
Research Article
Austin J Cancer Clin Res. 2022; 9(1): 1102.
Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection among Women in Beijing, China: A Two-Year Cross-Sectional Comparative Study
Qiu XM1#, Liu RZ1#, Wang SZ2 and Zhang XF2*
¹Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
²Department of Obstetrics and Gynecology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
#These authors have contributed equally to this article
*Corresponding author: Zhang XF, Department of Obstetrics and Gynecology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Received: May 09, 2022; Accepted: June 27, 2022; Published: July 04, 2022
Abstract
Objective: Our study aimed to observe the dynamic epidemiological characteristics of high-risk human papillomavirus (HR-HPV) infection among women in Beijing, China, between 2015 and 2020.
Methods: A retrospective analysis was performed on all collected cervical specimens from women who underwent HR-HPV examination in the outpatient clinic, ward, and physical examination center of Beijing Chao yang Hospital, Capital Medical University, from April to December 2015, and from April to December 2020. Real-time polymerase chain reaction (PCR) was applied to detect 15 HR-HPV genotypes.
Results: A total of 26003 patients were enrolled in the study. No statistical difference was detected in the HR-HPV infection rate between the two years (22.1% versus 23.1%, P>0.05). The top five genotypes were HPV52/58, 16, 56 and 51, in descending order in both years. Single HR-HPV infection was the most frequent infection type in both years. The proportion of single infection in 2015 and 2020 were 73.32% (2218/3025) and 76.22% (2167/2843), respectively. There was no significant difference in infection rates across age groups in 2015, but the infection rate curve of 2020 was “bimodal”, with two peaks in young women (≤24 years-old group) group and the 60-64 years-old (y) group, with the trough in the 45-49y group.
Conclusions: There was no significant change in the overall HR-HPV infection rate of women in Beijing, and the genotype distribution of HR-HPV seldom changed except for the age-related infection rate during the last 5 years. These findings may provide baseline information for local administrations topromote targeted HPV screening and HPV vaccination.
Keywords: Human Papillomavirus; Infection; Prevalence Rate; Genotype
Abbreviations
AIS: Adenocarcinoma in Situ; CIN: Cervical Intraepithelial Neoplasia; CSCCP: Chinese Society for Colposcopy and Cervical Pathology of China Healthy Birth Science Association; GCO: Global Cancer Observatory; HPV: Human Papillomavirus; HR-HPV: High- Risk HPV; HSIL: High-Grade Squamous Intraepithelial Lesions; LRHPV: Low-Risk HPV; PCR: Polymerase Chain Reaction; Y: Yearsold.
Introduction
According to the Global Cancer Observatory (GCO), the incidence of cervical cancer in women ranked fourth (8.4%) in 2020, and the mortality rate also ranked fourth (7.7%). Cervical cancer is also the global cancer model’s leading cause of death in 36 countries. Affected by the increase in socio-economic level, the improvement of reproductive health, related publicity and education work, and the continuous implementation of HPV vaccination work, the incidence of cervical cancer in developed countries showed a downward trend [1]. However, cervical cancer remains a burden in China. In the past 15 years, the incidence and mortality rate of cervical cancer have shown an increasing trend. The incidence rate has increased from 11.26/100,000 in 2003 to 13.73/100,000 in 2018, and the mortality rate has risen from 4.88/100,000 in 2003 to 5.89/100,000 in 2018 [2]. So cervical cancer continues to be one of the leading causes of death in Chinese women.
HPV is a diverse group of small DNA viruses consisting of double-stranded cyclic DNA with 8,000 base pairs. The persistence of viral infections and the uncontrolled expression of E6 and E7 viral oncogenes are critical to its transformation. Since the discovery in the 1990s that persistent HR-HPV infection is a necessary but inadequate condition for invasive cervical cancer [3], HPV testing has gradually become an essential form of secondary prevention of cervical cancer. 80% of women are infected with HPV throughout their lifetime, but 90% of these infections will go away automatically [4]. Among the more than 100 HPV subtypes discovered so far, they can be subdivided into HR-HPV, possible HR-HPV, and low-risk HPV (LR-HPV), according to the degree of malignancy of the disease. Of the 15 types of HR-HPV, HPV16, 18, 45, 31, 33, 52, 58, and 35 were responsible for 95 percent of HPV DNA-positive cervical squamous cell carcinomas [5].
