
Research Article
Austin J Cancer Clin Res. 2016; 3(2): 1070.
Analysis of Prognostic and Related Factors in Hepatocellular Carcinoma Patients with Portal Vein Thrombosis after Radiotherapy
Lee YC1,2, Chang HW3, Tseng HC4,5 and Chang WW6,7*
¹Department of Radiation Oncology, Chung Shan Medical University Hospital, Taiwan
²Institute of Medicine, Chung Shan Medical University, Taiwan
³Department of Radiation Oncology, Chung Shan Medical University Hospital, Taiwan
4Department of Radiation Oncology, Chung Shan Medical University Hospital, Taiwan
5School of Medicine of Chung Shan Medical University, Taiwan
6School of Biomedical Sciences, Chung Shan Medical University, Taiwan
7Department of Medical Research, Chung Shan Medical University Hospital, Taiwan
*Corresponding author: Wen-Wei Chang, Department of Medical Research, Chung Shan Medical University Hospital, Taichung City, Taiwan
Received: August 22, 2016; Accepted: October 14, 2016; Published: October 19, 2016
Abstract
Aim: Patients with hepatocellular carcinoma (HCC) with portal vein or inferior vena cava tumor thrombosis (PVT or IVCT) are deemed to have poorer treatment outcomes than those without. Radiotherapy (RT) is the main treatment for HCC patients with PVT or IVCT. This study aimed to clarify the prognostic factors, safety, and quality of RT in these patients for improved therapeutic design.
Materials and Methods: Patients with HCC who had PVT or IVCT and received RT were enrolled in this study. Demographic variables, laboratory values, tumor characteristics, and RT modalities were determined before and after RT. The primary endpoint was overall survival. Predicted factors of survival were identified by univariate and multivariate analyses. The planning target volume was used to evaluate the safety margin. The imaging records of Tomo Therapy in the treatment of abdomen or pelvic tumors were used to evaluate daily different motions of the liver.
Results: Ten patients with HCC with PVT or IVCT received RT were enrolled. Pretreatment unfavorable predictors included advanced stage, positive HBsAg, higher aspartate aminotransferase (AST), and poor Child-Pugh classification. Post-treatment unfavorable predictors were higher total bilirubin, lower albumin, and higher AST (p<0.05). Gross tumor volume safety maximal margin at the different directions of X (right/left), Y (up/down), and Z (in/out) were 4, 8, and 8 mm, respectively.
Conclusion: These results provided the potential factors that influenced the survival of patients with HCC after RT. RT was effective for PVT or IVCT, and careful addition of adequate margin could safely overcome daily motions.
Keywords: Hepatocellular carcinoma; Portal vein thrombosis; Radiotherapy; Prognostic factors; Gross tumor volume
Introduction
Cancer is the top cause of death in Taiwan in the last 34 years. According to information from the Taiwan Department of Health and the Health Promotion Administration, Ministry of Health and Welfare, hepatocellular carcinoma (HCC) is the second most common malignancy. In 2012, the number of newly diagnosed HCC reached more than 11 000, and more than 8000 died because of HCC [1]. HCC male patient ratio is about 2.35 times higher than women (male = 7920; female = 3372). Liver cancer is often diagnosed at its terminal stage, and the 5-year survival rate remains <12% in patients with additional complications [2].
According to the data from the Surveillance, Epidemiology, and End Results (SEER) of the American Cancer Society, of the treatment of liver cancer, the five-year relative survival of local invasion tumor, regional invasion and distant metastases were 21%, 6%, and 2%, respectively. Chung-Shan Medical University Hospital’s annual report of the treatment of cancer from 2004 to 2011 show that the five-year survival rate of patients with AJCC stage I, II, IIIA, IIIB, and IV were 46.4%, 26.9%, 8.9%, 6.6%, and 5.8%, respectively [3].
The status of stage III HCC invasions to the surrounding blood vessels resulting in hepatic portal vein thrombosis (PVT or IVCT), normal liver cells without oxygen, and nutrient supply are considered as poorer treatment outcomes. Patient could receive external beam radiation therapy (RT) to irradiate blocking portal vein tumor, and subsequent embolization. [4-9]. Radiotherapy (RT) using highenergy X-ray can be used to irradiate tumor lesions, kill cancer cells or stop proliferation. Due to normal liver cells also being sensitive to high-dose external beam radiation, we need to assess the tumor size, lymph node, violations of organs and position, and liver function of the patients before RT.
