
Review Article
Austin J Cancer Clin Res. 2016; 3(2): 1072.
Nanomaterials Facilitate Tumor Targeting and Drug Delivery
Peng X1,2, Rahman MA1,2, Mao H2,3 and Shin DM1,2*
¹Department of Hematology and Medical Oncology, USA
²Emory Winship Cancer Institute, USA
³Department of Radiology and Imaging Sciences, Emory University School of Medicine, USA
*Corresponding author: Shin DM, Winship Cancer Institute, Rm 3090, 1365-C Clifton Road, Atlanta, GA 30322, USA
Received: November 23, 2016; Accepted: December 29, 2016; Published: December 30, 2016
Abstract
Current anticancer therapeutic drugs are restricted by: 1) relatively lower drug concentrations in tumor tissues; 2) lack of targeted delivery; 3) side effects resulting from the nonspecific accumulation of drugs in normaltissues; 4) acquired drug resistance. The targeted or selective delivery of anticancer drugs to tumor cells by nanoparticles has been demonstrated to potentially reduce the limitations of current drug delivery. In recent years, the development of tumor-targeted nanoparticles as the next generation of drug carriers has been extensively studied. Results from largely preclinical studies of various types of engineered nano carriers show that targeted nanomaterial-facilitated delivery may further increase the intracellular accumulation of drugs and improve their antitumor effects compared with non-targeted nanoparticles. However, developing tumor-targeted nanoparticles for selective anticancer delivery in vivo requires further understanding on how nanoparticle carriers behave and function in the complex biological systems, various physiological conditions and heterogeneous tumor microenvironment in order to better design and functionalize a nanoparticle delivery system. In this review, we will focus on tumor targeting strategies with discussions on tumor specific biomarkers, promising nanomaterials developed and tested for targeted delivery of therapeutics and imaging, and important issues and challenges for future clinical applications in oncology.
Keywords: Tumor; Drug Delivery; Nanomaterial
Introduction
Tumor targeting is a key strategy in personalized cancer diagnosis and treatment. The concept of tumor targeting was originally proposed decades ago to guide the development of “tumor seeking missiles” or “magic bullets” for cancer treatment [1]. With our increasing understanding of tumor biology and physiology at cellular and molecular levels and the discovery of new biomarkers and target specific treatment and drugs, tumor targeting is now applied in many major areas of cancer management, including diagnostic imaging, drug delivery and, most recently “theranostics”, which combines both diagnosis and therapy [2-6] in a shared platform. In parallel with tremendous advances in biomedical engineering, tumor biology and molecular oncology, one major focus in biomedical research is the development of new functional materials that enable highly efficient tumor targeting for imaging and delivery of therapeutic agents. Novel nanotechnologies and nanomaterials with unique properties and functions have increasingly shown capabilities and advantages in tumor targeted applications, particularly in the effort to overcome the challenges and limitations of tumor targeted anticancer drug delivery [7-10]. With tremendous progress made in the last decade some of the nanoparticles have already been approved by the FDA or are in clinical trials [11,12].
Conventional drug delivery routes applied in most current clinical practices include oral, transdermal, transmuscular and intravenous delivery, which are not designed to specifically target to certain cancer cells or cells with specific molecular characteristics.
In contrast, drug delivery with engineered nanoparticles can be accomplished in a targeted fashion using both passive and active targeting strategies as illustrated in Figure 1. Passive targeting takes advantage of the enhanced permeability and retention effect (EPR) [13,14] from leaky blood vessels and limited lymphatic drainage in tumors, which are commonly associated with tumor growth, to increase the accumulation of drug-carrying nanoparticles in the tumor mass. Thus, passive targeting enhances the anti-tumor effects and reduces the side effects of cytotoxic agents through changing the distribution and pharmacokinetics of nanoparticle-loadeddrugs without using tumor targeted ligands.
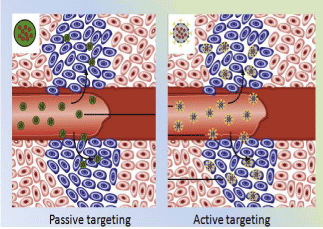
Figure 1: Passive Targeting and Active Targeting for Drug Delivery with
Nanocarriers. Nano carriers are expected to stay in the blood for a long
time, accumulate in tumor sites with affected and leaky vasculatures via the
enhanced permeability and retention (EPR) effect, and facilitate internalization
into cells via active targeting using specific ligand-receptor interactions.
However, counting solely on the increased accumulation of drugs in the tumor massto deliver drugs is insufficient to attain maximal cytotoxic effects in cancer cells since most drugs must be taken up by the tumor cells to exert theiractions. Furthermore, the EPR effect mainly depends on the presence and the distribution of the tumor vasculature and its structures. Most solid tumors are highly heterogeneous with well-vascularized and poorly perfused portions mixed within the same tumor mass, limiting intra-tumoral delivery and distribution of drug-carrying nanoparticles. More importantly, non-targeted delivery vehicles are mainly localized in the extracellular matrix [15]. Payload drugs may be released and rapidly cleared from the tumor mass due to increased interstitial pressure before reaching their cellular targets. In comparison, active targeting based on the interaction of a targeting ligand and a biomarker, e.g., a cell surface receptor, not only enables tumor-targeted drug-loaded nanoparticles to accumulate in the tumor mass via the EPR effect but also facilitates the uptake of drugs into targeted cancer cells from the extracellular space via receptor-mediated internalization [7,16,17]. Increasing evidence has shown that targeted nanoparticles have greater antitumor activity compared with non-targeted nanoparticles [8,18- 22].
In this article, we will mostly focus on drug delivery using tumortargeted nanoparticles with a brief overview of tumor biomarkers that are specifically suited for targeted drug delivery, and the current status of nanoparticles for targeted delivery of therapeutics. We will discuss future prospects of the translational and clinical applications of nanomaterials, focusing on several important issues and challenges.
Biomarker targets and Targeting strategies
Cancer-associated biomarkers are logical choices for the development of targeted delivery vehicles for selective drug delivery to tumor cells. While biomarkers can be found in a variety of forms and systems, many may not be suitable for engineering tumor-targeted nanoparticles in drug delivery applications. Clinical translation using nanomaterials as delivery vehicles requires careful consideration of several important issues and limitations related to tumor targeting [23-48]. Table 1 provides examples of cancer biomarkers and their targets that have been used for targeted drug delivery and diagnostic imaging.
Cancer Cells
Targeted Biomarker
Tumor Types
Epidermal growth factor receptor (EGFR)
Breast cancer (14-91%), non-small lung cancer (40-80%), esophageal cancer (65-88%), pancreatic cancer (30–50%), gastric cancer (33–83%), colon cancer (25-77%), ovarian cancer (35-70%), cervical cancer (71-91%), bladder cancer (31–72%), hepatocellular carcinoma (32.4-66%), headand neck cancers (83-100%), renal cancer (50-90%), glioma (40–63%) [23,49-51]
Human epidermal growth factor receptor 2 (Her-2/neu receptor)
Breast cancer (15-30%), non–small cell lung cancer (30–50%), colon cancer (11-20%), gastric cancer (8-53%), pancreatic cancer (19–45%), renal cancer (16.7-40%), ovarian cancer (25–32%), cervical cancer (10-80%), bladder cancer (40-70%), prostate adenocarcinomas (34-86%), glioma (20–54%) [23, 52].
Folate receptor
Breast cancer (29-48%), non-small lung cancer (66-78%), renal cancer (64-75%), ovarian cancer (82-90%), uterine cancer (90%), headand neck cancers (45-50%), brain tumors (90%) [53,54].
Transferrin receptor (TfR)
Breast cancer (65.8-72.7%), non–small cell lung cancer (76-93%), pancreatic cancer (80-93%), hepatocellular carcinoma (32.4-97%), colon cancer (48%), non-Hodgkin's lymphoma (34.5%), bladder cancer (31.6–78.9%), gliomas (66%) [26,27,55,56]
Urokinase plasminogen activator receptor (uPAR)
Breast cancer (54-90%), lung cancer (80%), pancreatic cancer (40-86%) [28].
Prostate-specific membrane antigen (PSMA)
Prostate adenocarcinomas (66-94%), bladder cancer (14%) and the neovasculature of many other solid tumors [29,30].
Underglycosylated mucin-1 antigen (uMUC-1)
Breast cancer (72.7%), gastric cancer (65%), ovarian cancer (90%), non–small cell lung cancer (43-98%), prostate adenocarcinomas (17-78%), colon cancer (55%), hepatocellular carcinoma (77%), bladder cancer (93%) [31,32].
Interleukin-4 receptors (IL-4Rs)
Lung cancer (66-79%), headand neck cancers (33%), colon cancer (33%), ovarian cancer (60%) [33-35,57]
CXCR4 receptors
Breast cancer (77-83%), pancreatic cancer (55-90%), hepatocellular carcinoma (47.6%), ovarian cancer (36-59%), prostate adenocarcinomas (79%), colorectal cancer (22%), hepatocellular carcinoma (77%), gastric cancer (30%) [36,37,58,59]
Carcinoembryonic antigen (CEA)
Breast cancer (60%), colorectal cancer (98.8%), gastric cancer (91.1%), lung cancer (25.4-93.7%), pancreatic cancer (63.3%) [39,60]
Luteinizing hormone-releasing hormone receptor (LHRH)
Breast cancer (52%), ovarian cancer (80%), prostate adenocarcinomas (86-95.7%), ovarian cancer (80%) [41]
CD133
Breast cancer, ovarian cancer, colorectal cancer, prostate adenocarcinomas, melanoma, hepatocellular carcinoma [42]
CD44
Breast cancer, colorectal cancer, gastric cancer, ovarian cancer, melanoma [43]
Tumor Stroma
Vascular endothelial growth factor receptors (VEGFR)
Neovascular endothelial cells, various solid cancers [61]
αvβ3 integrin
Lung cancer, breast cancer neuroblastomas, glioblastomas, melanomas [45,62]
Fibroblast activation protein (FAP)
Epithelial cancers, breast cancer [46].
