect preoperative axillary metastases with ultrasound (US)-guided fine needle aspiration biopsy (FNAB), eliminate the need for sentinel lymph node (SLN) scintigraphy and biopsy, and in that of with suspicious US findings, and to evaluate the accuracy of preoperative US-guided FNAB for patients with suspicious lymph node metastases on US.
88.46
100
100
66.6
Torres Sousa MY et al. Radiología 2011 [19]
To investigate the sensitivity, NPV and PPV of the axillary biopsy using different morphologic LN parameters
94
NR
100
(in the presence of both cortical thickening and non-hilar cortical vascular flow)
Cortical thickening 68%
Changes in the hilar fat 90%
NHCVF (nonhilar cortical vascular flow) 78%
88.9
Mainiero MB et al. American Journal of Roentgenology 2010 [8]
To evaluate the utility of ultrasound-guided fine-needle aspiration (FNA) of the axillary lymph nodes in breast cancer patients depending on the size of the primary tumor and the appearance of the lymph nodes.
Overall= 59
Primary tumor
T0, T1a-b=29
T1c=50
T2= 69
T3,T4=100
LN appearance
Benign =11
Intermediate=44
Suspicious=93
Overall= 100
NR
NR
Nathanson SD et al. Annals of surgical oncology 2007 [5]
To investigate the use of ultrasound-guided core needle biopsy of a morphologically normal or abnormal lymph node in the anatomic position of the SLN would accomplish the goal of a single axillary operation.
77.3
94.4
94.4
77.3
Krishnamurthy S et al. Cancer 2002 [20]
To determine diagnostic accuracy of US guided FNA of indeterminate, suspicious or metastatic appearing ALNs during initial staging
86.4
100
100
67
PPV: Positive Predictive Value; NPV: Negative Predictive Value; NR: Not Recorded
Table 2: Axillary ultrasound and ultrasound guided lymph node biopsy.
Size of the primary tumor, size of the metastatic deposit in the lymph node, and increasing number of affected nodes will increase the sensitivity and specificity of AUS and USLNB. In a study of 224 patients, sensitivity of USLNB was 100% with primary tumors >5cm, 69% with primary tumors >2 to = 5cm and 29% in patients with tumors = 1 cm [8]. Based on this finding, the authors recommended axillary ultrasound in patients with tumors >1cm and USLNB in patients with LN that have indeterminate or suspicious features [8] (Table 1). In another study evaluating the diagnostic accuracy of US-guided FNA of suspicious or metastatic appearing lymph nodes in early breast cancer, characteristics of the cortex and hilum were examined (Table 1). All cases with three or more axillary lymph nodes harboring metastasis and 93% of metastatic deposits measuring >0.5 mm were detected by USLNB. The probability of detecting nodes with smaller metastatic deposit less than 0.5mm was 44% [20]. Recent studies suggest that patients with nodal disease detected by ultrasound have significantly more axillary nodal involvement and larger number of axillary lymph nodes containing metastasis than those who have a negative AUS and USLNB but positive SLNB [21]. A meta-analysis comparing 532 patients with positive USLNB to 248 patients with a negative USLNB but positive SLNB demonstrated that the number of involved nodes was significantly higher in patients whom axillary metastasis was detected by ultrasound guided biopsy (p<0.001) [10].
Marking the biopsied lymph node
In breast cancer, the conventional definition of the SLN is the first node (or nodes) in the axilla to receive lymphatic drainage from the primary tumor in the breast. Identification of the SLN in the operating room depends on the reliable lymphatic uptake of tracers (blue dye, radio-labeled colloid, indocyanine green) as well as the gradient of that lymphatic flow from the tumor to the first node. Occasionally, nodal tumor infiltration can obstruct the orderly flow of lymph and high nodal pressure in the SLN can divert the lymphatic stream to other axillary non-SLNs [22], leading to a false positive identification. To identify any nodal burden in the axilla that may not be identified by optical or radioactive tracers, the surgeon palpates the soft tissue in the axilla and any hard, matted, or suspicious lymph nodes are considered abnormal and removed.
