
Research Article
Austin J Cancer Clin Res. 2021; 8(2): 1091.
Antitumor Activities of Immune Costimulatory Molecules of TNF Superfamily in Colorectal and Lung Cancers
Liming Gui1, Zhixue Wang1, Pan Yin1, Bin Ma1,2*
¹School of Biomedical Engineering, Med-X Research Institute, Shanghai Jiao Tong University, Shanghai, China
²Clinical Stem Cell Research Center, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
*Corresponding author: Bin Ma, School of Biomedical Engineering, Med-X Research Institute, Shanghai Jiao Tong University, Clinical Stem Cell Research Center, Renji Hospital, School of Medicine, Shanghai, China
Received: May 28, 2021; Accepted: June 21, 2021; Published: June 28, 2021
Abstract
Cancer immunotherapy has emerged as a promising treatment that utilizes the innate or adaptive immunity to generate a robust killing of malignant cells. However, only a limited number of cancer patients showed responsiveness to immunotherapies such as checkpoint inhibitors, suggesting the need for potent alternative strategies. In the present study, we explored the therapeutic potentials of costimulatory molecules including TNF superfamily member 4 (TNFSF4), member 9 (TNFSF9), and member 18 (TNFSF18). In tumor samples from human colorectal and lung cancer patients, expression of these factors positively correlated with lymphocyte infiltration and expression of several immune effector genes. In syngeneic mouse tumor models, overexpression of the TNF superfamily costimulatory factors in murine colorectal or lung cancer cells significantly suppressed tumor progression. Especially, TNFSF9 and 18 showed stronger antitumor effects than TNFSF4. Together, our study demonstrated the great potential of cancer immunotherapy targeting these immune costimulatory molecules.
Keywords: Costimulatory molecules; TNF superfamily; Immunotherapy; Colorectal cancer; Lung cancer
Introduction
The recent development of immunotherapies has revolutionized cancer treatment. Checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 antibodies have been used to treat various cancers [1]. However, the overall rate of responsiveness remains limited [2]. Both tumor-intrinsic and -extrinsic factors may lead to the resistance to checkpoint blockade, including low expression of immune signaling molecules (e.g. PD-L1, type I interferon), epigenetic silencing of chemokines, low tumor-infiltrating lymphocytes, and the prominence of immunosuppressive cells [3]. Therefore, it is warranted to explore other effective immunotherapeutic targets.
Costimulatory molecules play a crucial role in effector immune response, providing costimulatory signals for immune cell proliferation, differentiation, activation, and cytokine production [4,5]. Moreover, costimulatory pathways are essential in driving effective antitumor immunity [6]. The Tumor Necrosis Factor (TNF) and TNF Receptor (TNFR) superfamilies (TNFSF/TNFRSF) are principal parts of costimulatory molecules [7-9]. The coordinated surface expression of TNFSF/TNFRSF ligand-receptor pairs on Antigen Presentation Cells (APCs), antigen-specific T cells, and NK cells shape the T/NK cell-mediated immune response. Several studies have shown that stimulation of the TNFSF/TNFRSF pathway promotes impressive antitumor immunity in various murine tumor models by activating the downstream NF-κB and MAPK pathways that upregulate the effector cytokine production and prolong cytotoxic cells survival [10,11]. Besides, agonists of these costimulatory molecules are now being tested in ongoing clinical trials have shown promising outcomes [12-14]. However, a comparison of the antitumor activities of these TNF superfamilies factors hasn’t been performed.
Materials and Methods
Database analysis
The correlation between TNFSF4/9/18 expression and immune cell infiltration in colorectal adenocarcinoma and lung adenocarcinoma was analyzed by a gene module in the TIMER2.0 database (www.timer.cistrome.org) [15,16].
The cBioPortal [17] database (www.cbioportal.org) was used to analyze the correlation between the expression of TNFSF4/9/18 between CD69, GZMA and PRF1 in colorectal adenocarcinoma and lung adenocarcinoma.