In cancer RT planning, a safety margin should be added around gross tumor volume (GTV) to overcome uncertainties in planning or treatment delivery. The aim of this study is to clarify prognostic factors, safety, and quality of RT in these patients and use this for further therapeutic design.
Materials and Methods
Enrolled patients
Patients met the following criteria in Chung-Shan Medical University Hospital Cancer Registry database from 2009 to 2012 were enrolled:
A. Clinician diagnosed with liver cancer by puncture biopsies proved or tumors larger than 1 cm with two classical image enhancements (3-phase CT or MRI showed typical vascular characteristics) [10-13].
B. The clinical diagnosis with portal vein invasion, PVT or IVCT.
C. Receiving RT, and the radiation field must contain the portal vein tumor Patients’ characteristics with clinical AJCC-TNM Stage and Age were shown in Table 1.
Patient
Numbers
cT
cN
cM
Age
1
3
0
0
55
2
2
0
0
79
3
4
0
0
70
4
3
0
0
83
5
3
0
0
55
6
3
0
0
60
8
3
0
0
72
7
3
0
0
52
9
3
0
0
36
10
3
0
0
57
Table 1: The characteristicsof HCC Patients with clinical AJCC-TNM Stage and Age.
Prognostic factors evaluation
The following parameters of patients with re- and post-RT were recorded: Age, HBsAg, Total Bilirubin, Albumin, AST, ALT, Child- Pugh classification Radiation dose (Gy), Target Tumor volume, Tumor with whole liver volume ratio (T-L Ratio), Response and Overall survival. The date of diagnosis was used as a reference to calculate the date of death or last contact date (depending on database records), which was defined as the interval between the survival time Patients’ characteristics with Pre-RT and Post-RT were shown in Table 2 and Table 3.
Patient No.
HBsAg
Bilirubin
(mg/dL)Albumin
(g/dL)ALT
(U/L)AST
(U/L)C-P
Class*
Dose
(Gy)
GTV
(cc)
Liver Volume
(cc)T-L Ratio#
1
+
2.43
4.1
63
48
A
55
22.5
357.8
6.3%
2
-
0.49
3.2
31
55
A
57.2
379.1
986.7
38.4%
3
+
2.92
3.1
52
59
B
57.2
386.4
1825.9
21.2%
4
+
0.77
4.4
32
56
A
57.2
106.1
830.7
12.8%
5
+
2.21
2.7
22
55
B
55
286.8
1217.6
23.6%
6
+
0.69
3.9
55
68
A
57.5
304.3
1092.3
27.9%
8
+
1.95
2.9
55
72
B
52
1004.1
1704.3
58.9%
7
+
0.9
2.8
40
45
B
55
201.3
1636.9
12.3%
9
+
1.35
3.7
248
182
B
46.2
146.5
1654.3
8.9%
10
+
2.99
2.4
30
100
B
55
224.7
775.6
29.0%
*C-P class: Child-Pugh classification
#T-L Ratio: Tumor with whole liver volume ratio
Table 2: Lab data, RT dose and tumor volume of enrolled patients before RT.
Patient
Bilirubin
(mg/dL)Albumin
(g/dL)ALT
(U/L)AST
(U/L)Response*
Survival
time
(months)1
1.13
3.5
53
53
CR
15
2
0.76
3.7
25
28
>PR
24
3
1.74
2.9
67
111
>PR
7
4
0.7
3.4
42
93
>PR
8
5
1.57
2
16
34
>PR
17
6
0.59
3.9
39
44
>PR
20
8
1.85
2.5
20
32
>PR
8
7
2.23
2.3
87
123
<PR
4
9
7.9
2.2
20
114
DP
4
10
4.28
2.4
34
110
DP
5
*CR: Complete Response; PR: Partial Response; DP: Disease Progress
Table 3: Lab data, Response and Survival time of enrolled patient’s post-RT.