Accurate examination of the clinically negative axilla by ultrasound relies on the anatomic location, morphology, size and Doppler flow characteristics in the nodes. Debate regarding SLN identification by AUS has revolved around the concept of the “true SLN”. Some argue that the true SLN is the suspicious, cancer-containing node on preoperative ultrasound and percutaneous biopsy. Others believe that the true SLN is the “hot and blue node” identified by the orderly lymphatics with the combined use of radiocolloid and blue dye.
Marking the biopsied axillary node with a microchip [5] or percutaneous tattooing [9] is helpful to identify previously biopsied node. A microchip is a reliable way to determine if the lymph node that was initially biopsied is the same node that is removed during SLNB [5]. The “true SLN” (as identified in the operating room) was the node that was preoperatively biopsied in 47 of 60 (78.3%) of patients in whom a microchip was placed [5]. The microchip can easily be seen on an x-ray of the specimen if present. Evidence of percutaneous biopsy can sometimes be noted during open SLNB, including presence small hematoma, seroma or inflammatory reaction. But the main disadvantage of marking the node with a clip is that it is placed inside the body of the node and not visually apparent intraoperatively. To provide the surgeon with visual confirmation, one study injected black ink into the percutaneously biopsied node. The black ink was found to be easily distinguishable from blue dye, and tattooed ALNs were identified in all patients treated with neoadjuvant chemotherapy [9]. Clip placement in combination with tattooing allows for both radiological and visual confirmation and may increase the identification rate when compared to each individual modality alone.
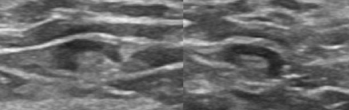
Figure 1: Normal lymph nodes.
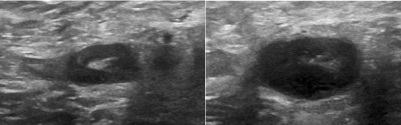
Figure 2: Invasive ductal carcinoma metastatic to axillary nodes. Sonography
demonstrates circumferential thickened cortex and compressed hilum.
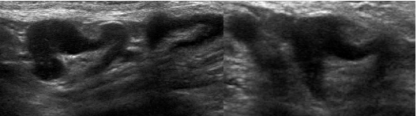
Figure 3: Metastatic intraductal carcinoma with eccentric node thickening.
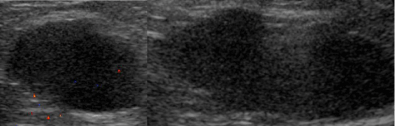
Figure 4: Metastatic invasive ductal carcinoma. Axillary nodes replaced with
tumor. No fatty hilum.
Targeted Axillary Node Dissection
Axillary ultrasound and USLNB may accurately define axillary status before surgery. For node positive patients receiving neoadjuvant chemotherapy, a pathologic complete response (PCR) is seen in approximately 40% of cases [23]. The benefit of complete axillary nodal dissection in patients without residual axillary node disease after chemotherapy has been questioned and the accuracy of performing SLNB following neoadjuvant chemotherapy has been investigated.
The European SENTINA study (Sentinel Lymph Node Biopsy in patients with breast cancer before and after neoadjuvant chemotherapy) found a higher false negative rate and lower detection rate of SLN in patients who received chemotherapy versus those who did not. All patients underwent preoperative physical examination with axillary palpation and AUS. An axilla was considered negative if the AUS was negative. There was no universally accepted standard used to define lymph node morphology, and USLNB was recommended but not mandatory [24]. Results from the ACOSOG Z1071 trial, conducted to evaluate SLNB in patients with node positive disease who received chemotherapy, demonstrated a false negative rate of 12.6% that was considered higher than was acceptable [23]. However, a decrease in the false negative rate to 7.4% and 10.8% was observed when a preoperative biopsy clip was placed and dual tracer technique was used, respectively [23]. New recommendations by the National Comprehensive Cancer Network soon followed to include placing a clip in a biopsied lymph node and removing all clip containing axillary nodes during definitive axillary surgery [3].