Cervical cytology, HPV vaccination, and HPV infection screening are all effective methods of preventing cervical cancer and precancerous lesions [6], with HPV vaccination providing a 66 to 100 percent protective effect against cervical cancer [7]. However, HPV infection rates, HPV subtype distribution, and high-risk age groups can vary between different countries and regions around the world or within a country due to demographic, geographic, socioeconomic level, and ethnic differences [8]. At the same time, with the development of vaccine immunization and herd immunity effects, the distribution of significant subtypes of HPV will also change [9,10]. As a result, understanding the epidemiological characteristics and trends of HR-HPV infection is critical for HPV screening and vaccine development. Although there are many epidemiological studies of HR-HPV infection in different regions in China, the existing studies still have insufficient data and a lack of comparison over time. Therefore, this study conducted a retrospective crosssectional investigation in 2015 and 2020, respectively, and obtained the epidemiological characteristics and changing trends of HR-HPV infection in two years, which provided an essential basis for the prevention and treatment of cervical cancer and HPV vaccination in women in Beijing. To the best of our knowledge, this is the first study to compare a two-year cross-sectional HR-HPV infection status, which provides horizontal and longitudinal perspectives for further study.
Materials and Methods
Clinical Data Collection
A total of 26933 women aged 16-95y presented to the outpatient clinics, wards, and physical examination center of Beijing Chao yang Hospital from April to December in 2015 and from April to December in 2020 were tested for type-specific HR-HPV analyses. Inclusion criteria: previous sexual history; non-menstrual period; non-pregnancy; non-breastfeeding period. Exclusion criteria: no prior history of sexual life; women who were menstruating, pregnant, or lactating when sampling. Patients’ information was obtained from medical records. The Ethics Committee approved this study of Chao yang Hospital, affiliated with Capital Medical University.
Sampling Method
Patients did not use intravaginal medications, vaginal douching, or sterilization for 3 days before testing; they were a sexual within 24 hours. Sampling method: use an aseptic cotton swab to wipe the cervical orifice secretions clean; take a cervical sample from each woman with a cell brush; re-suspend the sample in 2.5mL of liquid cytology medium; and send it for testing for type-specific HR-HPV.
HPV DNA Genotyping
HPV DNA genotype testing was performed using a HPV genotyping Real-time PCR kit (Shanghai ZJ Bio-Tech Co., LTD.) according to standard instructions as described in previous studies [11]. Fifteen HPV genotypes were detected (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 82).
Statistical Analysis
Analyses were conducted using the commercially available statistical SPSS25.0 software. The positive rate and infection rate were used as descriptive indicators; the Chi-square test was used for statistical analysis. Statistical significance was accepted if the p value was less than 0.05.
Results
Overall Prevalence Rate of HR-HPV Infection
In 2015, 13692 women were enrolled in the study, of which 3025 were HR-HPV positive, with an infection rate of 22.1%. In 2020, 12311 women were enrolled in this study, of which 2843 were HRHPV positive, with an infection rate of 23.1%. The results showed no statistical differences (P>0.05).
The Distribution of HR-HPV Genotypes
In 2015, the top five positive rates of HR-HPV infection genotypes were HPV58, 52, 16, 56 and 51, in descending order. However, in 2020, the top five HR-HPV genotypes from high to low were HPV52, 58, 16, 56 and 51. Overall, the most prevalent HPV genotypes in both years were HPV52/58, followed by HPV16. The positive rates of HPV18 and HPV 16 had a mild decline in 2020 compared with that in 2015, but there was no significant difference between the two years (Table 1). The prevalence rate of HPV58 and 68 in 2020 was lower than that in 2015 (P<0.05).In contrast, the prevalence rate of HPV66 and 82 was higher than that in 2015 (P<0.05) (Figure 1).
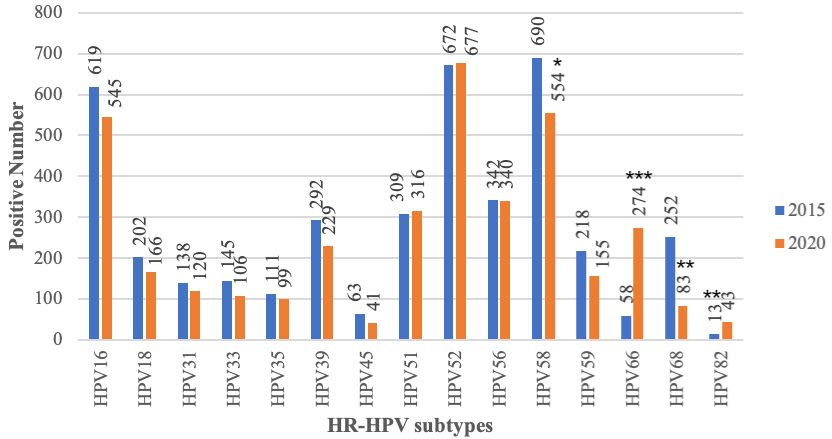
Figure 1: Comparison of HR-HPV infection subtypes in 2015 and 2020.The
blue column represented the infection amount in 15 subtypes of HR-HPV in
2015. The orange column represented the infection amount in 15 subtypes of
HR-HPV in 2020. (*P<0.05; **P<0.01; ***P<0.001).