Intercellular adhesion molecule (ICAM)-1
Breast cancer, colon cancer, non-small cell lung cancer, gastric cancer [47].
Lymphocyte function-associated antigen-1 (LFA-1)
Various types of leukemia [48]
Fibrin–fibronectin complexes
Various solid cancers [48]
Table 1: Biomarkers used to engineer tumor-targeted nanoparticles.
Selection of tumor biomarkers for tumor targeting
Cancer-specific receptors and antigens, which have high expression on the tumor cell membrane but negligible or low expression on normal cells, are generally considered to be promising cell surface targets for tumor-targeted delivery. Nanoparticle-targeted drug delivery facilitates greater cellular internalization compared to simple administration of free drugs, which leads to much higher intracellular drug concentrations and release within the cells, and consequently enhanced anti-tumor effects. Thus, tumor biomarkers that facilitate cellular internalization are the preferred choice for the development of tumor-targeted nanoparticles to carry drugs that act intracellularly.
EGFR, HER2, FR, PSMA and TfR, as listed in Table 1, have been extensively studied for tumor cell targeting. Each of these biomarkers has unique properties, which confer certain advantages and disadvantages for tumor targeting. For example, EGFR is one of the most commonly used biomarkers for targeting tumor cells due to its presence and over expression in almost all solid tumors and its well-known biological characteristics. However, one concern about targeting EGFR is that targeting ligands may also induce activation of the EGFR signaling pathway, which in turn may promote tumor cell proliferation [63]. One study showed that EGFR-binding GE11 peptide-conjugated nanoparticles could be taken up into cells without activating the EGFR pathway or reducing the level of EGFR on the cell surface [63]. TfR is another biomarker that has been successfully used to engineer tumor-targeted nanoparticles for drug delivery. Among five targeted nanoparticle drugs in the stage of clinical studies, three target TfR [8,64].
One exceptionally convenient tumor target for receptor targeted drug delivery is the Folate Receptor (FR). FR has been demonstrated to be highly expressed in various solid tumors but absent in normal tissues. The FR-targeted ligand, vitamin folic acid (FA, molecular weight: 441), is a small molecule that binds to FR with very high affinity (Kd = 10-10 M), and thengets internalized into cells via receptor mediated endocytosis [65]. The ability to deliver therapeutic agents into cells is a key motivation in the development of FR-targeted drug delivery that directly affects the intracellular processes. In addition to its biological significance, there are other practical advantages of using FA as a targeting ligand for FR, such as: 1) low cost, 2) stable molecule comparing to other ligands, e.g., antibodies, 3) robust chemistry and compatibility with both organic and aqueous solvents for conjugation of FA with therapeutic agents and nanocarriers, and 4) less concern regarding immunogenicity [53]. It is anticipated that some FR-targeted nanoparticles may be moved forward to the clinic in the near future.
Selection of targeting ligands to engineer tumor-targeted nanoparticles
A targeting ligand should render the drug-loaded nanoparticles able to selectively bind to the surface target and then to be internalized into the targeted cells. A variety of targeting ligands, including intact antibodies, antibody fragments, affibodies, peptides, proteins, small molecules and DNA aptamers, have been used to construct tumortargeted nanoparticles for drug delivery [8,66-70]. The size, molecular weight, stability, binding specificity and affinity, conjugation process, tissue penetration, immunogenicity and cost of the ligands should be considered when selecting the targeting ligand. Table 2 summarizes the advantages and limitations of the different types of targeting ligands for in vivo tumor targeting.
Targeting Ligands
Advantages
Disadvantages
Monoclonal antibodies (Mab)
High affinity and specificity for their targets;
Commercially available
Immunogenicity;
High molecular weight;
Low penetration ability;
Expensive cost;
Antibody fragments
Small size;
Low immunogenicity;
Easily obtained at low costs
Low target affinity;
Susceptibility to proteolytic cleavage
Peptides
Low molecular weight;
Good tissue penetration ability,
Lack of immunogenicity;
Easily obtained;
Relative flexibility in chemical conjugation processes;
High stability
Lower binding affinity to
surface receptors;
Nonspecific adhesion;
Affibodies
Small size;
Ability to penetrate tumor tissue and cell;
High receptor affinity
Short half-life;
Small molecules
Many diverse structures and properties;
Low cost;
Small size;
Less immunogenic effects in vivo;
Reproducible and scalable manufacturing
Lower binding affinity to surface receptors;
Lower tumor selectivity
Aptamers
High specificity;
Small size and molecular weight;
Low immunogenicity;
Easy to obtain;
Rapid blood clearance;
Susceptibility to nuclease degradation;
Low stability
Endogenous proteins
Low immunogenicity;
High binding affinities to targeted receptor;
Low cost;
Susceptible to early clearance in vivo;
Off-target adverse effects;
Table 2: Targeting Ligands Used to Engineer Tumor-Targeted Nanoparticles.
Targeting thetumor stroma
The tumor microenvironment is complicated and highly heterogeneous. A large number of the cells within the tumor mass are actually not tumor cells but tumor stroma, consisting of fibroblasts and macrophages. Tumor stroma not only plays important roles in tumor progression and invasion but is also the major barrier for tumor cell specific drug delivery. On the other hand, targeting the stroma is also an attractive strategy for improving the tumor targeting efficiency [71-74]. Because various cell components and biomarkers are present in the stroma, targeting and treating tumors can be accomplished through targeting multiple markers and disrupting the tumor microenvironment. ICAM-1, as listed in Table 1, is over expressed in various solid tumors and stroma; one recent study showed that paclitaxel-loaded ICAM-1-targeted nanoparticles internalized efficiently in ICAM-1 positive tumor cells, tumor-associated endothelium, and macrophages [47]. Other studies using nanoparticles targeting the stroma markers FAP and Jagged1 also showed enhanced antitumor efficacy [75,76]. Thus, targeting molecular markers localized in the tumor stroma may provide an ideal strategy for the treatment of different types of tumors. More importantly, targeting tumor markers co-existing in the tumor stroma and tumor cells may provide synergistic advantages in improving the drug efficacy and treating drug resistant tumors [46].
Targeting the blood brain barrier (BBB)
Delivery of drugs to the brain represents a major challenge as most conventional chemotherapeutics cannot cross the BBB, which is composed of diverse cell types such as endothelial and microglial cells. In addition to this physiological barrier that protects the brain, there are a number of active efflux mechanisms, such as the P-glycoprotein (P-gp), that pump out drugs. Therefore, the application of chemotherapy to brain tumors is limited by significant delivery challenges. Brain tumor-targeted nanoparticles have the potential to circumvent this biological barrier without modifying the structure of the drug molecule. Several mechanisms have been explored for specific drug delivery to the brain [77,78], which include: 1) masking the unfavorable physicochemical characteristics of the loadeddrugs; 2) binding or absorbing the drug-nanoparticles to the surface of apolipoproteins; 3) taking advantage of receptor-mediated endocytosis by the brain capillary endothelial cells [79]. Using TfRtargeted nanoparticles, Zhang, et al. has shown the successful delivery of a significantly higher amount of drugs to the brain and enhanced antitumor efficacy compared to free drugs [80]. They demonstrated that Tf-modified paclitaxel-loaded polyphosphoester hybrid micelles (TPM) enhanced cellular uptake and brain accumulation, which were 2 and 1.8-fold of non-targeted PM, respectively. TPM exhibited substantial anti-glioma activity, and the mean survival was significantly longer than those treated with TaxolR80.
Targeting drug resistance
Targeted delivery of drug-loaded nanoparticles may also provide a potential strategy to overcome the drug resistance that cancer cells develop against conventional chemotherapy. There are many mechanisms involved in the development of drug resistance in cancer cells, such as: 1) reduced influx and/or increased efflux of drugs; 2) enhanced DNA repair and/or increased damage tolerance; and 3) failure of cell-death pathways. The decreased accumulation of drugs may result from either active efflux or impaired influx. Thus, increasing cellular uptake of drugs by using tumor-targeted nanoparticles may circumvent drug resistance [81-83]. Another important cause of drug resistance is that small molecule drugs have limited ability to penetrate tumor tissue and to reach the tumor cells [84]. However, tumor-targeted nanoparticles can alter the pharmacokinetic properties and biodistribution of drugs in vivo, thus, can deliver higher concentrations of drugs to the tumor sites. Circumventing drug resistance of tumor cells using nanoparticles can be accomplished through: 1) inhibiting the function of efflux pumps; 2) improved drug retention in tumor cells;3) co-delivery of efflux pump inhibitors with chemotherapeutics; 4) restoring proper apoptotic signaling; 5) co-delivery of multiple cytotoxic drugs with different mechanisms, and controlling drug exposure sequence [24,85-88].