Despite a high false negative rate in SLNB after neoadjuvant chemotherapy, results from these trials are being incorporated into clinical practice by way of targeted axillary dissection (TAD). TAD allows for biopsy proven lymph node positive patients who underwent chemotherapy to have the biopsied nodes selectively removed. Preoperative image guided wire or I125 radioactive seed localization allows the biopsy proven positive axillary node to be identified and removed at the time of definitive axillary surgery following neoadjuvant chemotherapy and has been shown to be safe, effective and fairly easy to perform [11]. The false negative rate and correlation between the previously biopsied (clipped) nodes versus the operative SLN is still being investigated so TAD is accompanied by complete axillary dissection [25]. TAD offers a promising and less invasive approach to restaging the axilla following neoadjuvant chemotherapy and further investigation is needed to determine its impact on recurrence and survival.
Conclusion
In certain patients with early breast cancer and small volume locoregional disease, complete axillary dissection has been shown to offer no survival benefit and has largely been abandoned. As continued improvements in systemic therapy and radiation lead to pathologic complete response, management of the axilla strives to be less invasive even in the setting of later stage breast cancer. Some randomized prospective trials are now investigating the utility of SLNB altogether. These studies rely on the high specificity of AUS and USLNB to identify the presence of nodal disease. New prospective randomized trials aim to determine if AUS is a reliable preoperative staging modality in hopes that AUS will be a minimally invasive replacement of the SLNB. The SOUND, INSEMA and other randomized trials are now examining the axillary recurrence, disease free and overall survival of patients with early stage breast cancer (T1-T2) with negative AUS who will be randomized to undergo SLNB versus no further axillary staging (INSEMA) [4,26,27]. Future research that utilizes ultrasound to differentiate between patients with minimal axillary nodal metastasis from those with extensive axillary nodal disease will contribute substantially to the less invasive surgical management of the axilla.
References
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011; 305: 569-575.
- Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014; 15: 1303-1310.
- The National Comprehensive Cancer Network Guidelines for Treatment of Breast Cancer. https://www.nccn.org/.
- Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast. 2012; 21: 678-681.
- Nathanson SD, Burke M, Slater R, Kapke A. Preoperative identification of the sentinel lymph node in breast cancer. Ann Surg Oncol. 2007; 14: 3102-3110.
- Britton P, Moyle P, Benson JR, Goud A, Sinnatamby R, Barter S, et al. Ultrasound of the axilla: where to look for the sentinel lymph node. Clin Radiol. 2010; 65: 373-376.
- Hieken TJ. The promise of axillary imaging in individualized surgical management of breast cancer patients: another step forward. Ann Surg Oncol. 2014; 21: 3369-3371.
- Mainiero MB, Cinelli CM, Koelliker SL, Graves TA, Chung MA. Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol. 2010; 195: 1261-1267.
- Choy N, Lipson J, Porter C, Ozawa M, Kieryn A, Pal S, et al. Initial Results with Preoperative Tattooing of Biopsied Axillary Lymph Nodes and Correlation to Sentinel Lymph Nodes in Breast Cancer Patients. Ann Surg Oncol. 2015; 22: 377-382.
- van Wely BJ, de Wilt JH, Francissen C, Teerenstra S, Strobbe LJ. Meta-analysis of ultrasound-guided biopsy of suspicious axillary lymph nodes in the selection of patients with extensive axillary tumour burden in breast cancer. Br J Surg. 2015; 102: 159-168.
- Caudle AS, Kuerer HM, Le-Petross HT, Yang W, Yi M, Bedrosian I, et al. Predicting the extent of nodal disease in early-stage breast cancer. Ann Surg Oncol. 2014; 21: 3440-3447.