HR-HPV subtypes
2015
2020
P-value
N
Positive
(N)Prevalence (%)
N
Positive
(N)Prevalence (%)
HPV16
HPV18
HPV31
HPV33
HPV35
HPV39
HPV45
HPV51
HPV52
HPV56
HPV58
HPV59
HPV66
HPV68
HPV8213692
619
202
138
145
111
292
63
309
672
342
690
218
58
252
134.5
1.5
1.0
1.1
0.8
2.1
0.5
2.3
4.9
2.5
5.0
1.6
0.4
1.8
0.10
12311
545
166
120
106
99
229
41
316
677
340
554
155
274
83
434.4
1.3
1.0
0.9
0.8
1.9
0.3
2.6
5.5
2.8
4.5
1.3
2.2
0.7
0.30.542
0.818
0.149
0.211
0.070
0.061
0.062
0.064
0.202
0.251
0.034
0.129
<0.001
0.006
0.003
Table 1: Comparisons of HR-HPV infection subtypes in 2015 and 2020.
The Distribution Characteristics of Single and Multiple Infections
Single infection was the main form of HR-HPV infection, accounting for 73.3% in 2015and 76.2% in 2015, respectively. Among the multi-infected individuals, as the number of genotypes increased, the number of people decreased. The rate of triple infections in 2020 was obviously lower than that in 2015 (P=0.006). Notably, one case was infected with 8 HR-HPV subtypes simultaneously in 2015 (Table 2).
Numbers of Genotypes
2015
2020
P-value
Positive number
Prevalence (%)
Positive number
Prevalence (%)
1
2218
73.32
2167
76.22
0.011
2
587
19.40
511
18.00
0.160
3
168
5.56
114
4.01
0.006
4
38
1.26
40
1.41
0.614
5
11
0.36
9
0.32
0.757
6
1
0.03
2
0.07
0.528
7
1
0.03
0
0.00
0.332
8
1
0.03
0
0.00
0.332
Total
3025
100.0
2843
100
Table 2: Distribution characteristics of single and multiple HR-HPV infections in 2015 and 2020.
In 2015, HPV39, 68 and 59 were prone to be co-infected with other subtypes, while the most prevalent HR-HPV subtypes, HPV58, 52, and 16, were mostly single-infected. In 2020, each HPV subtype was mainly single infected (Figure 2). The proportion of multiple infections with HPV39 and HPV68 decreased significantly, from 71.9% in 2015 to 41.9% in 2020 (P<0.001), and from 70.2% to 50.6% (P=0.001), respectively (Table 3). Additionally, the common combinations of genotypes for double HPV infections were HPV39+68 in 2015 and HPV16+58 in 2020 (Table 4).
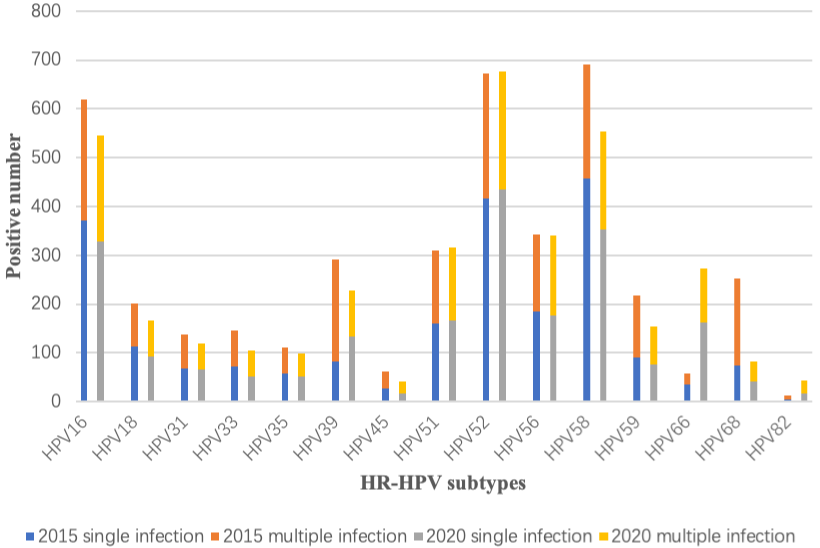
Figure 2: Comparison of single and multiple infections of HR-HPV subtypes
in 2015 and 2020. The blue column represented the singe HR-HPV infection
amount in 2015. The orange column represented the multiple HR-HPV
infection amounts in 2015. The grey represented the singe HR-HPV infection
amount in 2020. The yellow column represented the multiple HR-HPV
infection amounts in 2020.
HPV subtypes
2015
2020
Positive number
Prevalence (%)
Positive number
Prevalence (%)
P-value
HPV39
210
71.9
96
41.9
<0.001
HPV68
75
70.2
42
50.6
0.001
Table 3: Comparison of multiple infections of HR-HPV subtypes in 2015 and 2020.
Sequence
2015
2020
Combination of subtypes
Positive number
Prevalence (%)
Combination of subtypes
Positive number
Prevalence (%)
1
39+68
64
10.9
16+58
31
6.07
2
52+58
34
5.79
52+58
25
4.89
3
16+58
30
5.11
52+56
23
4.50
4
16+52
28
4.77
16+52
22
4.31
5
16+51
19
3.24
16+51
21
4.12
Table 4: Common combination HR-HPV subtypes in double infections.