Targeting tumor metastases
Tumor metastasis is one of the main causes of cancer patient death, thus, it is a rational therapeutic target for cancer [89]. However, there is still no efficient therapy for metastasis to date. Although loaded liposomal nanoparticles encapsulated with doxorubicin (DoxilR) without specific targeting have been applied in the clinic for the treatment of patients with metastatic breast cancer, there was no significant difference in progression-free survival between the DoxilR and free doxorubicin treated group [90]. Using engineered HER-2- targeted iron oxide nanoparticles, Zhang, et al. demonstrated that the targeted nanoparticles could selectively accumulate in lung, liver and bone marrow metastasis in a transgenic mouse model of metastatic breast cancer. Compared with targeting the primary tumor, the tumor-targeted nanoparticles showed remarkably higher targeting efficacy in the micrometastasis than the non-targeted nanoparticles [91]. Another study showed that doxorubicin-loaded Tumor Metastasis Targeting (TMT) peptide-conjugated nanoparticles could accumulate at a significantly higher concentration in the lung metastasis of breast cancer, resulting in enhanced antitumor efficacy [92]. Typically, drug-loaded nanoparticles must first reach the site of tumor metastasis, and then target the metastatic cancer cells. Thus, the binding affinity and specificity of the nano particles for the relevant targets as well as the physicochemical characteristics of the nanocarriers have to be considered when developing tumor metastases-targeted nanoparticles [93].
Targeting intracellular organelles
Active targeting enables drug molecules to be more efficiently transported to intracellular organelles, facilitating drug action for targeted and individualized treatments. Therefore, the controlled intracellular delivery of drugs may provide a significantly higher antitumor effect using a lower dose of drug. The intracellular distribution of drug-loaded nanoparticles can be controlled by using organelle-specific targeting ligands via endogenous intracellular trafficking mechanisms [94-96]. For example, nuclear DNA is a target of chemotherapy drugs interfering with DNA replication and synthesis, such as anthracyclines and cisplatin, however, most of the nanoparticles are localized in cytoplasmic organelles rather than the nucleus after entering the cells. Nanoparticles conjugated with both receptor-targeted and nuclear-targeted ligands have been demonstrated to achieve significantly greater nuclear accumulation in vitro [97,98].
Regardless of which target is selected for tumor targeting, only a small fraction of tumor cells or stroma can be targeted due to biological barriers and heterogeneity in the tumor mass. There are many factors that may significantly affect the targeting efficacy of tumor targeted nanoparticles, such as: 1) the number of available cell surface receptors; 2) the densities of attached ligands; 3) the orientation of the ligands and nanoparticles; 4) the chemistry of linking, which should be considered when engineering tumortargeted nanoparticles for delivery of therapeutics [8,66]. In addition to the physicochemical properties of the nanoparticles, the tumortargeting efficacy is also affected by the Mononuclear Phagocytic System (MPS) and the biological characteristics of the tumor. Thus, many different strategies can be developed to improve the tumor targeting efficacy by modulating the above factors, such as: 1) downregulating related macrophage scavenger receptors and reducing receptor recycling [99]; 2) eliminating plasma opsonins by injecting decoy particles [100]; 3) targeting biomarkers shared by the tumor cells and tumor stroma [47]; 4) co-administering nanoparticles with tumor homing and penetrating peptide [101,102]; 5) decreasing the pericyte coverage of the tumor vasculature [103]; 6) targeting multiple biomarkers simultaneously [104]; 7) developing a multistage nanoparticle system to control the size of the nanoparticles in the tumor microenvironment [105]; and 8) depleting Kupffer cells ahead of nanoparticle administration [106].
Nanomaterials and Functionalization
Clinically applicable drug delivery routes include oral, transdermal, transmuscular and intravenous, with intravenous delivery providing arguably the highest efficiency. All approaches share common challenges in overcoming physical and physiological barriers, target specific delivery, and controlled and sustained release of therapeutic agents. Among various materials developed for tumor targeted drug delivery, nanomaterials offer unique properties that can address those challenges and new capabilities to enhance tumor targeting and drug delivery efficiency. Current nanotechnology enables engineering nanoconstructs with complicated and multifunctional properties that take full advantages of the surface chemistry of nanomaterials (Figure 2). Nanoparticles have been demonstrated to significantly improve drug specificity and action. Living Radical Polymerization (LRP) techniques have been gaining interest in the field of polymeric micelle based delivery systems for cancer therapeutics and diagnostic tools. The ability to prepare complex polymeric structures with highly controlled molecular weights and defined architectures enables multiple functionalities such as hydrophobic/hydrophilic blocks needed for self-assembly, stimuli-responsive regions (responding to CO2, pH and temperature) for triggered drug release, and reactive groups for drug conjugation, cross-linking and ‘click’ chemistry [107- 109].

Figure 2: Nanoconstructs with multi-surface chemistry to serve as targeting
ligands, imaging agentsor therapeutic agents.
Nanocarriers used for drug delivery can be divided into two main families, namely organic and inorganic, according to their composition and materials. Organic nanocarriers, including liposomes, micelles, protein-based nanocarriers, DNA-RNA-based nanostructures and synthetic polymers, are mainly composed of carbon and can be biodegradable in vivo. In contrast, inorganic nanocarriers usually consist of metals or metal oxides, such as gold, silver and iron, bringing unique physical properties and other functional capabilities, such as imaging and therapeutic interventions. Each type of nanocarrier is unique in terms of composition, size, charge, shape, and interaction with the biological medium. Currently several classes of nanoparticle delivery platforms have been developed for targeted delivery and image-guided drug delivery, including liposomal micelles, polymeric nanoparticles and metal or metaloxide nanoparticles (Figure 3).
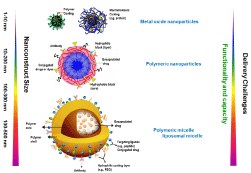
Figure 3: Classes of Targeted Nano constructs.
Classes of Nanoparticles
Advantages
Limitations
Liposomes
Structure is comparable to the phospholipid membranes of living cells;
Deliver both hydrophobic and hydrophilic drugs;
Relatively nontoxic compared to polymeric nanoparticles;
pH and temperature-sensitive liposomes can be used for controlled drug release;
Reproducibility limited;
Poor drug encapsulation and retention;
Low stability;
Aggregation and fusion;
Premature drug release due to leakage.
Micelles
Size, charge, and surface properties can easily be controlled;
Generally used for the delivery of hydrophobic drugs;
Tendency to break up upon dilution
Dendrimers
Large number of surface groups,
Multivalent interactions;
Delivery of either hydrophilic or hydrophobic drugs;
Functional groups can be tuned;
Synthesis is difficult and quite expensive;
The molecules are not well trapped;
Premature drug release;
Protein-based nanoparticles
Biocompatible;
Biodegradable;
Low toxicity;
Synthesis of uniform size of nanoparticles is difficult;
Immunogenic reactions
Iron Oxide Nanoparticles
High T2 MRI contrast agents;
Biodegradable and low toxicity;
Suitable for imaging-guided drug delivery in vivo;
Weak interaction between ligand and particle;
Easily aggregates and precipitates;
Gold Nanoparticles
Easily attached to cancer cells;
Scatter and absorb light very strongly;
Plasmonic photo-thermal therapy;
High stability in vivo;
Toxicity concerns,
Photo-thermal therapy is only applicable to superficial tumors
Quantum Dots
High levels of brightness and excellent photostability;
Multicolor QDs nanoparticles can be used for simultaneous imaging and tracking multiple tumor markers;
Simultaneous estimation of tissue drug levels and monitoring of therapeutic response in vitro;
Toxicity concerns
Carbon Nanotube
Ability to be functionalized with various moieties;
Biocompatible hollow structures with a large surface area;
High aspect ratio, with metallic or semi-metallic behavior;
Penetration into the cell;
Toxicity concerns;
Limited control over functionalized-carbon nanotube behavior;
DNA-RNA
Nanostructures
High biocompatibility;
High design capability;
High biodegradability;
Easy to tune up;
Low stability;
Genome integration risk;
Relatively high cost;
Table 3: Nanoparticles used for drug delivery.
When selecting nanoparticles for drug delivery, an ideal nanocarrier should possess the following properties: 1) no or low toxicities; 2) biocompatible and degradable; 3) easily and reproducibly prepared; 4) high drug loading efficacy; 5) controlled drug release system; 6) selective accumulation in tumor mass; 7) ability to monitor biodistribution and drug release in real-time; 8) cost-effective and easily obtained on a large scale. The structure, properties, synthesis and surface modification of such nanocarriers have been well described in many published literature including the selected references [110-121]. Here, their main advantages and limitations in their applications to drug delivery are summarized in Table 3.
The size, surface charge and shape of nanoparticles have been demonstrated to have significant effect on their biodistribution in vivo, which in turn affects their tumor accumulation capacity. Perrault, et al. investigated the tumor accumulation of gold nanoparticles with different sizes (e.g., 20, 40, 60, 80 and 100nm) but the same surface coating [122]. Gold nanoparticles of 100 nm size achieved the greatest accumulation, which was 9, 4.3, 40 and 38 times greater than that of nanoparticles sized 80, 60, 40 and 20 nm, respectively. Furthermore, tumor accumulation was significantly correlated with the blood halflife of the nanoparticles. The permeation of nanoparticles within the tumor is also size dependent, such that larger nanoparticles are mainly localized around the vasculature, while smaller nanoparticles (less than 20 nm) can diffuse throughout the tumor tissue matrix [123]. In addition, nanoparticles with relatively higher molecular weight usually have higher intra-tumoral accumulation compared to those with lower molecular weight. Nanoparticle permeation is also related to the tumor type and grade; some tumors have much more permeable vasculature than others. When the accumulation and effectiveness of drug-loaded polymeric micelles of different size (e.g.,30, 50, 70 and 100 nm) were compared in highly and poorly permeable tumors [124], the results showed that all the particles could penetrate the highly permeable tumor, while only the smaller size nanoparticles (30 nm) were able to penetrate the poorly permeable tumor. For targeting the stroma, especially for antiangiogenic therapy, relatively larger nanoparticles (60-100nm) coated with large mPEG (5 or 10 kDa) may be a better choice, while for tumor-targeted therapy, nanoparticles with a size of 20 nm may be more suitable.