RT-Planning evaluation
We further analyzed radiotherapy planning, design different clinical treatment volume (PTV), to assess the doses received by normal liver tissue. The radiotherapy PTV enclosed the Gross Tumor Volume (GTV) with anisotropic margins to account for possible uncertainties in beam alignment, patient positioning, organ motion, and organ deformation. In the RT-Plan, GTV of liver and portal vein. The addition of variable margin of GTV at different directions of X (Right/Left), Y (Up/Down), Z (In/Out), respectively was shown in Table 4. Radiotherapy planning must consider critical normal tissue structures; include bilateral Lung, Kidney, Spinal Cord, Small Intestine and Normal Liver, known as organs at risk (ORs) [14-16]. This study used Intensity Modulation Radiation Therapy (IMRT) technicality, given five angular direction 10MV energy beams, 0, 35, 240, 280, 320 degrees, respectively. The dose prescribed to V55Gy>99% in 22 fractions. RT-Planning was determined by Pinnacle3 Planning System. Dose-Volume-Histogram (DVH) was used to evaluate the RT-Planning quality by senior radiation oncologist. Tolerance of normal tissues and organs to the radiation dose was assessed by the criteria of Radiation Therapy Oncology Group/ European Organization for Research and Treatment of Cancer (RTOG/ EORTC) Late Radiation Morbidity Scoring Schema (2007). Patients receiving radiation therapy of the abdominal or pelvic tumor were enrolled for such analysis. The normal liver dose-volume percentage (V5, V10, V15, V20, V25, V26, V30, V35, V40, %) and the average dose received (Mean dose, Gy) was shown in Table 5. The kilovolt computer tomography images including KVCT Images for positioning and MVCT Images before treatment were obtained and used for calculation of daily changes. The variables of daily changes at different directions of X (Right/Left), Y (Up/Down), Z (In/Out) were analyzed according to a report from Kristy, et al. [17].
X*
Y*
Z*
PTV-1
2
2
2
PTV-2
3
3
3
PTV-3
3
5
5
PTV-4
5
10
10
PTV-5
5
15
15
* X (Right/Left), Y (Up/Down), Z (In/Out)
Table 4: The PTV planning with addition of variable margin (mm) of GTV.
V5 (%)
V10 (%)
V15 (%)
V20 (%)
V25 (%)
V30 (%)
V35 (%)
Mean (cGy)
PTV-1
49.1
43.9
35.6
32.7
25.2
17.9
13.1
1424.7
PTV-2
53.5
47.6
42.2
36.4
28.8
20.9
15.5
1582.6
PTV-3
53.9
48.7
43.7
38.5
30.7
22.8
16.5
1649.4
PTV-4
59.3
53.7
49.6
44.8
36.9
27.7
19.9
1896.2
PTV-5
63.2
58.0
53.4
48.5
40.7
32.1
23.7
2090.1
Normal Tissue
Tolerance Dose86%
68%
59%
49%
35%
28%
25%
2300
Table 5: Normal liver dose-volume percentage and the average dose received.
Statistical analysis
Kaplan-Meier curves were generated for survival. For univariate and multivariate analysis were used for analyzing factors correlated with the survival time, including pre- and post-RT. P<0.05 was considered statistically significant. All calculations were performed with SPSS 19.0 for Windows (SPSS, Chicago, IL, USA).
Results
Evaluating the prognostic factors of HCC patients with PVT or IVCT received RT
Among 42 patients with HCC who had received RT between April 2009 and October 2012, only 10 patients had PVT or IVCT received RT and were enrolled in this study. The number of complete response (CR) was 1 (10%); 6 (60%) patients had more than partial response status (>PR) and 3(30%) patients had less than partial response status (<PR). Overall median survival in partial response group was 14.1 months versus 4.3 months in stable or progress group (p =0.001) (Figure 1).
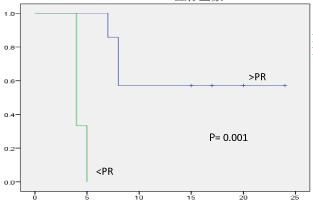
Figure 1: Overall median survival of enrolled patients. The overall median
survival period of partial response (>PR) group was 14.1 months versus 4.3
months in stable or progress group (
In univariate analysis, only higher post-RT AST (GOT) was
considered as a significant predictor of poor survival (95% confidence
interval, p =0.04). The median survival times in patients with normal
AST level (<37 U/L) or not more than twice of the normal value (<76 U/L) post-RT were 16.3 ± 6.5 months and 17.5 ± 2.5 months,
respectively. The average survival of patients with AST more than
twice of the normal value (>76 U/L) was only 5.60 ± 1.6 months
(Table 6).