There was no significant difference between 2015 and 2020 in terms of the distribution of single or multiple infections by age group, except for the 40-44y group. The proportion of multiple infections within this age group decreased from 27.4% in 2015 to 15.1% in 2020 (P<0.001) (Figure 3).
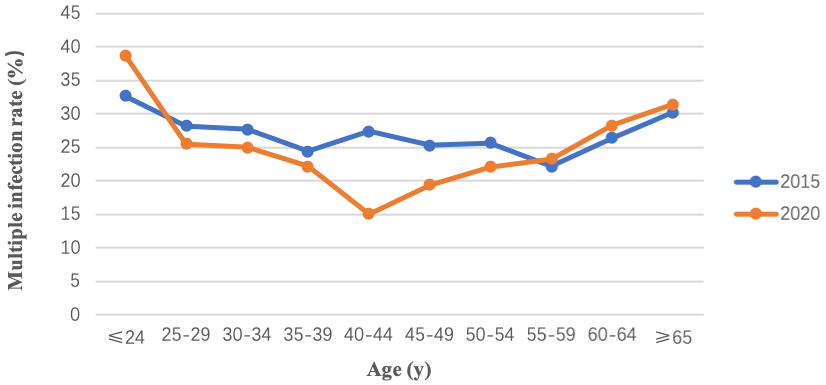
Figure 3: Comparison of multiple HR-HPV infection rates by age groups in
2015 and 2020. The blue broken line represented multiple HR-HPV infection
rates of different age groups in 2015.
The orange broken line represented multiple HR-HPV infection rates of
different age groups in 2020.
Age Distribution Characteristics of HR-HPV Infection
Comparison of infection rates by age group over two year: We divided the participants into 10 groups by age, according to the HR-HPV screening age range and intervals from previous studies [11,12]. There was no significant difference in infection rate between age groups in 2015 (P>0.05)(data not shown, while the age-related infection rate curve in 2020 showed a double-peak pattern, and the infection rate between age groups had statistical differences within the group (P<0.001) (Figure 4). The first peak was in the ≤ 24y group, with an age-related infection rate of 34.47%, and the second peak was in the 60-64y group with an age-related infection rate of 27.79%. There were statistical differences in HR-HPV infection rates between the two years in the ≤ 24y groups (P<0.001), 45-49y (P=0.029), and 60-64y (P=0.005) (Figure 4).

Figure 4: Comparison of infection rates by age groups in 2015 and 2020.
The blue broken line represented the total HR-HPV infection rates of different
age groups in 2015. The orange broken line represented the total HR-HPV
infection rates of different age groups in 2020.
The proportion of HR-HPV subtypes in all ages: There was no statistically significant difference in the proportion of age groups in each subtype among positive patients in 2015. However, the proportion of age groups in HPV16, 35, 39, 51, 56, 58, and 82 subtypes showed statistical differences among the positive patients in 2020.When comparing the proportion of each age group in each HPV subtype, the ≤24y group accounted for the highest proportion in HPV16 (P=0.038), HPV35 (P=0.002), HPV 39 (P=0.044), and HPV 82 (P=0.005) subtypes; the 25-29y group accounted for the highest proportion in HPV 51 subtype(P=0.024); the 55-59y group accounted for the highest proportion in HPV56 subtype (P<0.001); and the60-64ygroup accounted for the highest proportion in HPV58 subtype (P=0.006) (Table 5).
HPV subtype
Age groups
P-value
≤24(%)
25-29(%)
30-34(%)
35-39(%)
40-44(%)
45-49(%)
50-54(%)
55-59(%)
60-64(%)
≥65(%)
HPV16
27.9
20.4
20.5
18.1
17.1
12.0
18.8
17.8
19.1
25.5
0.038
HPV18
5.4
6.5
6.2
5.8
6.5
6.5
5.2
4.0
5.3
3.9
0.956
HPV31
2.7
3.5
5.0
5.0
5.8
3.7
1.9
4.0
4.6
2.0
0.429
HPV33
5.4
4.2
3.3
5.2
2.7
1.9
2.3
5.9
2.0
3.9
0.202
HPV35
7.2
2.1
2.7
2.8
5.5
5.6
5.2
0.5
2.6
6.9
0.002
HPV39
11.7
6.7
9.2
9.7
7.2
9.7
9.4
3.5
5.3
3.9
0.044
HPV45
2.7
0.7
1.1
2.6
0.3
2.3
2.3
0.5
0.7
2.9
0.061
HPV51
11.7
16.0
11.1
10.8
9.6
6.9
8.0
8.9
12.5
13.7
0.024
HPV52
27.0
26.5
24.8
22.2
18.8
24.5
20.7
28.2
21.7
23.5
0.269
HPV56
6.3
11.8
10.8
9.5
11.3
9.3
12.7
23.3
14.5
17.6
<0.001
HPV58
20.7
16.9
19.8
19.2
17.5
23.6
18.3
12.9
29.6
25.5
0.006
HPV59
8.1
4.9
5.9
4.5
7.2
4.2
6.6
5.4
3.9
3.9
0.648
HPV66
11.7
11.6
9.4
8.8
7.9
8.8
12.7
7.9
9.2
8.8
0.626
HPV68
2.7
3.7
2.6
1.7
3.4
5.6
1.4
4.5
2.6
1.0
0.124
HPV82
6.3
2.1
1.4
1.7
0.3
1.4
1.4
0.5
0.7
1.0
0.005
Table 5: Comparison of the proportion of HR-HPV positive patients in 2020 by age groups.