Although the physicochemical properties of nanoparticles may not be directly involved in the tumor targeting process, they play key roles in the interaction of nanoparticles with the biological environment and are particularly critical to the pharmacokinetics of targeted nanoparticles in living systems. Increasing evidence over the past years suggests that the size of nanoparticles may affect their distribution in vivo. Relatively large nanoparticles (> 200 nm) are usually taken up by the RES system in liver and spleen, while smaller size particles (< 6 nm) are quickly eliminated by the kidney [125]. Nanoparticles ranging from 10 to 100 nm are considered to offer the most effective distribution in certain tissues, especially in tumors [81,126]. Nanoparticles with positive or negative charge may result in nonspecific binding and shorter blood circulation time compared to nanoparticles with neutral surfaces. The shape of nanoparticle is another important factor that affects cellular internalization, blood circulation and biodistribution of the nanoparticles in vivo. Spherical shaped nanoparticles are more easily taken up by cells than nanorods, while non-spherical nanoparticles may have longer circulation times than spherical nanoparticles [127]. Drugs requires an estimated 6 hours of blood circulation to enter cells through the EPR effect, thus, the ideal blood half-time of nanoparticles should be longer than 6 hours [128].
Surface coating and functionalization is one of the key strategies to alter and improve nanoparticle pharmacokinetics, and thus enhance targeting. PEGylation is the most common approach for coating the surface of nanoparticles to minimize or eliminate opsonization of nanoparticles, thus increase the blood circulation time of nanoparticles. The blood circulation time, organ distribution and tumor uptake and potential toxicity of nanoparticles can be regulated by adjusting PEG density, polymer chain architecture and molecular weight [112,129-132]. However, there are some drawbacks when applying PEG to the development of nanotherapeutics, such as: 1) immune reaction resulting from anti-PEG antibodies on PEG conjugates; 2) accelerated blood clearance after a second dose; and 3) relative ease of degradation compared to polymers with a carbon backbone [133,134]. To overcome these drawbacks, different synthetic polymers have been studied and used as alternative polymers to PEG in the development of nanocarriers. These include poly (glutamic acid) (PGA), poly (hydroxyethyl-l-asparagine) (PHEA), poly (hydroxyethyl-l-glutamine) (PHEG), L-poly (glycerol) (PG), poly (acrylamide) (PAAm), poly (vinylpyrrolidone) (PVP), and poly (N-(2-hydroxypropyl) methacrylamide (PHPMA) and poly (2-oxazoline) s (POx) (PEG-REPLACE) [134] Among these, the POx nanocarrierexhibits unique properties, such as: 1) excellent water solubility; 2) variation of size structure and chemical functionality; 3) high loading capacity, as much as 45 wt.% of active drug such as paclitaxel can be loaded; 4) high stability; and 5) limited complement activation [135]. It is expected that POx-based therapeutics may be moved forward to clinical trials very soon [135-137].
For tumor targeting, a lower density of the targeting ligand on the nanoparticle surface are recommended [7,138], particularly when using antibodies and proteins as targeting ligands, as early studies showed that high ligand densities may not enhance the targeting ability but rather increase the immunogenicity of nanoparticles, resulting in acceleration of their opsonization-mediated clearance [8,111]. Thus, the ligand density and surface properties should be optimized for maximum targeting effects. The number of receptors on targeted cells, the binding affinity of ligands to receptors and the molecular weight and size of ligands should be considered when determining the ratio of ligands and nanoparticles.
Stimuli-responsive delivery is one of the interesting systems, which is designed in response to external stimuli (e.g., pH, light, and magnetic field). Here, the drug release is triggered only at the desired time and location. When exposed under external stimuli, the polymers will undergo physiochemical structural changes, and therefore lose the well-defined nanoarchitectures releasing drugs directly onto the tumor cells. For example, pH gradients have been widely used for controlled release, relying on the abnormally low pH of endosomes or in tumor cells compared with healthy cells [139-143].
More recently, multifunctional targeted nanoparticles have become increasingly attractive for various imaging applications. Taking advantage of their abundant surface area, accommodation of multiple moieties of targeting ligands and drugs andutilization of physical properties in imaging, such nanoconstructs enable imageassisted delivery to monitor the accumulation, retention and clearance of the delivery vehicle in real time, and to potentially quantify the drug delivery efficiency (Figure 4).
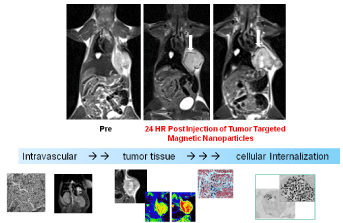
Figure 4: Targeted delivery of nanoconstructs and monitoring their
movements from intravascular, to tumor sites via internalization into tumor
cells demonstrated by imaging.
Translation to Clinical Applications
More than 50 anti-cancer drugs are commonly used for cancer treatment at present. In general, drugs such as docetaxel/paclitaxel, doxorubicin and platinum-drugs are widely used in the clinic but with severe side effects. Small molecules, such as small interfering RNA (siRNA) and nucleic acids that may have high anti-cancer efficacy are promising candidates for delivery by a nanocarrier system. Since the first nano-drug Doxil® was approved by the FDA in 1995 [11], more than 40 different targeted and non-targeted nanoparticle therapeutics have reached the market or are in clinical development. These are mainly based on liposome, micelle, and polymeric nanoparticle delivery systems, and promising clinical results have been obtained with several new agents [8,114,144-146].
As shown in Table 4, most of the first generation nanotherapeutics are non-tumor targeted drugs. The enhanced tumor accumulation of drugs results mainly from the EPR effect with additional contributions from changes in the PK/PD of loaded drugs. However, the antitumor efficacy is limited in clinical applications due to the lack of active targeting. To date, five tumor-targeted nanotherapeutics have been developed for clinical applications, which pave the way for further development of targeted nanoparticle delivery systems. Preclinical studies have demonstrated these tumor-targeted nanotherapeutics to have significantly enhanced antitumor effects and to be better tolerated than non-targeted nanotherapeutics and free drugs at the equivalent doses, and Phase I studies have further demonstrated the prolongation of circulation time and active targeting to the tumor [147,148,149]. The potential use of RNAi specific for important cancer-related genes has several implications. Systemic administration of siRNA by nanoparticles has opened new windows for clinical use. Several promising siRNA delivery nanoformulations [150-154] are under clinical trials and are listed in Table 4. CALAA-01 is the first tumor-targeted nanoparticle to be used to deliver siRNA in humans [64,15]. Analysis of tumor samples from patients treated with CALAA-01 showed that the nanoparticles were internalized into the tumor cells and delivered to the specific targeted site for cleaving the mRNA in vivo [155]. This study demonstrated the feasibility of using tumor-targeted nanoparticles as a carrier to selectively deliver antitumor agents to their targeted locations in vivo. Further results such as the antitumor effects are expected to be obtained in the future. In our own study, when applying CALAA-01 in a xenograft mouse model of head and neck cancer, we observed that tumor progression was suppressed significantly by decreasing cell proliferation and inducing apoptosis [156].
Nano-formulations
Payload Drug
Product or Commercial Name
Indication
Development Status
Non-targeted nanotherapeutic
Liposomes
Doxorubicin
Doxil/Caelyx
Breast cancer, ovarian cancer, multiple myeloma, Kaposi’s sarcoma
Approved
Myocet
Breast cancer
Approved
MCC-465
Gastric cancer
Phase I
ThermoDox
Liver cancer, breast cancer
Phase III
Daunorubicin
DaunoXome
Kaposi’s sarcoma
Approved
Cytarabine
DepoCyt
Lymphomatus meningitis, leukaemia, glioblastoma
Approved
Cisplatin
SPI-077
Head and neck cancer, lung cancer
Phase II
Lipoplatin
Various solid cancer
Phase III
CKD-602
S-CKD602
Various solid cancer
Phase I/II
NL CPT-11
Irinotecan (CPT-11)
Glioma
Phase I
CPX-1
Irinotecan
Colorectal cancer
Phase II
Paclitaxel
Endo-Tag-1
Triple negative breast cancer
phase II
Topotecan
Brakiva
Relapsed solid cancer
Phase I
Vinorelbine
Alocrest™
Newly diagnosed or relapsed solid cancer
Phase I
LE-SN38
SN-38
Colorectal cancer
Phase II
Micelles
Paclitaxel
Genexol-PM
Breast cancer, lung cancer, ovarian cancer
Approved
NK105
Gastric cancer
Phase II
Paclical
Ovarian cancer
Phase III
Doxorubicin
NK911
Various solid cancer
Phase III
SP1049C
Various cancers
Phase II
SN-38
NK012
Various solid cancer
Phase II
Oxaliplatin
NC-4016
Various solid cancer
Phase I
Protein-based nanoparticles
L-asparaginase
Oncaspar
Leukaemia
Approved
Doxorubicin
PK1
Breast cancer, lung cancer, colorectal cancer
Phase II
DOX-OXD
Various cancers
Phase I
PK2
Hepatocellular carcinoma
phase I/II
Paclitaxel
Abraxane
Breast cancer
Approved
Opaxio
Lung cancer, ovarian cancer
Phase III
Docetaxel
Docetaxel-PNP
Various solid cancer
Phase I
Platinum
AP5280
Various solid cancer
Phase II
Oxaliplatin
ProLindac
Ovarian cancer
Phase II
Methotrexate
MTX-HSA
Kidney cancer
Phase II
Camptothecin
CRLX101
Various malignancies
Phase II
XMT-1001
Gastric cancer, lung cancer
Phase I
Pegamotecan
Gastric cancer
Phase II
Irinotecan
NKTR-102
Breast cancer, ovarian cancer, colorectal cancer
Phase III
DX-8951
DE-310
Various cancers
Phase I/II
RNAi-based
nanoparticles
VEGF and KSP
siRNA
ALN-VSP
Solid tumors
Phase I
PKN3 siRNA
Atu027
Advanced solid cancer
Phase I/II
PLK1 siRNA
TKM-080301
Hepatocellular carcinoma
Phase I/II
BCL2 siRNA
PNT2258
Lymphoma
Phase I/II
KRAS siRNA
siG12D LODER
Pancreatic cancer
Phase I
eIF5A siRNA
SNS01-T
Multiple myeloma, leukemia
Phase II
miR-34 siRNA
MRX34
Liver cancer, small cell lung cancer, lymphoma, melanoma, multiple myeloma, renal cell carcinoma, non-small cell lung cancer
Phase I
MYC siRNA
DCR-MYC
Solid tumor, multiple myeloma, non-hodgkins lymphoma, pancreatic neuroendocrine tumor
Phase I
EPHA2 siRNA
siRNA-EPHA2-DOPC
Advanced cancer
Phase I
Tumor-targeted nanotherapeutics
Nano-formulations
Payload Drug
Product or Commercial Name
Target
Indication
Status
Liposome
p53 gene
SGT53-01
Transferrin receptor
Solid tumors
Phase I
Doxorubicin
MCC-465
Tumor antigen
Metastatic stomach cancer
Phase I
Oxaliplatin
MBP-426
Transferrin receptor
Various cancers
Phase I
Protein-based nanoparticles
Docetaxel
BIND-014
PSMA
Various cancers
Phase I
RNAi-based
nanoparticles
RRM2 siRNA
CALAA-01
Transferrin receptor
Solid tumors
Phase I
Table 4: Nanotherapeutics approved and in clinical trials.