- Farrell TP, Adams NC, Stenson M, Carroll PA, Griffin M, Connolly EM, et al. The Z0011 Trial: Is this the end of axillary ultrasound in the pre-operative assessment of breast cancer patients? Eur Radiol. 2015;.
- Fung AD, Collins JA, Campassi C, Ioffe OB and Staats PN. Performance characteristics of ultrasound-guided fineneedle aspiration of axillary lymph nodes for metastatic breast cancer employing rapid on-site evaluation of adequacy: Analysis of 136 cases and review of the literature. Cancer Cytopathol. 2014; 122: 282–291.
- Houssami N, Turner RM. Staging the axilla in women with breast cancer: the utility of preoperative ultrasound-guided needle biopsy. Cancer Biol Med. 2014; 11: 69-77.
- Cools-Lartigue J, Sinclair A, Trabulsi N, Meguerditchian A, Mesurolle B, Fuhrer R, et al. Preoperative axillary ultrasound and fine-needle aspiration biopsy in the diagnosis of axillary metastases in patients with breast cancer: predictors of accuracy and future implications. Ann Surg Oncol. 2013; 20: 819-827.
- Ibrahim-Zada I, Grant CS, Glazebrook KN, Boughey JC. Preoperative axillary ultrasound in breast cancer: safely avoiding frozen section of sentinel lymph nodes in breast-conserving surgery. J Am Coll Surg. 2013; 217: 7-15.
- Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol. 2012; 19: 1825-1830.
- Oz A, Demirkazik FB, Akpinar MG, Soygur I, Baykal A, Onder SC, et al. Efficiency of ultrasound and ultrasound-guided fine needle aspiration cytology in preoperative assessment of axillary lymph node metastases in breast cancer. J Breast Cancer. 2012; 15: 211-217.
- Torres Sousa MY, BanegasIllescas ME, Rozas Rodríguez ML, Arias Ortega M, González López LM, Martín García JJ, et al. Preoperative staging of axillary lymph nodes in breast cancer: ultrasonographic parameters and ultrasound-guided core needle biopsy. Radiología. 2011; 53: 544-551.
- Krishnamurthy S, Sneige N, Bedi DG, Edieken BS, Fornage BD, Kuerer HM, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002; 95: 982-988.
- Kondo N, Fujita T, Sawaki M, Hattori M, Yoshimura A, Ichikawa M, et al. Preoperative axillary imaging with ultrasonography: Among the breast cancer patients with lymph node metastases, can we identify the patients who may omit axillary dissection? [abstract]. In: Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2014. San Antonio, TX. Philadelphia (PA): AACR; Cancer Res. 2015; 75: P2-01-18.
- Nathanson SD, Shah R, Chitale DA, Mahan M. Intraoperative clinical assessment and pressure measurements of sentinel lymph nodes in breast cancer. Ann Surg Oncol. 2014; 21: 81-85.
- Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American College of Surgeons Oncology Group Z1071 Trial (Alliance). J Clin Oncol. 2015: JCO-2014.
- Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013; 14: 609–618.
- Mittendorf EA, Caudle AS, Yang W, Krishnamurthy S, Shaitelman S, Chavez-MacGregor M, et al. Implementation of the American college of surgeons oncology group z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014; 21: 2468-2473.
- Tucker NS, Gillanders WE, Eberlein T, Aft R, Margenthaler J, Gao F, et al. Abstract OT3-5-01: A prospective, randomized trial of sentinel lymph node biopsy versus no additional staging in patients with T1-T2 invasive breast cancer and negative axillary ultrasound. Cancer Research. 2015; 75: OT3-5.
- Reimer T, Hartmann S, Stachs A, Gerber B. Local treatment of the axilla in early breast cancer: concepts from the national surgical adjuvant breast and bowel project B-04 to the planned intergroup sentinel mamma trial. Breast Care (Basel). 2014; 9: 87-95.