
Research Article
Austin J Cancer Clin Res. 2022; 9(1): 1100.
PRPF8 Expression is an Independent Predictor for Poor Prognosis in Patients with Kidney Renalclear Cell Carcinoma
Zhu D1#, Qiqi Z2,3#, Qi J2,4, Sima M2,5, Li C6, Li Y2, Li Z2,7, Wang T2* and Lv C2,4*
1Sichuan Agricultural University, Chengdu, China
2Changchun Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Changchun, PR China
3North Sichuan College of Preschool Education Teacher, Guangyuan, China
4The Key Laboratory of Molecular Epigenetic, Institute of Genetics and Cytology, Northeast Normal University, Changchun, China
5College of Basic Medicine, Changchun University of Chinese Medicine, Changchun, PR China
6Fuxin Higher Training College, Fuxin, China
7College of Animal Medicine, Jilin University, Changchun, PR China
#Contributed Equally to this work
*Corresponding author: Tiecheng Wang, Changchun Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Changchun, 130122, PR China
Chaoxiang Lv, Changchun Veterinary Research Institute, Chinese Academy of Agricultural Sciences, 130122, PR China; The Key Laboratory of Molecular Epigenetic, Institute of Genetics and Cytology, Northeast Normal University, Changchun, 130024, China
Received: February 21, 2022; Accepted: March 17, 2022; Published: March 24, 2022
Abstract
Background: Alternative splicing is an important process associated with disease including tumors. PRPF8 is a conserved protein in splice some component U5 snRNP that plays an important role in tumor cell growth.
Materials and Methods: The data in The Cancer Genome Atlas was downloaded and analyzed by R Studio. The box plots showed the expression pattern of PRPF8. The chi-square test was used to manifest the association between PRPF8 expression and clinical parameters. The diagnostic value was assessed using ROC curve. The Kaplan-Meier curves and the Cox regression analysis elucidated the difference of overall survival and relapse-free survival between high expression and low expression and the prognostic value.
Results: We downloaded the PRPF8 expression and the clinical data of 50 healthy individuals and 537 patients from TCGA database. We found PRPF8 expression is lower in tumor tissues than that in normal tissues and is related to age, histologic grade, pathologic stage, M classification, T classification and vital status. The Kaplan Meier curves showed the patients with lower PRPF8 expression has a poorer overall survival and relapse-free survival. The Univariate and Multivariate analysis suggested PRPF8 expression was an independent prognostic factor for overall survival and relapse-free survival in kidney renal clear cell carcinoma.
Conclusion: Low PRPF8 expression is an independent predictor for overall survival and relapse-free survival in kidney renal clear cell carcinoma.
Keywords: PRPF8; Predictor; Kidney renal clear cell carcinoma; TCGA; Molecular marker; Diagnosis
Introduction
Kidney cancer is one of the most common malignancies, accounting for 2% of all cancers, and usually has a poor prognosis [1]. The most common type of kidney cancer is renal cell carcinoma accounting for 90% of all kidney cancers [2]. Renal cell carcinoma includes Kidney Renal Clear Cell Carcinoma (KIRC), Kidney Renal Papillary Cell Carcinoma (KIRP) and Kidney Chromophobe (KICG) [3-6]. Among them, KIRC accounts for 70-75% of all renal cell carcinomas [7]. Therapy for KIRC includes radiation therapy, chemotherapy, and surgery. Nephrectomy remains the primary treatment for clinically localized disease, but 20% to 30% of patients with localized disease relapse after nephrectomy, and the majority of these patients die from the disease [8]. Unfortunately, there are currently no approved treatments to reduce the risk of renal cancer recurrence, progression, or death after primary treatment for the localized disease. Therefore, it is very important to search for markers that indicate the prognosis of renal cell carcinoma.
Alternative splicing is an important process in eukaryotes and is also of great value in diseases, especially in tumors. Alternative splicing can lead to the appearance of various abnormally expressed proteins, which may be the key to the development of disease. It is mainly performed in spliceosomes, which are composed of U1, U2, U4/U6, U5 and more than 200 proteins. PRPF8 is a key protein in the component U5 [9,10]. In eukaryotes, deletion of PRPF8 leads to the development of many physiological or pathological diseases. For example, studies have reported that decreased expression of PRPF8 leads to increased cellular proliferation in K542 and CD 34+ cells [11]. The PRPF8 depletion disrupts Homology-Directed Repair (HDR), Single Strand Annealing (SSA), and end resection [12].In HCC caused by different causes, PRPF8 over expression is related to the invasion of HCC cell lines in vitro [13].