Despite this progress, it remains challenging to move most tumor-targeted nanoparticles forward to clinical applications, mainly due to the lack of a “standardized system” to optimize the properties of tumor-targeted nanoparticles, such as highly effective tumortargeting, evasion of the immune system and controlled drug release. In addition, the formulation of nanoparticles through a multi-step synthesis process may produce a large number of nanoparticles with varying physicochemical properties, such as size, charge, ligand density and drug loading efficacy. Since these physical and biological properties may affect the in vivo fate of nanoparticles and treatment outcomes, such variations can lead to irreproducible results. Taking this into consideration, one of the successful examples is the development of Bind-014, which was selected from a combinatorial library of more than 100 tumor-targeted nanoparticles formulations from a reproducible, scalable, one-step self-assembly process [148].
Challenges and Future Directions
With unique chemical and physical properties of nanomaterials and their advantages in biomarker targeting and drug delivery, it is anticipated that more and variety of engineered nanomaterials will be developed for biomedical applications in the new era of the precision and personalizaed medicine in which targeting and tumor specific delivery will play critical roles. However, the future material innovation and development need to overcome several major challenges that have been learned in the past decade.
Improving the delivery efficiency
The engineered nanoparticles developed for in vivo applications in imaging and drug delivery are subject to closely interact with a range of physiological environments and various biological materials that can alter the properties and functions of the nanocarrierdrug complex and induce the responses from the biological systems, including the immune system [157]. These interactions can affect pharmacokinetic parameters and toxicity profiles of the nanoparticles and carried drugs which may lead to significant clinical consequences. It is now well recognized that whether are composed of polymeric, liposomal or metal oxide materials, are rapidly cleared by the Mononuclear Pagocyte System (MPS) after being captured and engulfed by phagocytic cells, such as hepatic Kupffer cells and splenic macrophages once intravenously administered. It has been reported that the peripheral blood monocyte count and phagocytic function have been shown to correlate with nanocarrier clearance rates with pegylated liposomal formulations (S-CKD-602, and SPI- 077) in preclinical rodent and canine models [158] and similarly in patients [159]. Thus uptake and sequestration of nanoparticles in cells and organs of the MPS is a major barrier limiting the circulation half-life. Furthermore, engineered nano-carriers can quickly absorb serum proteins in the blood, including IgG, IgM, promoting the opsonization of the carrier and clearance by the MPS. Therefore, the nanocarrier-drug complex delivered to tumors is often below 5% of the administered doses. While pegylation or coating of nanoparticles with poly-ethylene glycol (PEG) is the most common strategy used to reduce opsonization, improve stability in plasma, and prolong circulation time, the results have been not satisfactory. In addition, recent studies have shown that PEG is not immunologically inert [160,161]. Clinically, it was shown that PLD activates complement in the peripheral blood of cancer patients and that the extent of complement activation correlated with the development of acute infusion reactions [160]. Administration of pegylated nanoparticles induces production of anti-PEG IgM antibodies that enhance immune recognition and clearance of the second dose of nanoparticles [162]. Therefore, undesired interactions with circulating serum proteins can affect the pharmacokinetics and tolerability of carriermediated drugs. There is a need of careful investigation and better understanding of nanoparticle interactions with biological media and clearance mechanism to guide the development of novel and improved nanoparticle coating to address this critical shortcoming of the conventional PEG coating.
Efforts have also been directed to develop ultra-small nanoparticles that have sizes below the 10 nm to improve the delivery as these sub-10 nm nanoparticles may have more efficient extravasation with enhanced EPR effect [163, 164] and less hindrance to reach the tumors and are less susceptible to the MPS clearance [165,166]. Therefore, new synthetic and size control approaches for preparing and functionalizing these ultra-small nanoparticles are needed, although the issues of filtration through kidney tubules need to be further studied.
Improving specificity and targeting efficiency
As targeting with high specificity remains to be a major area of focus in developing nanomaterials for precision medicine and biomarker specific treatment, the current efforts in discovering and validating new biomarkers, developing new targeting ligands and chemistry for surface functionalization may also need to consider other factors important to the passive and active targeting of the tumors. One issue that has increasingly recognized is that the nonspecific interactions between nanoparticles and biological materials as discussed above also leads to the changes of nanoparticle surface and functional properties [167]. The interactions of nanoparticles with biomacromolecules, such as proteins in blood, interstitial fluid, and cellular cytoplasm after systemic administration cause rapid nonspecific adsorption of these proteins, leading to the formation of a protein coating, also known as corona, on the nanoparticle surface. The presence of protein corona is responsible for attenuation of the targeting specificity [168] in addition to promoting the fast clearance of nanoparticles by the MPS and RES. The surface absorption of biomacromolecues and non-specific uptake of normal tissue and cells, as known as biofouling effect, results in the substantial reduction in targeting efficiency as the protein corona can cover the functional moiety or targeting ligands on the nanoparticle surfaces. Given the immunogenicity concern of PEG and its less optimal performance in reducing protein corona formation, other coating materials, such as polysaccharides, and zwitterionic polymers, have been investigated to provide antifouling capability to limit the protein corona [169-171]. Recently, Li, et al. [172] developed an antifouling amphiphilic diblock coating polymer that incorporates PEG chain with NH2 groups for surface functionalization and hydrophobic allyl glycidyl ether (AGE) moieties that provide a control over hydrophobic percentage and ligand density of the coating polymer with allyl groups of AGE used for conjugating a variety of functional moieties. This PEG-b-AGE copolymer was used for coating iron oxide nanoparticles (IONPs) to demonstrate the excellent anti-fouling effect in preventing the formation of protein corona and the nonspecific uptake by a variety of cell lines, including macrophages. The targeting ligand modified PEG-b-AGE polymer coated IONP showed excellent targeting specificity with improved targeting efficiency as the result of reduced non-specific interactions and formation of the protein corona. It is expected that the new approaches and materials that enable not only targeting but also reducing the non-specificity will gain more attentions in the future as provide the benefits of both improved targeting and low non-specific uptake of normal tissues and organs with reduced toxicity.
Reducing toxicity
It is widely recognized that the physical and chemical properties of a nanomaterial directly influence a variety of functional performances and outcomes, including biodistribution, clearance, and immunotoxicity [173-175]. To date, the toxicity of tumortargeted nanoparticles in vivo has not been as intensively studied as the antitumor efficacy. Nanoparticles have been reported to be able to stimulate and/or suppress immune responses by binding to proteins in the blood, and to affect the physiology and interrupt the structure of organs and tissues, which may lead to side-effects [133,146,176]. In addition, only a small fraction of the injected nanoparticles actually accumulate in the tumor (1–10%) [125], no matter which type of tumor-targeted nanoparticles are used. The majority of the injected dose iscaptured in the liver and spleen, thus the long-term fate of the nanoparticles and the effects of such accumulation on the body remain unknown. Our ongoing study showed that about 70% of injected gold nanorods still remain in the liver and spleen up to 15 months after treatment. A complicating factor is that even for the same kind of nanoparticle, different research groups perform their toxicity studies independently, in which the doses and method of administration, time of sample collection for pharmacokinetic analysis, toxicity evaluation and methods used for analyzing samples may vary, yielding results that are inconsistent or even controversial. As mentioned before, surface properties such as size, charge, PEG coating, and ligand density, may have significant effects on the biodistribution of nanoparticles in vivo, which may also introduce different toxicity effects. Furthermore, the animal models used in most current toxicity studies are inappropriate for addressing some toxicity questions. White rodent models are the most conventional models used for toxicity studies, studies in larger animals and nonhuman primates would allow the more accurate evaluation of toxicity.