Cell lines
CT26 cell line is murine colon adenocarcinoma cells derived from BALB/c mice, and LLC1 cell line is murine lung adenocarcinoma cells derived from C57BL/6 mice. CT26 and LLC1 cells were cultured in RPMI 1640 and DMEM, respectively, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cell lines were authenticated by Shanghai Biowing Applied Biotechnology using short tandem repeat analysis and routinely tested for mycoplasma.
Antibodies
Antibodies for flow cytometry analysis were purchased from BioLegend. The following antibodies were used: APC-conjugated antibodies to mouse TNFSF4 (clone RM134L); PE-conjugated antibodies to mouse TNFSF9 (clone TKS-1) and TNFSF18 (clone YGL386).
Lentivirus production and transduction
cDNAs for mouse TNFSF4, TNFSF9, and TNFSF18 were cloned into lentiviral vectors co-expressing ZsGreen. Lentiviruses were produced and titrated by OBiO Technology (Shanghai). CT26 and LLC1 cell lines were infected with lentiviruses in the presence of 8 μg/mL polybrene (Sigma-Aldrich). A transduction efficiency of >90% in each tumor cell line was validated by FACS analysis of ZsGreen expression before use. Successful expression of TNFSF4, TNFSF9, and TNFSF18 in tumor cell lines was also validated by FACS.
In vitro cell proliferation/viability assay
Proliferation/viability of CT26 and LLC1 cell lines overexpressing mouse TNFSF4, TNFSF9, and TNFSF18 were assessed by Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) according to the manufacturer’s instructions. Absorbance was measured using a microplate reader (Tecan).
Flow cytometry analysis
To validate the expression of costimulatory molecules, CT26 and LLC1 cell lines transduced with lentiviruses expressing TNFSF4, TNFSF9, and TNFSF18 were detached with 20 mM EDTA, washed twice with PBS containing 1% BSA and stained with corresponding antibodies. Fluorescence data were acquired on a BD LSRFortessa cell analyzer (BD Biosciences) and analyzed using FlowJo software.
Syngeneic mouse models
To establish syngeneic tumor models, CT26 (0.5×106/mouse) and LLC1(1×106/mouse) cell lines overexpressing mouse TNSFS4, TNFSF9, TNFSF18, or empty vector were injected subcutaneously at the right lower flank of 6- to 8-week-old BALB/c and C57BL/6 mice, respectively. Tumors were measured every three days using a digital caliper, and the tumor volume was calculated using the following formula: V=L×W²/2, where L and W are the long and short diameters of the tumor, respectively.
Statistical analysis
Data are shown as mean values ± SEM. Two-tailed t-tests were used to compare treatment groups with control groups or, when means of more than two groups were compared, by two-way ANOVA followed by a Bonferroni multiple comparison test. Statistical analyses were performed with GraphPad Prism 8 (GraphPad Software). P<0.05 was considered statistically significant.
Study approval
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University, Shanghai, China.
Results
The expression of costimulatory molecules positively correlated with lymphocyte infiltration and immune effector factor levels
To examine the role of costimulatory molecules in the process of tumor immunity, we analyzed the correlation between the expression of TNFSF4, TNFSF9, and TNFSF18 and lymphocyte infiltration levels in colorectal adenocarcinoma and lung adenocarcinoma from TIMER database [15,16]. The results indicated that tumors with high costimulatory molecule expression showed significantly elevated infiltration of CD8+ T and NK cells in colorectal adenocarcinoma patients. Meanwhile, the expression of TNFSF4 and TNFSF18 positively correlated with the infiltration of CD4+ T cells (Figure 1). Furthermore, we observed a similar correlation between costimulatory molecule expression and immune infiltration of CD8+ T and NK cells in lung adenocarcinoma patients, although it seemed that the expression of TNFSF4, TNFSF9, and TNFSF18 had little association with CD4+ T cells infiltration (Figure 1).
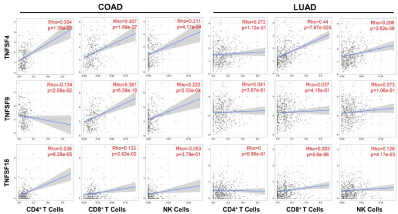
Figure 1: Correlation of TNFSF4, TNFSF9 or TNFSF18 expression with levels of tumor-infiltrated lymphocytes in human COAD (colorectal adenocarcinoma) and
LUAD (lung adenocarcinoma).