In our study, we evaluated the expression pattern of PRPF8 in kidney renal clear cell carcinoma patients and the relation between PRPF8 expression and clinical pathological parameters. In addition, we also assessed the diagnostic value of PRPF8 expression and prognostic significance of PRPF8 expression for overall survival and relapse-free survival in kidney renal clear cell carcinoma patients.
Materials and Methods
Data downloading from the Cancer Genome Atlas (TCGA) database We downloaded PRPF8 mRNA expression data of normal tissues and tumor tissues and obtained the clinical information of patients from TCGA database by RTCGA Tool box package in R Studio [14,15].
Statistical analysis
Box plots were performed to analyze the expression pattern of PRPF8 and the difference of PRPF8 expression according to clinical variables by ggplot2 package in R. Chi-square test was used to evaluate the relationship of PRPF8 expression with clinical characteristics. We generated ROC curves to predict the diagnostic value of PRPF8 expression by pROC package in R [16]. Then according to ROC curve, we divided PRPF8 expression into two groups, high PRPF8 expression and low PRPF8 expression. And Kaplan-Meier curve was plotted to describe the effect of high PRPF8 expression and low PRPF8 expression on overall survival and relapse-free survival by using survival package in R. Univariate Cox analysis was used to determine the factors affecting OS and RFS [17]. Multivariate Cox analysis was applied for to judge the influence of PRPF8expression on the overall survival and relapse-free survival of patients.
Results
Characteristics of patients in TCGA-KIRC database
We downloaded PRPF8 expression and clinical information of 537 patients from TCGA-KIRC cohort. The clinical information included age, gender, histologic grade, pathologic stage, TNM stage, vital status, and recurrence of kidney renal clear cell carcinoma patients (Table 1).
Parameters
Numbers (%)
Age
≥55
365(67.97)
<55
172(32.03)
Gender
Male
346(64.43)
Female
191(35.57)
Histologic grade
NA
3(0.56)
G1
14(2.61)
G2
230(42.83)
G3
207(38.55)
G4
78(14.52)
GX
5(0.93)
Pathologic stage
Ⅰ
270(50.28)
Ⅱ
57(10.61)
Ⅲ
126(23.46)
Ⅳ
84 (15.64)
T classification
T1
275(51.21)
T2
69(12.85)
T3
182(33.89)
T4
11(2.05)
N classification
N0
240(44.69)
N1
17(3.17)
NX
280(52.14)
M classification
NA
2(0.37)
M0
426 (79.33)
M1
79(14.71)
MX
30(5.59)
Vital status
Dead
162(30.17)
Survival
375(69.83)
Relapse
NA
28(5.21)
NO
364(67.78)
YES
145(27.01)
PRPF8 expression
NA
4(0.74)
High
258(48.05)
Low
275(51.21)
NA: Not Available.
Table 1: The clinical characteristics of patients in the present study.
The PRPF8 expression and the relationship between its expression and clinical variables
The PRPF8 expression is lower in tumor tissues than in normal tissues (P=0.001599). The box plots showed that significant difference of PRPF8 expression was observed according to histologic grade, pathologic stage, T classification, M classification and vital status (Figure 1). Moreover, we analyzed the association of PRPF8 expression with clinical variables by chi-square test. The results revealed PRPF8 expression is closely related to age (P=0.011), histologic grade (P<0.001), pathologic stage (P<0.001), M classification (P<0.001), T classification (P<0.001) and vital status (P<0.001) (Table 2).
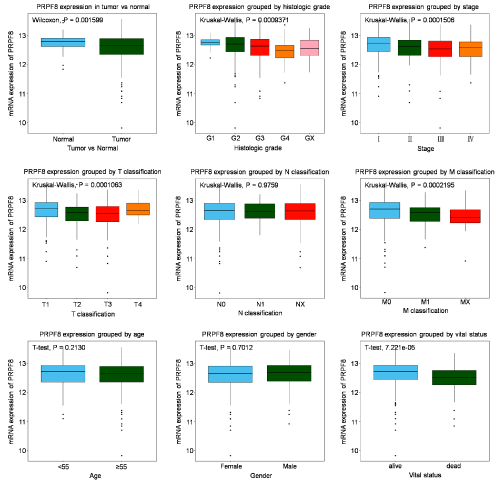
Figure 1: Expression of PRPF8 in KIRC. Expression of PRPF8 between tumor and normal tissue was compared. The expression of PRPF8 was compared
according to different age, gender, histologic grade, histological type, T/N/M classification, as well as radiation therapy, residual tumor, sample type, stage and
vital status.