Optimizing formulation
Contamination and standardization are critical issues in the synthesis of nanoparticles for human applications and should be considered before the conduct of preclinical studies. The NIH Nanotechnology Characterization Laboratory (NCL) reported that almost half of the 75 nanoparticles submitted to them have been contaminated with bacteria or high levels of endotoxin, which may come from the synthesis and purification procedure [146]. Such contamination could have significant effects on the biodistribution, toxicity, and immunological profile in vivo, and lead to improper study results. In addition, residual manufacturing components in the final product may also contribute to side effects [146]. Thus, the synthesis and purification of nanoparticles must be performed under sterile conditions, and all of the unwanted materials must be removed before preclinical studies are initiated. Resources for scaleup, standardization and GMP manufacture of nanomedicines remain a vital area of further development to address this critical gap in the translation of nanomaterials for biomedical applications.
Other outstanding concerns
There are still many obstacles to overcome when constructing nanoparticles for drug delivery, which include: 1) nanotherapeutic stability; 2) nanotherapeutic reproducibility; 3) specific accumulation in the tumor but minimal uptake in normal tissue and organs through the selection of ideal tumor-targeted ligands; 4) selection of approproiate nanoparticles for particular drug delivery; 5) preclinical pharmacokinetic/pharmacodynamic (PK/PD) evaluation of nanotherapeutic; 6) optimization of the dosing schedule;7) selection of tumor model (subcutaneous, orthotopic, metastatic tumor) for evaluating the efficacy of nanoparticles; and 8) regulatory and approval issues of nanoparticles.
In recent years, significant progress has been made in the field of nanotechnology and nanoparticles. Encouraged by the clinical study of several tumor-targeted nanotherapeutic agents, we believe that increasing numbers of tumor-targeted nanoparticles will be applied in the clinic and potentially being recognized as a unique entity among therapeutics in the following decades. Tumor-targeted nanoparticle agents have great potential to enhance antitumor efficacy and reduce the side effects of the delivered drugs, thus increasing the survival for patients with many types of cancer.
References
- Schwartz RS. Paul Ehrlich's magic bullets. N Engl J Med. 2004; 350: 1079-1080.
- Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc Chem Res. 2011; 44: 1061-1070.
- Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012; 338: 903-910.
- Li Y, Lin TY, Luo Y, Liu Q, Xiao W, Guo W, et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat Commun. 2014; 5: 4712.
- Lukianova-Hleb EY, Ren X, Sawant RR, Wu X, Torchilin VP, Lapotko DO. On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nat Med. 2014; 20: 778-784.
- Yaari Z, da Silva D, Zinger A, Goldman E, Kajal A, Tshuva R, et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat Commun. 2016; 7: 13325.
- Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 2010; 107: 1235-1240.
- Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012; 41: 2971-3010.
- Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007; 9: 257-288.
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012; 63: 185-198.
- Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012; 160: 117-134.
- Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005; 23: 7794-7803.
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986; 46: 6387-6392.
- Chan E, Wang H. Introduction for Design of Nanoparticle Based Drug Delivery Systems. Curr Pharm Des. 2016.
- Bae YH. Drug targeting and tumor heterogeneity. J Control Release. 2009; 133: 2-3.
- Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006; 66: 6732-6740.
- Huang X, Peng X, Wang Y, Shin DM, El-Sayed MA, Nie S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano. 2010; 4: 5887-5896.
- Christie RJ, Matsumoto Y, Miyata K, Nomoto T, Fukushima S, Osada K, et al. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano. 2012; 6: 5174-5189.
- Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006; 103: 6315-6320.
- Peng XH, Wang Y, Huang D, Shin HJ, Chen Z, Spewak MB, et al. Targeted delivery of cisplatin to lung cancer using ScFvEGFR-heparin-cisplatin nanoparticles. ACS Nano. 2011; 5: 9480-9493.
- Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci U S A. 2008; 105: 17356-17361.
- Liang X, Shi B, Wang K, Fan M, Jiao D, Ao J, et al. Development of self-assembling peptide nanovesicle with bilayers for enhanced EGFR-targeted drug and gene delivery. Biomaterials. 2016; 82: 194-207.
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995; 19: 183-232.
- Wang X, Li J, Wang Y, Koenig L, Gjyrezi A, Giannakakou P, et al. A folate receptor-targeting nanoparticle minimizes drug resistance in a human cancer model. ACS Nano. 2011; 5: 6184-6194.
- Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005; 338: 284-293.
- Ryschich E, Huszty G, Knaebel HP, Hartel M, Buchler MW, Schmidt J. Transferrin receptor is a marker of malignant phenotype in human pancreatic cancer and in neuroendocrine carcinoma of the pancreas. Eur J Cancer. 2004; 40: 1418-1422.
- Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006; 121: 144-158.
- Yang L, Peng XH, Wang YA, Wang X, Cao Z, Ni C, et al. Receptor-targeted nanoparticles for in vivo imaging of breast cancer. Clin Cancer Res. 2009; 15: 4722-4732.
- Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007; 50: 472-483.
- Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997; 3: 81-85.
- Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004; 122: 61-69.
- Sadeqzadeh E, Rahbarizadeh F, Ahmadvand D, Rasaee MJ, Parhamifar L, Moghimi SM. Combined MUC1-specific nanobody-tagged PEG-polyethylenimine polyplex targeting and transcriptional targeting of tBid transgene for directed killing of MUC1 over-expressing tumour cells. J Control Release. 2011; 156: 85-91.
- Formentini A, Braun P, Fricke H, Link KH, Henne-Bruns D, Kornmann M. Expression of interleukin-4 and interleukin-13 and their receptors in colorectal cancer. Int J Colorectal Dis. 2012; 27: 1369-1376.
- Kioi M, Takahashi S, Kawakami M, Kawakami K, Kreitman RJ, Puri RK. Expression and targeting of interleukin-4 receptor for primary and advanced ovarian cancer therapy. Cancer Res. 2005; 65: 8388-8396.
- Kawakami M, Kawakami K, Kasperbauer JL, Hinkley LL, Tsukuda M, Strome SE, et al. Interleukin-13 receptor alpha2 chain in human head and neck cancer serves as a unique diagnostic marker. Clin Cancer Res. 2003; 9: 638-6388.
- Xiang ZL, Zeng ZC, Tang ZY, Fan J, Zhuang PY, Liang Y, et al. Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer. 2009; 9: 176.
- Liu Y, Ji R, Li J, Gu Q, Zhao X, Sun T, et al. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res. 2010; 29: 16.
- Fischer T, Nagel F, Jacobs S, Stumm R, Schulz S. Reassessment of CXCR4 chemokine receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-2. PLoS One. 2008; 3: e4069.
- Allum WH, Stokes HJ, Macdonald F, Fielding JW. Demonstration of carcinoembryonic antigen (CEA) expression in normal, chronically inflamed, and malignant pancreatic tissue by immunohistochemistry. J Clin Pathol. 1986; 39: 610-614.
- Wang J, Ma Y, Zhu ZH, Situ DR, Hu Y, Rong TH. Expression and prognostic relevance of tumor carcinoembryonic antigen in stage IB non-small cell lung cancer. J Thorac Dis. 2012; 4: 490-496.
- Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol. 2000; 163: 623-629.
- Wu Y, Wu PY. CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev. 2009; 18: 1127-1134.
- Marhaba R, Klingbeil P, Nuebel T, Nazarenko I, Buechler MW, Zoeller M. CD44 and EpCAM: cancer-initiating cell markers. Curr Mol Med. 2008; 8: 784-804.
- Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res. 2010; 16: 3548-3561.
- Graf N, Bielenberg DR, Kolishetti N, Muus C, Banyard J, Farokhzad OC, et al. alpha(V)beta(3) integrin-targeted PLGA-PEG nanoparticles for enhanced anti-tumor efficacy of a Pt (IV) prodrug. ACS Nano. 2012; 6: 4530-4539.
- Hofmeister V, Schrama D, Becker JC. Anti-cancer therapies targeting the tumor stroma. Cancer Immunol Immunother. 2008; 57: 1-17.
- Park S, Kang S, Chen X, Kim EJ, Kim J, Kim N, et al. Tumor suppression via paclitaxel-loaded drug carriers that target inflammation marker upregulated in tumor vasculature and macrophages. Biomaterials. 2013; 34: 598-605.
- Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010; 188: 759-768.
- Abedi-Ardekani B, Dar NA, Mir MM, Zargar SA, Lone MM, Martel-Planche G, et al. Epidermal growth factor receptor (EGFR) mutations and expression in squamous cell carcinoma of the esophagus in central Asia. BMC Cancer. 2012; 12: 602.
- Buckley AF, Burgart LJ, Sahai V, Kakar S. Epidermal growth factor receptor expression and gene copy number in conventional hepatocellular carcinoma. Am J Clin Pathol. 2008; 129: 245-251.
- Dordevic G, Matusan Ilijas K, Hadzisejdic I, Maricic A, Grahovac B, Jonjic N. EGFR protein overexpression correlates with chromosome 7 polysomy and poor prognostic parameters in clear cell renal cell carcinoma. J Biomed Sci. 2012; 19: 40.
- Wang H, Liu C, Han J, Zhen L, Zhang T, He X, et al. HER2 expression in renal cell carcinoma is rare and negatively correlated with that in normal renal tissue. Oncol Lett. 2012; 4: 194-198.
- Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009; 13: 256-262.
- Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010; 53: 6811-6824.