Comparison of susceptible HR-HPV subtypes by age group: Compared with 2015, the proportion of HPV52, 66, and 82 infections increased in ≤ the 24y group in 2020(P=0.030, P=0.002, P=0.042), but the proportion of HPV68 infections decreased (P=0.009). In the 60-64y group, the infection rate of HPV66 increased significantly in 2020, compared with 2015(P=0.014). The overall decrease of infection rates in 2020 in the 45-49y group may be due to decreased infection rates for HPV16 (P=0.036) and HPV33 (P=0.012) (Table 6).
Year
HR-HPV subtypes
16(%)
18(%)
31(%)
33(%)
35(%)
39(%)
45(%)
51(%)
52(%)
56(%)
58(%)
59(%)
66(%)
68(%)
82(%)
2015
20.8
9.9
4.0
4.0
5.9
13.9
4.0
8.9
14.9
11.9
21.8
7.9
1.0
11.9
1.0
2020
27.9
5.4
2.7
5.4
7.2
11.7
2.7
11.7
27
6.3
20.7
8.1
11.7
2.7
6.3
P-value
0.228
0.216
0.609
0.62
0.711
0.639
0.609
0.504
0.03
0.156
0.850
0.960
0.002
0.009
0.042
45-49y group
Table 6: Comparison of susceptible HR-HPV subtypes by age groups in 2015 and 2020. ≤24y group.
Year
HR-HPV subtypes
16(%)
18(%)
31(%)
33(%)
35(%)
39(%)
45(%)
51(%)
52(%)
56(%)
58(%)
59(%)
66(%)
68(%)
82(%)
2015
19.0
8.8
3.3
6.6
1.5
8.1
1.8
9.2
23.4
11.4
27.5
5.9
2.6
6.2
0.4
2020
12.0
6.5
3.7
1.9
5.6
9.7
2.3
6.9
24.5
9.3
23.6
4.2
8.8
5.6
1.4
P-value
0.036
0.343
0.807
0.012
0.012
0.519
0.708
0.375
0.778
0.451
0.332
0.398
0.002
0.755
0.213
60-64y group
Table 6 off 1:
Year
HR-HPV subtypes
16(%)
18(%)
31(%)
33(%)
35(%)
39(%)
45(%)
51(%)
52(%)
56(%)
58(%)
59(%)
66(%)
68(%)
82(%)
2015
18.2
7.3
3.6
3.6
2.7
9.1
1.8
7.3
22.7
15.5
30.9
7.3
1.8
5.5
1.8
2020
19.1
5.3
4.6
2.0
2.6
5.3
0.7
12.5
21.7
14.5
29.6
3.9
9.2
2.6
0.7
P-value
0.854
0.503
0.700
0.410
0.962
0.227
0.384
0.170
0.845
0.826
0.820
0.238
0.014
0.239
0.384
60-64y group
Table 6 off 2:
Discussion
Our cross-sectional study assessed the prevalence rate of HRHPV infection and the distribution of HR-HPV subtypes in Beijing Chao yang Hospital and further compared the infection situation of 2015 with that of 2020. We expected to lay the foundation for a better understanding of the dynamic epidemiological characteristics in Beijing and provide baseline data for HPV screening and vaccination.
This study observed that the infection rate of women who underwent HR-HPV examinations in Beijing Chao yang Hospital in 2015 and 2020 was 22.1% and 23.1%, respectively. There was no significant change in the HR-HPV infection rate between the two years. Our HR-HPV infection rate data is supported by a study conducted by Beijing Friendship Hospital between 2017 and 2020 [13], but it is higher than11.78% reported by another Beijing-based HR-HPV infection study [14]. Such discrepancies may be due to the different inclusion criteria. The latter study only included healthy women who presented for regular check-ups, whereas our study population also included women from gynecological clinics and cervical cancer screening.