Table 6: Univariate or multivariate analysis of factors related to radiation therapy in enrolledpatients.
Variables
n
Survial status
P-values
Averge ± SD
Univariate
Multivariate
Stage
II
1
24.0 ± 0.0
0.185
<0.001*
III
8
10.1 ± 5.9
IV
1
7.00 ± 0.0
Age
≤60
6
10.8 ± 6.7
0.486
0.016*
>60
4
11.8 ± 7.1
HBsAg
Negative
1
24.0 ± 0.0
0.242
0.002*
Postive
9
9.80 ± 5.7
Total Biliribum
≤1.2
4
14.0 ± 8.2
0.645
0.111
>1.2
6
9.30 ± 4.9
Albumin
≥3.5
4
11.8 ± 6.2
0.645
0.111
<3.5
6
10.8 ± 7.2
ALT
≤38
4
13.5 ± 7.5
0.645
0.111
>38
6
9.70 ± 5.9
AST
38-76
7
13.6 ± 6.9
0.111
0.001*
>76
3
5.70 ± 1.7
Child-pugh classification
A
4
16.8 ± 6.0
0.07
0.037*
B
6
7.50 ± 4.5
Tumor size (cm)
≤5
4
14.0 ± 6.0
0.067
0.068
>10
4
10.8 ± 7.8
≥15 or Multiple
2
6.50 ± 1.5
POST-RT
≤1.2
4
16.8 ± 6.0
0.07
0.037*
Total Biliribum
>1.2
6
7.50 ± 4.5
POST-RT
≥3.5
2
22.0 ± 2.0
0.06
0.002*
Albumin
<3.5
8
8.50 ± 4.6
POST-RT
≤38
5
11.6 ± 7.7
1
0.179
ALT
>38
5
10.8 ± 5.8
POST-RT AST
≤38
3
16.3 ± 6.5
0.04*
0.012*
38-76
2
17.5 ± 2.5
>76
5
5.60 ± 1.6
EBRT Dose (Gy)
≤55
6
8.80 ± 5.3
0.645
0.111
>55
4
14.8 ± 7.4
EBRT Volume (cc)
≤200
3
9.00 ± 4.5
0.629
0.09
200-400
5
14.0 ± 8.1
>400
2
7.50 ± 0.5
Tumor/Liver Volume ratio (%)
≤20
4
7.80 ± 4.5
0.861
0.081
20-40
5
14.6 ± 7.4
>40
1
8.00 ± 0.0
Response to EBRT
CR or PR
7
14.1 ± 6.2
0.111
<PR or PD
3
4.30 ± 0.5
Table 6: Univariate or multivariate analysis of factors related to radiation therapy in enrolledpatients.
From multivariate analysis, the pretreatment unfavorable
predictors were advanced stage, old age, positive HBsAg, higher
AST, and poorer Child-Pugh classification (Table 6). Post-treatment
unfavorable predictors were higher post-RT total bilirubin, lower
albumin, and higher AST (95% confidence interval, p<0.05) (Table
6). The average survival of patients with normal total bilirubin level
(<1.2 mg/dL) after treatment was significantly higher than those with
abnormal level (>1.2 mg/dL) (16.8 ± 6. 0 months versus 7.50 ± 4.3
months, p<0.05).
Evaluating the RT-Planning among HCC patients
Statistical analysis results of the normal liver dose-volume
percentage (V5, V10, V15, V20, V25, V26, V30, V35, V40, %) and
the average dose received are shown in Table 5. We compared the
RTOG/EORTC criteria (V5<86%, V10<68%, V15<59%, V20<49%,
V25<35%, V30<28%, V35<25%, V40<20%, Mean<23Gy) of tolerance
radiation dose of normal liver, PTV1 (X+2 mm; Y+2 mm; Z+2 mm),
PTV2 (X+3 mm; Y+3 mm; Z+3 mm) and PTV3 (X+3 mm; Y+5 mm;
Z+5 mm) versus the dose-volume percentage and mean dose much
lower than criteria. The PTV4 (X+5 mm; Y+10 mm; Z+10 mm) plan
V25=36.9% is slightly higher by 1.9%, while in the V25 and V30,
the PTV5 (X+5 mm; Y+15 mm; Z+15 mm) plan V25 and V30 were
40.7% and 32.1%, higher by 5.7% and 4.1%, respectively. Therefore,
when patient received PTV4 and PTV5, plan shall carefully assess the
potential the side effects.