- Habashy HO, Powe DG, Staka CM, Rakha EA, Ball G, Green AR, et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat. 2010; 119: 283-293.
- Sciot R, Paterson AC, van Eyken P, Callea F, Kew MC, Desmet VJ. Transferrin receptor expression in human hepatocellular carcinoma: an immunohistochemical study of 34 cases. Histopathology. 1988; 12: 53-63.
- Koller FL, Hwang DG, Dozier EA, Fingleton B. Epithelial interleukin-4 receptor expression promotes colon tumor growth. Carcinogenesis. 2010; 31: 1010-1017.
- Lee HJ, Kim SW, Kim HY, Li S, Yun HJ, Song KS, et al. Chemokine receptor CXCR4 expression, function, and clinical implications in gastric cancer. Int J Oncol. 2009; 34: 473-480.
- Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol. 2006; 103: 226-233.
- Chung TH, Hsiao JK, Hsu SC, Yao M, Chen YC, Wang SW, et al. Iron oxide nanoparticle-induced epidermal growth factor receptor expression in human stem cells for tumor therapy. ACS Nano. 2011; 5: 9807-9816.
- Brave SR, Odedra R, James NH, Smith NR, Marshall GB, Acheson KL, et al. Vandetanib inhibits both VEGFR-2 and EGFR signalling at clinically relevant drug levels in preclinical models of human cancer. Int J Oncol. 2011; 39: 271-278.
- Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, et al. cRGD-functionalized, DOX-conjugated, and (6)(4)Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials. 2011; 32: 4151-4160.
- Mickler FM, Mockl L, Ruthardt N, Ogris M, Wagner E, Brauchle C. Tuning nanoparticle uptake: live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012; 12: 3417-3423.
- Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009; 6: 659-668.
- Vlahov IR, Leamon CP. Engineering folate-drug conjugates to target cancer: from chemistry to clinic. Bioconjug Chem. 2012; 23: 1357-1369.
- Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012; 2: 3-44.
- Kanwar JR, Mohan RR, Kanwar RK, Roy K, Bawa R. Applications of aptamers in nanodelivery systems in cancer, eye and inflammatory diseases. Nanomedicine (Lond). 2010; 5: 1435-1445.
- Xiao K, Li Y, Lee JS, Gonik AM, Dong T, Fung G, et al. "OA02" peptide facilitates the precise targeting of paclitaxel-loaded micellar nanoparticles to ovarian cancer in vivo. Cancer Res. 2012; 72: 2100-2110.
- Creixell M, Bohorquez AC, Torres-Lugo M, Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano. 2011; 5: 7124-7129.
- Karra N, Benita S. The ligand nanoparticle conjugation approach for targeted cancer therapy. Curr Drug Metab. 2012; 13: 22-41.
- Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci U S A. 2008; 105: 9343-9348.
- Fokong S, Theek B, Wu Z, Koczera P, Appold L, Jorge S, et al. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J Control Release. 2012; 163: 75-81.
- Galloway AL, Murphy A, Rolland JP, Herlihy KP, Petros RA, Napier ME, et al. Micromolding for the fabrication of biological microarrays. Methods Mol Biol. 2011; 671: 249-260.
- Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci U S A. 2002; 99: 12617-12621.
- Messerschmidt SK, Musyanovych A, Altvater M, Scheurich P, Pfizenmaier K, Landfester K, et al. Targeted lipid-coated nanoparticles: delivery of tumor necrosis factor-functionalized particles to tumor cells. J Control Release. 2009; 137: 69-77.
- Landen CN Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010; 9: 3186-3199.
- Cui Y, Zhang M, Zeng F, Jin H, Xu Q, Huang Y. Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy. ACS Appl Mater Interfaces. 2016.
- Li S, Amat D, Peng Z, Vanni S, Raskin S, De Angulo G, et al. Transferrin conjugated nontoxic carbon dots for doxorubicin delivery to target pediatric brain tumor cells. Nanoscale. 2016; 8: 16662-16669.
- Gelperina S, Maksimenko O, Khalansky A, Vanchugova L, Shipulo E, Abbasova K, et al. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur J Pharm Biopharm. 2010; 74: 157-163.
- Zhang P, Hu L, Yin Q, Zhang Z, Feng L, Li Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: synthesis, preparation and in vivo evaluation. J Control Release. 2012; 159: 429-434.
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008; 7: 771-782.
- Shapira A, Assaraf YG, Epstein D, Livney YD. Beta-casein nanoparticles as an oral delivery system for chemotherapeutic drugs: impact of drug structure and properties on co-assembly. Pharm Res. 2010; 27: 2175-2186.
- Cavaco M, Pereira C, Kreutzer B, Gouveia L, Silva-Lima B, Brito MA, et al. Evading P-glycoprotein mediated-efflux chemoresistance using Solid Lipid Nanoparticles. Eur J Pharm Biopharm. 2016.
- Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007; 99: 1441-1454.
- Chow EK, Zhang XQ, Chen M, Lam R, Robinson E, Huang H, et al. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med. 2011; 3: 73ra21.
- Milane L, Duan Z, Amiji M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol Pharm. 2011; 8: 185-203.
- Dong X, Mattingly CA, Tseng MT, Cho MJ, Liu Y, Adams VR, et al. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009; 69: 3918-3926.
- Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009; 136: 21-29.
- Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008; 5: 206-219.
- O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004; 15: 440-449.
- Kievit FM, Stephen ZR, Veiseh O, Arami H, Wang T, Lai VP, et al. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano. 2012; 6: 2591-2601.
- Wang Z, Yu Y, Dai W, Cui J, Wu H, Yuan L, et al. A specific peptide ligand-modified lipid nanoparticle carrier for the inhibition of tumor metastasis growth. Biomaterials. 2013; 34: 756-764.
- Heller DA, Levi Y, Pelet JM, Doloff JC, Wallas J, Pratt GW, et al. Modular 'Click-in-Emulsion' bone-targeted nanogels. Adv Mater. 2012.
- Kim JH, Bae SM, Na MH, Shin H, Yang YJ, Min KH, et al. Facilitated intracellular delivery of peptide-guided nanoparticles in tumor tissues. J Control Release. 2012; 157: 493-499.
- Boddapati SV, D'Souza GG, Erdogan S, Torchilin VP, Weissig V. Organelle-targeted nanocarriers: specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett. 2008; 8: 2559-2563.
- Kang B, Mackey MA, El-Sayed MA. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J Am Chem Soc. 2010; 132: 1517-1519.
- Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, et al. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J Am Chem Soc. 2003; 125: 4700-4701.
- Balasubramanian S, Kagan D, Hu CM, Campuzano S, Lobo-Castanon MJ, Lim N, et al. Micromachine-enabled capture and isolation of cancer cells in complex media. Angew Chem Int Ed Engl. 2011; 50: 4161-4164.
- Rogers WJ, Basu P. Factors regulating macrophage endocytosis of nanoparticles: implications for targeted magnetic resonance plaque imaging. Atherosclerosis. 2005; 178: 67-73.
- Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci U S A. 2007; 104: 932-936.
- Chen AM, Taratula O, Wei D, Yen HI, Thomas T, Thomas TJ, et al. Labile catalytic packaging of DNA/siRNA: control of gold nanoparticles "out" of DNA/siRNA complexes. ACS Nano. 2010; 4: 3679-3688.
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009; 16: 510-520.
- Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A. 2007; 104: 3460-3465.
- Kluza E, van der Schaft DW, Hautvast PA, Mulder WJ, Mayo KH, Griffioen AW, et al. Synergistic targeting of alphavbeta3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesis. Nano Lett. 2010; 10: 52-58.
- Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011; 108: 2426-2431.
- Ohara Y, Oda T, Yamada K, Hashimoto S, Akashi Y, Miyamoto R, et al. Effective delivery of chemotherapeutic nanoparticles by depleting host Kupffer cells. Int J Cancer. 2012; 131: 2402-2410.
- Isse AA, Gennaro A, Lin CY, Hodgson JL, Coote ML, Guliashvili T. Mechanism of carbon-halogen bond reductive cleavage in activated alkyl halide initiators relevant to living radical polymerization: theoretical and experimental study. J Am Chem Soc. 2011; 133: 6254-6264.
- Balaure PC, Grumezescu AM. Methods for synthesizing the macromolecular constituents of smart nanosized carriers for controlled drug delivery. Curr Med Chem. 2014; 21: 3333-3374.
- Kapishon V, Whitney RA, Champagne P, Cunningham MF, Neufeld RJ. Polymerization Induced Self-Assembly of Alginate Based Amphiphilic Graft Copolymers Synthesized by Single Electron Transfer Living Radical Polymerization. Biomacromolecules. 2015; 16: 2040-2048.
- Abdalla MO, Karna P, Sajja HK, Mao H, Yates C, Turner T, et al. Enhanced noscapine delivery using uPAR-targeted optical-MR imaging trackable nanoparticles for prostate cancer therapy. J Control Release. 2011; 149: 314-322.
- Agasti SS, Liong M, Tassa C, Chung HJ, Shaw SY, Lee H, et al. Supramolecular host-guest interaction for labeling and detection of cellular biomarkers. Angew Chem Int Ed Engl. 2012; 51: 450-454.
- Joralemon MJ, McRae S, Emrick T. PEGylated polymers for medicine: from conjugation to self-assembled systems. Chem Commun (Camb). 2010; 46: 1377-1393.
- Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012; 41: 2740-2779.
- Egusquiaguirre SP, Igartua M, Hernandez RM, Pedraz JL. Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin Transl Oncol. 2012; 14: 83-93.