Our study discovered that HPV52/58 were the dominant infected HR-HPV subtypes in both years, followed by HPV16, which was consistent with the findings of other studies in Beijing [14], Shanghai [15], and Fujian [16] in recent years. However, previous studies believed that HPV16 was the primary subtype in China, followed by HPV52/58 [17]. We considered distinctions in regions, time and the source of respondents were important factors leading to this difference. Shan Wei et al. [18] classified the surveyed population of HR-HPV infection and found that in the combined population of healthy women and women from the gynecological clinic, the HR-HPV subtypes were predominantly HPV52/58; while HPV 16 predominated in healthy women or women from the clinic. Although the main HR-HPV subtypes did not change in either year, there were fluctuations in specific subtypes. Compared with the year 2015, the prevalence of HPV58 and 68 infections showed a decreasing trend, whereas the prevalence of HPV66 and 82 infections exhibited a rising trend.
Single and multiple infections have different clinical implications. Studies have shown that the single infection is the risk factor for developing cervical intraepithelial neoplasia III (CIN III), cervical cancer, and cervical lesion progression. While multiple infections do not increase the risk of developing cervical cancer, they can cause persistent HR-HPV infection and are associated with CIN I-II lesions [19,20]. In the present study, the proportion of single infection was significantly higher than that of multiple infections and showed an upward trend, which was consistent with the findings of previous domestic studies [13,21]. This indicated that most women in China were infected with only one type of HR-HPV. This might be caused by competition between different HR-HPV subtypes. In both years, the common combinations of genotypes in multiple HR-HPV infections were HPV16+58, HPV52+58, HPV16+52, and HPV16+51. These subtypes were commonly detected genotypes. In addition, the most frequent combination of genotypes in 2015 was HPV39+68, which was also reported in a previous study [14]. It is worth noting that although HPV39 and HPV68 were not among the common genotypes of infection in this study, co-infection of the two subtypes accounted for 11.17% of the double infections. HPV 39 and HPV68 belong to the alpha-7 strain, and infection of this strain is a potential risk factor for developing endocervical gland lesions and cervical adenocarcinoma [22]. Therefore, future follow-up studies could focus on the mechanism of co-infection of these two genotypes. It is a question needing to be answered whether it is just a simple co-infection or there is a synergistic effect between the two subtypes.
Previous epidemiological studies of HR-HPV infection in Chinese women have revealed that the curve of age-related HR-HPV infection rate is bimodal, that is, the infection rate peaks in young women (< 21 years old or 20-24 years old), then declines, reaches a plateau, and then rises again in middle-aged and elderly women (> 50 years old) [21,23,24]. In our study, the first peak of the agerelated infection rate in 2020 was in ≤ 24y young women, and the second peak was slightly lower than the first peak and appeared in the 60-64y population, which was consistent with the above studies’ results [21,23,24]. Sexual debut and number of sexual partners are direct factors in the acquisition of the HPV [25]. Immune response including innate and adaptive immune response is co-players in the resolution of HPV infection, with cell-mediated immune response being the most common mechanism [26]. Moreover, estrogen is considered to have immunoenhancing effects, and transitioning into reproductive age may be associated with enhanced sex hormones [27]. Although young age is a risk factor for the presence of HPV, its specific role in the acquisition process of HPV has not been fully elucidated. Hou Lihan et al. reported a rapid acquisition and high incidence of HPV infection, as well as rapid resolution in young women after their first reported sex [28]. Here, we postulates the high infection rate in young women is associated with the fact that young women are in their initial sexual activity stage, they might have a weak sense of health and hygiene, and the high transmission nature of HPV [26,29]. Therefore, it is recommended that young women who have sex undergo gynecological physical examinations every year. The second peak in the 60-64y population may be associated with the following reasons: (1) Perimenopausal hormone fluctuations and physiological changes cause low immunity, which reactivates the previously infected virus in a low-replication state, resulting in a detect able number of replications; (2) the cumulative number of sexual partners in a lifetime, or changes in sexual behavior [6,30]; (3) > 60ywomen are not subjects of regular physical examinations and often go to the doctor because of having symptoms, thus resulting in a high positive rate. However, in 2015, no differences were found in the HR-HPV infection rate across age groups, so the influencing factors of the age-related HR-HPV infection rate need to be further elucidated in follow-up studies.
The two age groups with the highest prevalence of HR-HPV infections showed an upward trend, and this was mainly due to the increased HPV52, 66, and 82 infections. The 30-34y group had the highest number of detected cases, but the infection rate was not the highest. This might be due to the rigorous health awareness, regular physical examinations, and stable sexual partners in this age group. It should also be noted that the age-related HR-HPV infection rate in the ≥ 65y group was also at a relatively high level, higher than that of women in the 35-54y group. Therefore, we suggested that not only the routine screening of young women should be emphasized but also the education on HR-HPV screening awareness in older women should be strengthened. At present, the Colposcopy and Cervical Pathology Branch (CSCCP) of the Chinese Eugenics Science Association explained in 2018 that the expert consensus interpretation of issues related to cervical cancer screening and abnormal management in China [31] sets the initial screening age at 25 to 30 years old and the end screening age after 65 years old, if there is no abnormality in 3 consecutive cytology tests every 3 years in the past 10 years or 2 consecutive HR-HPV tests every 5 years, and there is no history of cervical endothelial neoplasia. Combined with our results, HR-HPV infection in young women is increasing, and it seems more reasonable to advance the screening age to before the age of 24. However, young women primarily have latent infections that will resolve spontaneously within 1 to 2 years [6]. Additionally, consideration should also be given as to whether to delay or cancel the screening termination age. However, if the above screening strategy is adopted, its socio-economic implications need to be further explored.