We next evaluated the daily changes of liver volume among
1442 fractions from patients receiving abdominal or pelvic radiation
therapy. The mean, median, and maximum motion changes in X
directions were 3.01, 2.52, and 14.00 mm, respectively. The mean,
median, and maximum motion changes in Y directions were 2.80,
1.71, and 24.46 mm, respectively. The mean, median, and maximum
motion changes in Z directions were 3.14, 2.31, and 30.07 mm,
respectively (Table 7). The mean, median, and maximum motion
changes in directions of roll angle were 0.41°, 0.20°, and 3.60°,
respectively (Table 7).
Table 7: The daily changes of liver volume among patients receiving abdominal
or pelvic radiation therapy.
X
(mm)
Y
(mm)
Z
(mm)
Roll
(degree)
Mean
3.01
2.8
3.14
0.41
Median
2.52
1.71
2.31
0.2
SD
2.33
3.1
3
0.53
Minimum
0
0
0
0
Max
14
26.46
30.07
3.6
Table 7: The daily changes of liver volume among patients receiving abdominal
or pelvic radiation therapy.
Discussion
The overall median survival in partial response group and in
stable or progress group of our study was 14.1 months versus 4.3
months (p=0.001) (Figure 1). A previous study also observed that the
survival time of 59 liver cancer patients with invasive hepatic portal
vein, or PVT, received radiation therapy was different according to
the response status [18]. The average survival was better in >PR group
than relatively poor response (<PR or PD) (10.7 months versus 5. 3
months) [18]. Both studies got similar results.
With univariate or multivariate analysis, several correlation
factors related to radiotherapy, such as radiation dose, target tumor
volume or T-L Ratio, were found to have no significant influence to
the survival time in our study (p >0.05). Given that this was only a
retrospective study and not a Phase I-II study, radiation oncology
physicians determined how much radiation dose and irradiated
volume administered. In fact, the dose and volume administered was
determined according to clinical experience and the overall condition
of patients with liver cancer, including the severity of liver damage
and residual normal liver tissue tolerance dose, as an adjustment to
the RT planning. Results showed no significant differences in the
final analysis of survival time. Another conclusion was that these 10
patients that received complete RT were safe and showed no evidence
of variation factors in RT plans.
Patients treated using a mold fixed, although the average and
median motion value were small, still had a large maximum error
and it affected the accuracy of cancer treatment. Therefore, this
study confirmed the benefits of using daily image-guided technology
to find and correct daily random motions. We also considered the
GTV and added maximal safe margin by calculation via interpolation
optimized to obtain a better plan target volume (called PTV6, Table
8). The directions of X, Y, and Z axis were not more than 4, 8, and
8 mm, respectively. In this PTV6 plan, the total irradiation volume
was 263.7 cm3, normal liver dose-volume percentage of V5, V10, V15,
V20, V25, V26, V30, V35, V40 was 58.6% (<86%), 53.0% (<68%),
47.9% (59.0%), 42.3% (<49%), 34.3% (<35%) , 25.1% (<28%) , 18.8%
(<25%), and 14.1% (<20.0%), respectively; the mean dose was 18.1Gy
(<23Gy) (Table 8). All the calculated parameters were lower than
RTOG/EORTC Normal Tissue Tolerance dose criteria. It would be
expected to obtain better therapeutic outcome and less side effect if
patients received RT according this PTV6 plan.
Table 8: The RT-Planning quality of newly planned PTV.
V5 (%)
V10 (%)
V15 (%)
V20 (%)
V25 (%)
V30 (%)
V35 (%)
V40 (%)
Mean (cGy)
PTV-6*
58.6
53
47.9
42.3
34.3
25.1
18.8
14.1
1810
Normal Tissue
Tolerance Dose
86%
68%
59%
49%
35%
28%
25%
2300Gy
2300
*PTV6: Directions X, Y and Z were added 4 mm, 8 mm and 8 mm, respectively.