Parameters
Variables
Numbers
PRPF8
X2
P-value
high
Prop(%)
low
Prop(%)
age
≥55
363
162
62.79
201
73.09
6.5015
0.011
<55
170
96
37.21
74
26.91
Gender
Male
345
164
63.57
181
65.82
0.2958
0.587
Female
188
94
36.43
94
34.18
Histologic grade
G1
14
11
4.30
3
1.09
25.9856
0.000
G2
229
126
49.22
103
37.59
G3
206
98
38.28
108
39.42
G4
76
19
7.42
57
20.81
GX
5
2
0.78
3
1.09
Pathologic stage
Ⅰ
268
157
60.85
111
40.36
24.9067
0.000
Ⅱ
57
27
10.47
30
10.91
Ⅲ
124
45
17.44
79
28.73
Ⅳ
84
29
11.24
55
20
M classification
M0
422
224
86.82
198
72.53
16.9529
0.000
M1
79
26
10.08
53
19.41
MX
30
8
3.10
22
8.06
N classification
N0
240
115
44.57
125
45.45
0.0514
0.975
N1
16
8
3.10
8
2.91
NX
277
135
52.33
142
51.64
T classification
T1
273
160
62.01
113
41.09
25.0397
0.000
T2
69
30
11.63
39
14.18
T3
180
63
24.42
117
42.55
T4
11
5
1.94
6
2.18
Vital status
Dead
160
205
79.46
168
61.09
21.3748
0.000
Survival
373
53
20.54
107
38.91
Table 2: Associations between the clinic pathologic variables and PRPF8 expression in KIRC.
The diagnostic value of PRPF8 expression in KIRC
To evaluate the diagnostic value of PRPF8 expression, we made the ROC curve using the PRPF8 expression and then the Area under the ROC Curve (AUC) was statistically calculated. The ROC curve of all patients indicated AUC was 0.614, which demonstrated the PRPF8 expression had a modest diagnostic value in all patients. Then we performed the subgroup analysis and the results showed that the AUC of patients in stage I stage II, stage III and stage IV was 0.553, 0.640, 0.685 and 0.686, respectively (Table 3). This suggested that the expression of PRPF8 had different diagstic significance for patients at different stages (Figure 2).
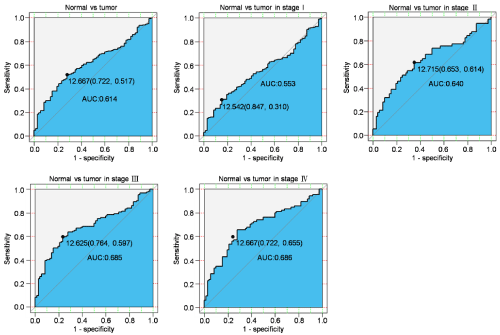
Figure 2: Diagnosis value of PRPF8 expression in KIRC. The ROC curve of PRPF8 expression in Cancerous vs. Normal liver tissues was generated. Cancerous
vs. Normal liver tissues was analyzed in different stages of KIRC.
Univariate analysis
Multivariate analysis
Hazard Ratio
CI95
P value
Hazard Ratio
CI95
P value
Age
1.889
1.298-2.748
0.001
1.530
1.03-2.26
0.03
Gender
1.037
0.753-1.427
0.826
-
-
-
Histologic grade
2.056
1.711-2.471
0.000
1.520
1.226-1.884
0.000
Pathologic stage
1.958
1.708-2.245
0.000
2.051
1.406-2.992
0.000
M classification
2.500
1.949-3.207
0.000
1.005
0.620-1.623
0.982
N classification
0.861
0.736-1.008
0.063
-
-
-
T classification
2.067
1.741-2.455
0.000
0.872
0.590-1.289
0.260
PRPF8
1.779
1.278-2.475
0.001
1.435
1.034-2.734
0.037
Table 3: Univariate and Multivariate analysis of Over Survival in patients with KIRC.