As the first vaccine to prevent tumors, the HR-HPV vaccine has attracted attention from all parties. China has also introduced bivalent, quadrivalent, and nine-valent HPV vaccines in 2016, 2017, and 2018, respectively, and successfully obtained marketing authorization [32,33]. Persistent infection with HPV16/18 strains is responsible for more than 70% of cervical cancers [34]. Moreover, the optimal time to get vaccinated is considered to be before the first sexual interaction. Between15 and 26 years old, women who are vaccinated and are HPV 16/18 negative prior to vaccination showed a reduced risk of developing high-grade squamous intraepithelial lesions (HSIL) and even HPV16/18-related adenocarcinoma in situ (AIS),compared with their HPV16/18 positive counterparts. However, all three doses must be given as required; otherwise, there is no protective effect [35]. The domestic HPV vaccine has been on the market for several years, and public awareness and willingness to be vaccinated has gradually increased. Yet, the awareness of the specific functions, protection range, and optimal vaccination time of HPV vaccines is still low among the public, and there is a lack of willingness of parents to get their daughters vaccinated [36], which is speculated to be related to the low attention to cervical cancer, bad HPV vaccine publicity and education, and high costs for non-national immunization programs.
The limitations of the study need to be addressed. Although a comparison of the two years was made, it wasn’t clear whether there were fluctuations between 2015 and 2020; there was a lack of analyses of the characteristics of the study population; and the correlation between HPV genotypes and cervical cytology remained unclear. Data from January to March were lacking, the climatic factors might play a part on the result [37]. In future studies, we are aiming to conduct continuous annual studies in multiple centers to reflect the epidemiological trend more realistically. Participants should be classified based on their source. Further HPV vaccination status should also be established, for example, valent, and vaccination time and completion status.
Conclusions
In conclusion, this retrospective study provided large scale data of dynamic characterize HR-HPV infections among women in Beijing in 2015 and 2020.There was no significant change in the overall HRHPV infection rate in the both years. Although single-infection still dominated in the population, specific combination of dual infections could not be neglected. Recent age-related infection rate curve showed a bimodal trend, emphasizing the importance of HR-HPV screening in both young and old population. These findings may provide baseline information for local administrations to promote targeted HPV screening and HPV vaccination.
Acknowledgments
All of the authors have no relationship with companies, and have no financial interest.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021; 71(3): 209-249. doi:10.3322/caac.21660.
- Zhang ZH, Liu CY, Ren HY, Liang SH. Analysis and prediction of the incidence and mortality trends of cervical cancer in Chinese women from 2003 to 2018. Chinese Journal of Disease Control & Prevention, 2022; 26(01): 14-20.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189(1): 12-9.
- Gong P, Wang Z, Geng J, Tan X. Comparative Study on Detection and Typing of Human Papillomavirus (HPV) Infection with Microarray Using Paraffin-Embedded Specimens from Squamous Cell Carcinoma and Cervical Precursor Lesions. Journal of nanoscience and nanotechnology. 2017; 17(2): 990-997. doi:10.1166/jnn.2017.12769.
- Muñoz N, Bosch FX, Sanjosé SD, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. The New England journal of medicine. 2003; 348(6): 518- 527. doi:10.1056/NEJMOA021641.
- Gravitt PE, Winer RL. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses. 2017; 9(10): 267. doi:10.3390/v9100267.
- Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, et al. HPV Vaccination and the Risk of Invasive Cervical Cancer. The New England journal of medicine. 2020; 383(14): 1340-1348. doi:10.1056/NEJMoa1917338.
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, Sanjosé SD. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. The Journal of infectious diseases. 2010; 202(12): 1789-1799. doi:10.1086/657321.
- Freire-Salinas J, Benito R, Azueta A, Gil J, Mendoza C, Nicolas M, et al. Genotype Distribution Change After Human Papillomavirus Vaccination in Two Autonomous Communities in Spain. Front Cell Infect Microbiol. 2021; 11: 633162.
- Drolet M, Bénard, Pérez N, Brisson M, Ali H, Boily M, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. The Lancet. 2019; 394(10197): 497-509. doi:10.1016/S0140-6736(19)30298-3.
- Liu Y, He X, Xu S, Qu J, Wang Y, Diao X, et al. Epidemiology and genotype distribution of high risk human papillomavirus in population of hospital opportunistic screening. International journal of clinical and experimental medicine. 2015; 8(9): 16007-14.