The immune effector molecules in the tumor microenvironment are predictors of the strength of local antitumor immunity [18,19]. Therefore, we investigated the connection between the expression of TNFSF4/9/18 and immune effector molecules. As expected, we found a significantly positive correlation between the expression of costimulatory molecules and T lymphocytes/NK cells activation marker CD69 and cytotoxic factors including granzyme A (GZMA) and perforin (PRF1) in both colorectal and lung adenocarcinoma samples (Figure 2).
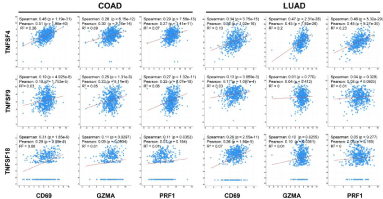
Figure 2: Correlation of TNFSF4, TNFSF9 or TNFSF18 expression with immune effector markers including CD69, GZMA and PFR1 in human COAD (colorectal
adenocarcinoma) and LUAD (lung adenocarcinoma).
Collectively, these results indicate that enhancement of costimulatory molecule levels in the tumor microenvironment may promote lymphocyte infiltration and elevate the expression of immune effector factors.
Tumoral expression of costimulatory molecules suppresses tumor progression
To explore the therapeutic potentials of the TNF superfamily costimulatory molecules, we established stable expression of mouse TNFSF4, TNFSF9, and TNFSF18 in mouse colorectal adenocarcinoma cell line CT26 and lung adenocarcinoma cell line LLC1 using lentiviral transduction. Overexpression of TNFSF4, TNFSF9, and TNFSF18 on the cell membranes was confirmed by FACS analysis (Figure 3A). Moreover, we found that overexpression of the above factors did not impact tumor cell proliferation/viability in vitro using Cell Counting Kit 8 (Figure 3B). Interestingly, tumoral overexpression TNFSF4, TNFSF9, and TNFSF18 resulted in a remarkable suppression of tumor growth in the syngeneic subcutaneous mouse tumor models. Notably, we observed a complete regression of TNFSF9-overexpressed tumors (in all 6 mice) on day 18 in the CT26 model (Figure 4A). 3 out 6 CT26-TNFSF18 tumors eventually disappeared. Similarly, the LLC1 cell lines overexpressing TNFSF9 (in 6 out of 7 mice) and TNFSF18 (in all 7 mice) even failed to form a palpable tumor in the mouse subcutaneous model (Figure 4B). In conclusion, our findings illustrate that the costimulatory molecules potently suppress tumor progression, and the antitumor effects of TNFSF9 and 18 appear to be superior to that of TNFSF4.
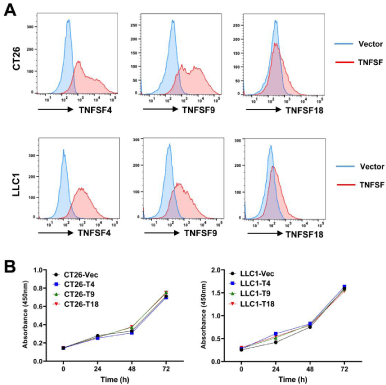
Figure 3: (A) FACS analysis of mouse TNFSF4, TNFSF9 and TNFSF18 expression in murine colon adenocarcinoma cell lines CT26 and lung adenocarcinoma cell
lines LLC1 transduced with lentiviruses overexpressing these factors or empty vector (Vec). (B) In vitro CCK-8 cell viability assay of CT26 and LLC1 overexpressing
mouse TNFSF4 (T4), TNFSF9 (T9) or TNFSF18 (T18) (n=5).