Low PRPF8 expression serves as an independent predictor for poor OS in patients with KIRC
To assess the effect of PRPF8expression on Overall Survival (OS), we generated two Kaplan-Meier curves according to PRPF8 expression. We found that low PRPF8 expression was closely associated with worse OS in all tumor patients. Furthermore, the subgroup analysis proved that low PRPF8 expression significantly correlated with poor OS of patients who was in female subgroup, histologic grade G3/G4/ GXsubgroup, N0 subgroup, N1/Nx subgroup, M0 subgroup, and age (<55) sub group. Univariate Cox analysis showed that age, histologic group, pathologic stage, M classification, T classification and PRPF8 expression was significantly associated with OS of patients with kidney renal clear cell carcinoma. Multivariate Cox analysis showed that PRPF8 expression was an independent biomarker for OS of kidney renal clear cell carcinoma patients (Hazard Ratio (HR)=1.435, 95% Confidence Interval (CI): 1.034-2.734, P=0.037) (Figure 3).
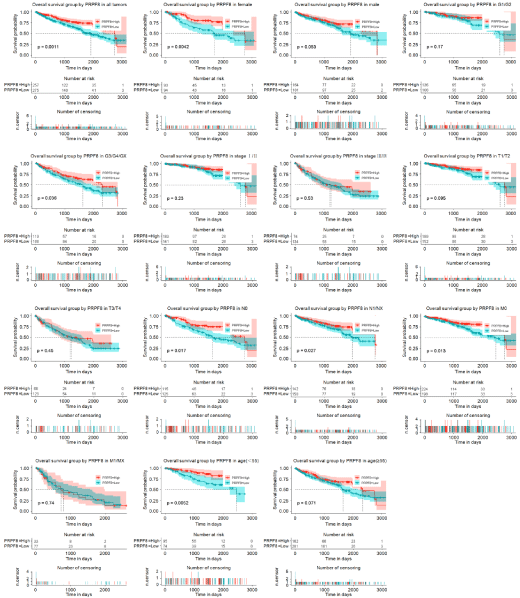
Figure 3: The effect of PRPF8 expression on OS in KIRC. Kaplan-Meier curves of PRPF8 expression in all patients with KIRC. Kaplan-Meier curves of PRPF8
expression in subgroup.
Low PRPF8 expression serves as an independent predictor for poor RFS in patients with KIRC
To assess the effect of PRPF8 expression on relapse-free survival (RFS), we generated two Kaplan-Meier curves according to PRPF8 expression. We found that low PRPF8 expression was closely associated with worse RFS in all tumor patients. Furthermore, the subgroup analysis proved that low PRPF8 expression significantly correlated with poor RFS of patients who was in male subgroup, histologic grade G3/G4/GXsubgroup, N1/Nx subgroup, and age (<55) subgroup. Univariate Cox analysis showed that age, histologic group, pathologic stage, M classification, T classification and PRPF8 expression was significantly associated with RFS of patients with kidney renal clear cell carcinoma. Multivariate Cox analysis showed that PRPF8 expression was an independent biomarker for RFS of kidney renal clear cell carcinoma patients (Hazard Ratio (HR)=1.209, 95% confidence interval (CI): 1.04-1.41, P=0.029) (Figure 4 and Table 4).
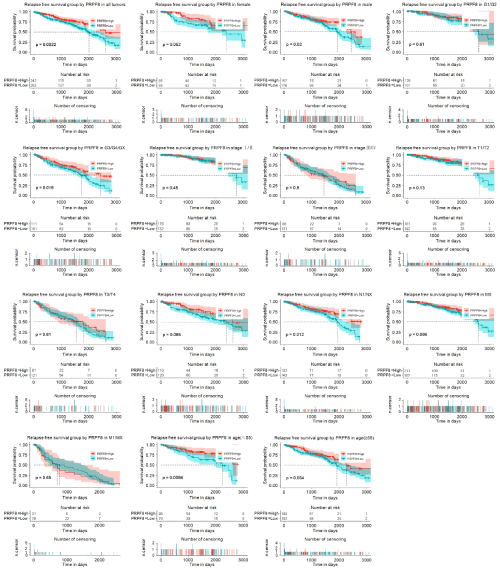
Figure 4: The effect of PRPF8 expression on RFS in KIRC. Kaplan-Meier curves of PRPF8 expression in all patients with KIRC. Kaplan-Meier curves of PRPF8
expression in subgroup.
Univariate analysis
Multivariate analysis
Hazard Ratio
CI95
P value
Hazard Ratio
CI95
P value
Age
1.906
1.304-2.786
0.001
1.535
1.042-2.263
0.03
Gender
1.049
0.760-1.448
0.769
-
-
-
Histologic grade
2.056
1.711-2.471
0.000
1.533
1.235-1.903
0.000
Pathologic stage
1.949
1.699-2.236
0.000
2.051
1.406-2.992
0.000
M classification
2.500
1.949-3.207
0.000
1.006
0.620-1.632
0.982
N classification
0.862
0.736-1.010
0.067
-
-
-
T classification
2.046
1.722-2.431
0.000
0.872
0.590-1.289
0.491
PRPF8
1.777
1.277-2.473
0.001
1.209
1.04-1.41
0.029
Table 4: Univariate and Multivariate analysis of Relapse-Free Survival in patients with KIRC.