Table 8: The RT-Planning quality of newly planned PTV.
Conclusion
Radiotherapy is effective for PVT or IVCT. Overall median survival
in partial response group was 14.1 months more than 4.3 months in
stable or progress group. Pretreatment unfavorable predictors were
advanced stage, old age, positive of HBsAg, higher AST and poorer
Child-Pugh classification. Post treatment unfavorable predictors
were higher total bilirubin, lower albumin, higher AST.
Careful addition of adequate margin could safely overcome the
side effect of RT caused by daily motions of liver. If there is no imageguided
technology to correct daily random motions (Directions of X,
Y and Z mean value were 3.01 mm, 2.8 mm and 3.14 mm respectively),
the GTV safety maximal margin at different directions of X (Right/
Left), Y (Up/Down), Z (In/Out) should not more than 4 mm, 8 mm
and 8 mm, respectively.
References
- https://www.mohw.gov.tw
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. 2010. CA Cancer J Clin. 2010; 60: 277-300.
- https://web.csh.org.tw
- Toya R, Murakami R, Baba Y, Nishimura R, Morishita S, Ikeda O, et al.
Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular
carcinoma. Radiother Oncol. 2007; 84: 266-271.
- Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma
accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;
12: 7561-7567.
- Lin CS, Jen YM, Chiu SY, Hwang JM, Chao HL, Lin HY, et al. Treatment of
portal vein tumor thrombosis of hepatoma patients with either stereotactic
radiotherapy or three-dimensional conformal radiotherapy. Jpn J Clin Oncol.
2006; 36: 212-217.
- Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, et al. Three-dimensional
conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma.
Cancer. 2005; 103: 2419-2426.
- Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, et al. A comparison
of treatment combinations with and without radiotherapy for hepatocellular
carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J
Radiat Oncol Biol Phys. 2005; 61: 432-443.
- Huang CJ, Lian SL, Chen SC, Wu DK, Wei SY, Huang MY, et al. External
beam radiation therapy for inoperable hepatocellular carcinoma with portal
vein thrombosis. Kaohsiung J Med Sci. 2001; 17: 610-614.
- Jang HJ, Lim JH, Lee SJ, Park CK, Park HS, Do YS. Hepatocellular carcinoma:
are combined CT during arterial portography and CT hepatic arteriography in
addition to triple-phase helical CT all necessary for preoperative evaluation?
Radiology. 2000; 215: 373-380.
- Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, et al.
CT and MRI improve detection of hepatocellular carcinoma, compared with
ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;
9: 161-167.
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK,
et al. Clinical management of hepatocellular carcinoma. Conclusions of the
Barcelona-2000 EASL conference. European Association for the Study of the
Liver. J Hepatol. 2001; 35: 421-430.
- Yamashita Y, Mitsuzaki K, Yi T, Ogata I, Nishiharu T, Urata J, et al. Small
hepatocellular carcinoma in patients with chronic liver damage: prospective
comparison of detection with dynamic MR imaging and helical CT of the
whole liver. Radiology. 1996; 200: 79-84.
- Lee MT, Purdie TG, Eccles CL, Sharpe MB, Dawson LA. Comparison of
simple and complex liver intensity modulated radiotherapy. Radiat Oncol.
2010; 5: 115.
- Hsieh CH, Liu CY, Shueng PW, Chong NS, Chen CJ, Chen MJ, et al.
Comparison of coplanar and non coplanar intensity-modulated radiation
therapy and helical tomotherapy for hepatocellular carcinoma. Radiat Oncol.
2010; 5:40.
- Ogino R, Hosono M, Ishii K, Tatsumi D, Tsutsumi S, Miki Y, et al. A dosevolume
intercomparison of volumetric-modulated arc therapy, 3D static
conformal, and rotational conformal techniques for portal vein tumor thrombus
in hepatocellular carcinoma. J Radiat Res. 2013; 54: 697-705.
- Brock KK, Dawson LA. Adaptive management of liver cancer radiotherapy.
Semin Radiat Oncol. 2010; 20: 107-115.
- Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, et al. Three-dimensional
conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma.
Cancer. 2005; 103: 2419-2426.