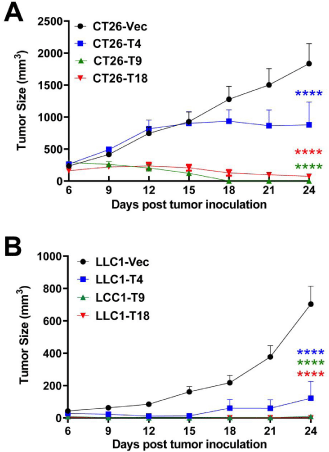
Figure 4: Tumor growth of vector (Vec)-, TNFSF4 (T4)-, TNFSF9 (T9)-or
TNFSF18 (T18)-overexpressed CT26 cells (n=6 mice/group) (A) or LLC1
cells (n=7 mice/group) (B) in syngeneic subcutaneous models. ****P<0.0001.
Discussion
Cancer immunotherapies such as immune checkpoint inhibitors (anti-PD-1/PD-L1 and anti-CTLA4) and adoptive cell therapy (CAR-T) emerge as novel strategies to overcome the obstacles of traditional cancer treatments and achieved considerable success in prolonging the survival of patient [20,21]. Nevertheless, these cancer immunotherapeutic strategies elicited robust antitumor immunity only in a limited cohort of patients [22]. The immune checkpoint inhibitors therapy utilizes specific antibodies to block the suppressive immune response “brake” signals and reverse the anergy of exhausted effector cells. However, the tumor microenvironment usually favors immune escape by downregulation of the costimulatory signals that prompt an insufficient activation of effector cells [20,23]. Similarly, the short half-life of the adoptive T cells in vivo is one of the primary obstacles of T cell-based cancer immunotherapy due to the lack of costimulatory signals in the tumor microenvironment [24,25]. Therefore, immunotherapy utilizing the costimulatory molecules may circumvent these problems. There are serval strategies to target the costimulatory pathway for clinical applications: Agonistic antibodies for costimulatory receptors (e.g. TNFRSF) and costimulatory ligands (e.g. TNFSF) fused to IgG Fc [26]; Delivery of sequences encoding costimulatory ligands by oncolytic viruses [27] or mesenchymal stem cells [18].
In the process of antitumor immune response, costimulatory molecules deliver essential activation signals in promoting effector cell proliferation, differentiation, and survival [28]. The ligands of costimulatory receptors are mainly founded on the surface of Antigen Presentation Cells (APCs), such as Dendritic Cells (DCs). The antigen-specific T cells are able to identify Major Histocompatibility Complex (MHC) conjugated tumor antigen and initiate the first activation signal pathway. Then, the costimulatory ligands on the APCs interact with the T cells’ costimulatory receptors, driving the activation of effector cells, provoking a potent antitumor response ultimately [5,29,30]. Furthermore, the interaction of costimulatory ligands and receptors also contributes to the activation of Natural Killing (NK) cells and promotes cytokine production [4,11].
In agreement with the essential role of costimulatory molecule in the initiation of adaptive immunity, current study revealed a positive correlation of costimulatory molecule expression with infiltration of lymphocytes including CD4+ T, CD8+ T, and NK cells, as well as the expression of immune effector markers including CD69, GZMA, and PFR1 in human colorectal and lung adenocarcinoma. In mouse syngeneic models for colorectal cancer and lung cancer, tumoral expression of costimulatory molecules potently suppressed tumor progression. Notably, TNFSF9 and TNFSF18 exhibited better therapeutic effects than TNFSF4. TNFRSF4 is induced on activated T cells after antigen recognition, mainly on the surface of CD4+ T cells. In comparison, the TNFRSF9 was founded on the surface T, B, NK cells, and DCs. The TNFRSF18 is highly expressed in Treg cells and can be induced upregulation on the surface of activated CD8+ and CD4+ T cells. Thus, TNFSF9 and TNFSF18 possess more diverse target cell populations, which may be one reasonable explanation for their higher antitumor activity as compared to TNFSF4.
In conclusion, our research highlighted the great promises of costimulatory molecule-based immunotherapy, which directly activates the immune effector cells to achieve robust antitumor effects.
References
- Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017; 17: 286-301.
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020; 20: 651-668.
- Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor- Intrinsic and -Extrinsic Factors. Immunity. 2016; 44: 1255-1269.