Discussion
In this study, it is demonstrated that PRPF8 may be a potential biomarker in kidney renal clear cell carcinoma. By analyzing the TCGA-KIRC data, we revealed that PRPF8 is down-regulated in KIRC. In addition, the expression of PRPF8 varies according to histologic grade, pathologic stage, T classification, M classification and vital status. What’s more, patients with low PRPF8 expression are associated with poor prognosis. The low PRPF8 expression has excellent clinical diagnostic value and can be used as a predictor of poor prognosis in patients with KIRC by Univariate and multivariate Cox regression analysis.
Many studies have reported the mutations of PRPF8 in the alternative splice process can lead to many diseases. Mutations in PRPF8 are known to be associated with human type 13 autosomal dominant mitochondrial retinal pigment [18,19]. In addition, the reduction of PRPF8 expression led to increased cell proliferation in K562 cells and CD34+ cells [20]. This is consistent with our findings. It has been reported that the expression of PRPF8 in a variety of breast cancer cells and found PRPF8 was elevated in the breast cancer cells examined [21]. This is not consistent with our findings that PRPF8 expression is lower in tissues of KIRC. The reason may be the cancer types and sample types studied were different, one is breast cancer cell, and the other one is kidney renal clear cell carcinoma tissues. In this study, we observed that the expression of PRPF8 gradually decreased from G1 to G4, from I to IV, from T1 to T3, from M1 to Mx, which suggested PRPF8 might affect the cancer progression and its expression could indicate the degree of disease progression. To our surprise, the expression of PRPF8 increased from G4 to Gx, from I to IV, from T3 to T4. We assume that the PRPF8 expression functions as different role in different stage of patients. In addition, we also found the expression of PRPF8 was associated with the vital status, which revealed the PRPF8 may associate with the survival of patients in kidney renal clear cell carcinoma.
The role of PRPF8 in tumor development has been reported. The increased PRPF8 expression promoted proliferation and inhibited apoptosis in ovarian cancer cells [22]. Silencing PRPF8 resulted in cell death in both breast cancer cell lines (Cal 51 and HCC 1954) and colon cancer cell lines (HCT116 and DLD1) [23,24]. Moreover, the silencing of PRPF8 leads to cancer cell apoptosis, including liver cancer [25], ovarian cancer [26] and breast cancer [27]. This is not consistent with what we have seen in renal cancer using TCGA. We need to verify it with experiments. To a certain extent, the role of PRPF8 in the proliferation and apoptosis of kidney renal clear carcinoma is provided. In our study, we found the PRPF8 expression was associated with the survival of patients and the patients with lower PRPF8 expression had a poorer overall survival and the relapse-free survival, especially in female, histologic grade G3/G4/ Gx, N classification N0/N1/Nx, M classification M0 and age≤55 subgroup of overall survival and in male, histologic grade G3/G4/Gx, N classification N1/Nx and age ≤55 subgroup of relapse-free survival. Clinically, this could be applied to more precise drug administration and personalized treatment according to the patient’s clinical information.
Conclusion
We first use TCGA database to analyze the expression pattern of PRPF8 in kidney renal clear cell carcinoma and the potential significance of PRPF8 for diagnosis and prognosis of patients clinically. In the future, relevant verification will be carried out through clinical sample experiments that are what we’re focusing on in the future.
Acknowledgements
This work was supported by grants from 2016YFD0501001 (National Key Research and Development Program of China). Thanks to TCGA database for providing the data for free.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018; 68: 394-424.
- Hu F, Zeng W, Liu X. A Gene Signature of Survival Prediction for Kidney Renal Cell Carcinoma by Multi-Omic Data Analysis. 2019; 20: 5720.
- Rathmell WK, Rathmell JC, Linehan WM. Metabolic Pathways in Kidney Cancer: Current Therapies and Future Directions. 2018: 36: 3540-3546.
- Linehan WM, Ricketts CJ. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications [J]. Nature Reviews Urology. 2019; 16: 539- 552.
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016; 70: 93-105.
- Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. 2017; 3: 17009.