- Smith AM, Ruan G, Rhyner MN, Nie S. Engineering luminescent quantum dots for in vivo molecular and cellular imaging. Ann Biomed Eng. 2006; 34: 3-14.
- Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res. 2008; 41: 60-68.
- An P, Wu Q, Wang H, Guan Y, Mu M, Liao Y, et al. TMPRSS6, but not TF, TFR2 or BMP2 variants are associated with increased risk of iron-deficiency anemia. Hum Mol Genet. 2012; 21: 2124-2131.
- Lee H, Lytton-Jean AK, Chen Y, Love KT, Park AI, Karagiannis ED, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012; 7: 389-393.
- Chen YJ, Groves B, Muscat RA, Seelig G. DNA nanotechnology from the test tube to the cell. Nat Nanotechnol. 2015; 10: 748-760.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007; 2: 751-760.
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010; 9: 615-627.
- Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009; 9: 1909-1915.
- Waite CL, Roth CM. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities. Crit Rev Biomed Eng. 2012; 40: 21-41.
- Baba M, Matsumoto Y, Kashio A, Cabral H, Nishiyama N, Kataoka K, et al. Micellization of cisplatin (NC-6004) reduces its ototoxicity in guinea pigs. J Control Release. 2012; 157: 112-117.
- Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012; 14: 1-16.
- Davis ME, Pun SH, Bellocq NC, Reineke TM, Popielarski SR, Mishra S, et al. Self-assembling nucleic acid delivery vehicles via linear, water-soluble, cyclodextrin-containing polymers. Curr Med Chem. 2004; 11: 179-197.
- Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007; 7: 1542-1550.
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001; 41: 189-207.
- Akiyama Y, Mori T, Katayama Y, Niidome T. The effects of PEG grafting level and injection dose on gold nanorod biodistribution in the tumor-bearing mice. J Control Release. 2009; 139: 81-84.
- van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res. 2007; 24: 1405-1414.
- Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, et al. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012; 12: 5304-5310.
- Cruz LJ, Tacken PJ, Bonetto F, Buschow SI, Croes HJ, Wijers M, et al. Multimodal imaging of nanovaccine carriers targeted to human dendritic cells. Mol Pharm. 2011; 8: 520-531.
- Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007; 2: 469-478.
- Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010; 49: 6288-6308.
- Barz M, Luxenhofer R, Zentel R, Kabanov AV. The uptake of N-(2-hydroxypropyl)-methacrylamide based homo, random and block copolymers by human multi-drug resistant breast adenocarcinoma cells. Biomaterials. 2009; 30: 5682-5690.
- Luxenhofer R, Han Y, Schulz A, Tong J, He Z, Kabanov AV, et al. Poly(2-oxazoline)s as polymer therapeutics. Macromol Rapid Commun. 2012; 33: 1613-1631.
- Luxenhofer R, Sahay G, Schulz A, Alakhova D, Bronich TK, Jordan R, et al. Structure-property relationship in cytotoxicity and cell uptake of poly(2-oxazoline) amphiphiles. J Control Release. 2011; 153: 73-82.
- Mullen DG, Borgmeier EL, Fang M, McNerny DQ, Desai A, Baker JR, Jr., et al. Effect of mass transport in the synthesis of partially acetylated dendrimer: implications for functional ligand-nanoparticle distributions. Macromolecules. 2010; 43: 6577-6587.
- Rejinold NS, Thomas RG, Muthiah M, Lee HJ, Jeong YY, Park IK, et al. Breast Tumor Targetable Fe3O4 Embedded Thermo-Responsive Nanoparticles for Radiofrequency Assisted Drug Delivery. J Biomed Nanotechnol. 2016; 12: 43-55.
- Gao W, Chan JM, Farokhzad OC. pH-Responsive nanoparticles for drug delivery. Mol Pharm. 2010; 7: 1913-1920.
- Henry SM, El-Sayed ME, Pirie CM, Hoffman AS, Stayton PS. pH-responsive poly(styrene-alt-maleic anhydride) alkylamide copolymers for intracellular drug delivery. Biomacromolecules. 2006; 7: 2407-2414.
- Lale SV, R GA, Aravind A, Kumar DS, Koul V. AS1411 aptamer and folic acid functionalized pH-responsive ATRP fabricated pPEGMA-PCL-pPEGMA polymeric nanoparticles for targeted drug delivery in cancer therapy. Biomacromolecules. 2014; 15: 1737-1752.
- Li X, McTaggart M, Malardier-Jugroot C. Synthesis and characterization of a pH responsive folic acid functionalized polymeric drug delivery system. Biophys Chem. 2016; 214-215: 17-26.
- Chan JM, Zhang L, Tong R, Ghosh D, Gao W, Liao G, et al. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc Natl Acad Sci U S A. 2010; 107: 2213-2218.
- Alexis F, Basto P, Levy-Nissenbaum E, Radovic-Moreno AF, Zhang L, Pridgen E, et al. HER-2-targeted nanoparticle-affibody bioconjugates for cancer therapy. ChemMedChem. 2008; 3: 1839-1843.
- Aguilar-Gallardo C, Rutledge EC, Martinez-Arroyo AM, Hidalgo JJ, Domingo S, Simon C. Overcoming challenges of ovarian cancer stem cells: novel therapeutic approaches. Stem Cell Rev. 2012; 8: 994-1010.
- Matsumura Y, Gotoh M, Muro K, Yamada Y, Shirao K, Shimada Y, et al. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol. 2004; 15: 517-525.
- Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012; 4: 128ra139.
- Rahman MA, Shin DM. CCR 20th Anniversary Commentary: Prospects and challenges of therapeutic nanoparticles in cancer. Clin Cancer Res. 2015; 21: 4499-4501.
- Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R, et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci U S A. 2014; 111: 11449-11454.
- Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010; 464: 1067-1070.
- Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013; 3: 406-417.
- Strumberg D, Schultheis B, Traugott U, Vank C, Santel A, Keil O, et al. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int J Clin Pharmacol Ther. 2012; 50: 76-78.
- Tolcher AW, Rodrigueza WV, Rasco DW, Patnaik A, Papadopoulos KP, Amaya A, et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014; 73: 363-371.
- Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007; 104: 15549-15554.
- Rahman MA, Amin AR, Wang X, Zuckerman JE, Choi CH, Zhou B, et al. Systemic delivery of siRNA nanoparticles targeting RRM2 suppresses head and neck tumor growth. J Control Release. 2012; 159: 384-392.
- Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008; 5: 487-495.
- Caron WP, Lay JC, Fong AM, La-Beck NM, Kumar P, Newman SE, et al. Translational studies of phenotypic probes for the mononuclear phagocyte system and liposomal pharmacology. J Pharmacol Exp Ther. 2013; 347: 599-606.
- La-Beck NM, Zamboni BA, Gabizon A, Schmeeda H, Amantea M, Gehrig PA, et al. Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother Pharmacol. 2012; 69: 43-50.
- Chanan-Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR, et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003; 14: 1430-1437.
- Ilinskaya AN, Dobrovolskaia MA. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol. 2014; 171: 3988-4000.
- Gabizon A, Isacson R, Rosengarten O, Tzemach D, Shmeeda H, Sapir R. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother Pharmacol. 2008; 61: 695-702.
- Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013; 73: 2412-2417.
- Huang K, Ma H, Liu J, Huo S, Kumar A, Wei T, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012; 6: 4483-4493.
- Tang L, Gabrielson NP, Uckun FM, Fan TM, Cheng J. Size-dependent tumor penetration and in vivo efficacy of monodisperse drug-silica nanoconjugates. Mol Pharm. 2013; 10: 883-892.
- Huang J, Wang L, Zhong X, Li Y, Yang L, Mao H. Facile non-hydrothermal synthesis of oligosaccharides coated sub-5 nm magnetic iron oxide nanoparticles with dual MRI contrast enhancement effect. J Mater Chem B Mater Biol Med. 2014.
- Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A. 2007; 104: 2050-2055.
- Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013; 8: 137-143.
- Zhang L, Cao Z, Bai T, Carr L, Ella-Menye JR, Irvin C, et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol. 2013; 31: 553-556.
- Wei H, Insin N, Lee J, Han HS, Cordero JM, Liu W, et al. Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano Lett. 2012; 12: 22-25.
- Chen H, Wang L, Yeh J, Wu X, Cao Z, Wang YA, et al. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PgammaMPS copolymer coating. Biomaterials. 2010; 31: 5397-5407.
- Li Y, Lin R, Wang L, Huang J, Wu H, Cheng G, et al. PEG-b-AGE Polymer Coated Magnetic Nanoparticle Probes with Facile Functionalization and Anti-fouling Properties for Reducing Non-specific Uptake and Improving Biomarker Targeting. J Mater Chem B Mater Biol Med. 2015; 3: 3591-3603.
- Ahlberg S, Antonopulos A, Diendorf J, Dringen R, Epple M, Flock R, et al. PVP-coated, negatively charged silver nanoparticles: A multi-center study of their physicochemical characteristics, cell culture and in vivo experiments. Beilstein J Nanotechnol. 2014; 5: 1944-1965.
- Braakhuis HM, Park MV, Gosens I, De Jong WH, Cassee FR. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part Fibre Toxicol. 2014; 11: 18.
- Otto DP, Otto A, de Villiers MM. Differences in physicochemical properties to consider in the design, evaluation and choice between microparticles and nanoparticles for drug delivery. Expert Opin Drug Deliv. 2015; 12: 763-777.
- David CA, Owen A, Liptrott NJ. Determining the relationship between nanoparticle characteristics and immunotoxicity: key challenges and approaches. Nanomedicine (Lond). 2016; 11: 1447-1464.