- Shissler SC, Lee MS, Webb TJ. Mixed Signals: Co-Stimulation in Invariant Natural Killer T Cell-Mediated Cancer Immunotherapy. Front Immunol. 2017; 8: 1447.
- O’Neill RE, Cao X. Co-stimulatory and co-inhibitory pathways in cancer immunotherapy. Adv Cancer Res. 2019; 143: 145-194.
- Marin-Acevedo JA, Kimbrough EO, Manochakian R, Zhao Y, Lou Y. Immunotherapies targeting stimulatory pathways and beyond. J Hematol Oncol. 2021; 14: 78.
- Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002; 100: 350-352.
- Kwon B, Kim BS, Cho HR, Park JE, Kwon BS. Involvement of tumor necrosis factor receptor superfamily (TNFRSF) members in the pathogenesis of inflammatory diseases. Exp Mol Med. 2003; 35: 8-16.
- Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004; 34: 613-622.
- Lubrano di Ricco M, Ronin E, Collares D, Divoux J, Gregoire S, Wajant H, et al. Tumor necrosis factor receptor family costimulation increases regulatory T-cell activation and function via NF-kappaB. Eur J Immunol. 2020; 50: 972- 985.
- Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005; 35: 1016-1022.
- Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004; 4: 420-431.
- Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013; 19: 1044-1053.
- Song Y, Margolles-Clark E, Bayer A, Buchwald P. Small-molecule modulators of the OX40-OX40 ligand co-stimulatory protein-protein interaction. Br J Pharmacol. 2014; 171: 4955-4969.
- Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017; 77: e108-e110.
- Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020; 48: W509-W514.
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012; 2: 401-404.
- Yin P, Gui L, Wang C, Yan J, Liu M, Ji L, et al. Targeted Delivery of CXCL9 and OX40L by Mesenchymal Stem Cells Elicits Potent Antitumor Immunity. Mol Ther. 2020; 28: 2553-2563.
- Nelson MA, Ngamcherdtrakul W, Luoh SW, Yantasee W. Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev. 2021.
- Hahn AW, Gill DM, Pal SK, Agarwal N. The future of immune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy. 2017; 9: 681-692.
- Liu G, Rui W, Zhao X, Lin X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol Immunol. 2021; 18: 1085- 1095.
- Walsh MJ, Dougan M. Checkpoint blockade toxicities: Insights into autoimmunity and treatment. Semin Immunol. 2021: 101473.
- Guo W, Zhang C, Qiao T, Zhao J, Shi C. Strategies for the Construction of Mouse Models With Humanized Immune System and Evaluation of Tumor Immune Checkpoint Inhibitor Therapy. Front Oncol. 2021; 11: 673199.
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021; 11: 69.
- Tang L, Zhang Y, Hu Y, Mei H. T Cell Exhaustion and CAR-T Immunotherapy in Hematological Malignancies. Biomed Res Int. 2021; 2021: 6616391.
- Emerson DA, Redmond WL. Overcoming Tumor-Induced Immune Suppression: From Relieving Inhibition to Providing Costimulation with T Cell Agonists. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2018; 32: 221-231.
- Zhang Y, Zhang H, Wei M, Mou T, Shi T, Ma Y, et al. Recombinant Adenovirus Expressing a Soluble Fusion Protein PD-1/CD137L Subverts the Suppression of CD8(+) T Cells in HCC. Mol Ther. 2019; 27: 1906-1918.
- Waight JD, Gombos RB, Wilson NS. Harnessing co-stimulatory TNF receptors for cancer immunotherapy: Current approaches and future opportunities. Hum Antibodies. 2017; 25: 87-109.
- Stoll A, Bruns H, Fuchs M, Volkl S, Nimmerjahn F, Kunz M, et al. CD137 (4- 1BB) stimulation leads to metabolic and functional reprogramming of human monocytes/macrophages enhancing their tumoricidal activity. Leukemia. 2021.
- Dammeijer F, De Gooijer CJ, van Gulijk M, Lukkes M, Klaase L, Lievense LA, et al. Immune monitoring in mesothelioma patients identifies novel immunemodulatory functions of gemcitabine associating with clinical response. EBioMedicine. 2021; 64: 103160.