- Huang MJ, Zhang T, Yao ZY, et al. MicroRNA related prognosis biomarkers from high throughput sequencing data of kidney renal clear cell carcinoma. Bmc Medical Genomics. 2021; 14: 9.
- Feng CY, Huang XL, Li XK, et al. The Roles of Base Modifications in Kidney Cancer. Front Oncol. 2020; 10: 6.
- Yan C, Hang J, Wan R, et al. Structure of yeast spliceosome at 3.6-angstrom resolution. Science (New York, NY). 2015; 349: 1182-1191.
- Bertram K, Agafonov DE, Liu WT, et al. Cryo-EM structure of a human spliceosome activated for step 2 of splicing. Nature, 2017; 542: 318-323.
- Kurtovic-Kozaric A, Przychodzen B, Singh J, et al. PRPF8 defects cause missplicing in myeloid malignancies. Leukemia. 2015; 29: 126-136.
- Onyango DO, Lee G, Stark JM. PRPF8 is important for BRCA1-mediated homologous recombination. Oncotarget. 2017; 8: 93319-93337.
- Natalia H-S, Juan LL-C, Antonio CF-F, et al. PRPF8 regulates FAK/AKT pathway and cytoskeleton remodeling through modulation of fibronectin 1 splicing in liver pathologies; proceedings of the Endocrine Abstracts F . Bioscientifica. 2021.
- Lv C, Li Y, Zhang Q, et al. Low REST Expression Indicates a Biomarker of Poor Prognosis in Patients with Renal Cell Carcinoma. BioMed Research International. 2021: 2021.
- Chen P, Zhang Q, Li Y, et al. CREBBP is a Novel Biomarker for Diagnostic and Prognostic of Patients with Renal Cell Carcinoma. Medical & Clinical Research. 2021; 6: 527-534.
- Liu J, Li Y, Zhang Q, et al. PVT1 Expression Is a Predictor for Poor Survival of Prostate Cancer Patients. Technology in Cancer Research and Treatment. 2021; 20: 1533033820971610.
- Jiao Y, Li Y, Ji B, et al. Clinical value of lncRNA LUCAT1 expression in liver cancer and its potential pathways. Journal of Gastrointestinal and Liver Diseases. 2019: 28; 439-447.
- Maubaret CG, Vaclavik V, Mukhopadhyay R, et al. Autosomal dominant retinitis pigmentosa with intrafamilial variability and incomplete penetrance in two families carrying mutations in PRPF8. Investigative ophthalmology and visual science. 2011; 52: 9304-9309.
- Xu G, Li T, Chen J, et al. Autosomal dominant retinitis pigmentosa-associated gene PRPF8 is essential for hypoxia-induced mitophagy through regulating ULK1 mRNA splicing. Autophagy. 2018; 14: 1818-1830.
- Kurtovic-Kozaric A, Przychodzen B, Singh J, et al. PRPF8 defects cause missplicing in myeloid malignancies. Leukemia. 2015; 29: 126-136.
- Cao Difei, Huang Guoqing, Xue Jiaying, et al. The Role of PRPF8 Protein in Alternative Splicing of Eukaryotes. Chinese Herbivore Science. 2021; 41: 49-51.
- Xu Q, Deng B, Li M, et al. circRNA-UBAP2 promotes the proliferation and inhibits apoptosis of ovarian cancer though miR-382-5p/PRPF8 axis %J Journal of Ovarian Research. 2020; 13: 81.
- Allende-Vega N, Dayal S, Agarwala U, et al. p53 is activated in response to disruption of the pre-mRNA splicing machinery. Oncogene. 2013; 32: 1-14.
- Rines DR, Gomez-Ferreria MA, Zhou Y, et al. Whole genome functional analysis identifies novel components required for mitotic spindle integrity in human cells. Genome Biol. 2008; 9: 12.
- Fan J, Zhang YQ, Li P, et al. Interaction between plasminogen activator inhibitor type-2 and pre-mRNA processing factor 8 . Acta Biochim Biophys Sin. 2004; 36: 623-628.
- Xu Q, Deng B, Li M, et al. circRNA-UBAP2 promotes the proliferation and inhibits apoptosis of ovarian cancer though miR-382-5p/PRPF8 axis. Journal of ovarian research. 2020; 13: 1-10.
- Chan S, Sridhar P, Kirchner R, et al. Basal-A triple-negative breast cancer cells selectively rely on RNA splicing for survival. Molecular cancer therapeutics. 2017; 16: 2849-2861.