- Rebolj M, Rimmer J, Denton K, Tidy J, Mathews C, Ellis K, et al. Primary cervical screening with high risk human papillomavirus testing: observational study. The BMJ. 2019; 364: l240. doi:10.1136/bmj.l240.
- Zhu X, Wang Y, Lv Z, Su J. Prevalence and genotype distribution of high-risk HPV infection among women in Beijing, China. Journal of Medical Virology. 2021; 93(8): 5103-5109. doi:10.1002/jmv.27013.
- Yu H, Yi J, Dou Y, Chen Y, Kong L, Wu J. Prevalence and Genotype Distribution of Human Papillomavirus Among Healthy Females in Beijing, China, 2016–2019. Infection and Drug Resistance. 2021; (14): 4173-4182. doi:10.2147/IDR.S332668.
- Meng J, Xu XS, Lu YY, Cai G. 2017-2020 survey of HPV infection subtypes in the cervical exfoliated cells in Shanghai. Journal of Diagnostics Concepts & Practice. 2021; 20(06): 567-72.
- Xu HJ, Liu K, Zheng JM, Jiang QL, Lu HD, Xu JF. Human papilloma virus (HPV) infection and cervical lesions screened in 3144 women at Xiamen, Fujian province, China. Basic and Clinical Medicine. 2021; 41(11): 1643-7.
- Li K, Li Q, Song L, Wang D, Yin R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer. 2019; 125(7): 1030- 1037. doi:10.1002/cncr.32003.
- Shan W, Zhang T, Zhang TJ, Zhao GM. The epidemiological situation of human papillomavirus (HPV) infection among women in China. Chinese Journal of Disease Control & Prevention. 2017; 21(01):89-93.
- Baloch Z, Li Y, Yuan T, Feng Y, Liu Y, Tai W, et al. Epidemiologic characterization of human papillomavirus (HPV) infection in various regions of Yunnan Province of China. BMC Infectious Diseases. 2016; 16(1). doi:10.1186/s12879-016-1562-7.
- Bruno MT, Scalia G, Cassaro N, Boemi S. Multiple HPV 16 infection with two strains: a possible marker of neoplastic progression. BMC Cancer. 2020; 20(1). doi:10.1186/s12885-020-06946-7.
- Bao H, Jin C, Wang S, Song Y, Xu Z, Yan X, et al. Prevalence of cervicovaginal human papillomavirus infection and genotypes in the pre-vaccine era in China: A nationwide population-based study. The Journal of infection. 2021; 82(4): 75-83. doi:10.1016/j.jinf.2021.02.017.
- Seoud M, Tjalma WAA, Ronsse V. Cervical adenocarcinoma: moving towards better prevention. Vaccine. 2011; 29(49): 9148-9158. doi:10.1016/j. vaccine.2011.09.115.
- Luo LP, He P, Liu QT, Jiang YH, Zhang YN, Li QZ, et al. Prevalence and genotype distribution of HPV infection among 214,715 women from Southern China, 2012-2018: baseline measures prior to mass HPV vaccination. BMC Infect Dis. 2021; 21(1): 328.
- Feng D, Wang Y, Zheng HY, Shen FJ. Analysis of HPV genotyping results of 74590 females. Medical Journal of Wuhan University: 1-6.
- Schneider A. Pathogenesis of genital HPV infection. Genitourinary Medicine. 1993; 69: 165-73.
- de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018; 47: 2-13.
- Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015; 14(3): 309-321. doi:10.1111/acel.12326.
- Houlihan CF, Baisley K, Bravo IG, Kapiga S, Sanjosé SD, Changalucha J, et al. Rapid acquisition of HPV around the time of sexual debut in adolescent girls in Tanzania. International Journal of Epidemiology. 2016; 45(3): 762- 773. doi:10.1093/ije/dyv367.
- Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecologic oncology. 2008; 110(3): S4-S7. doi:10.1016/j. ygyno.2008.07.045.
- Ryser MD, Rositch A, Gravitt PE. Modeling of US Human Papillomavirus (HPV) Seroprevalence by Age and Sexual Behavior Indicates an Increasing Trend of HPV Infection Following the Sexual Revolution. The Journal of Infectious Diseases. 2017; 216(5): 604-611. doi:10.1093/infdis/jix333.
- Zhao Y, Wei LH. CSCCP Expert Consensus Interpretation on Cervical Cancer Screening and Abnormal Management in China. Journal of Practical Obstetrics and Gynecology. 2018; 34(02):101-104.
- WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition: use of mRNA tests for human papillomavirus ( HPV) .
- National Medical Products Administration.
- Guan P, Howell-Jones R, Li N, Bruni L, Sanjosé SD, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: A metaanalysis from cervical infection to cancer. International Journal of Cancer. 2012; 131(10): 2349-2359. doi:10.1002/ijc.27485.
- Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. The Cochrane database of systematic reviews. 2018; 2020(3): CD009069. doi:10.1002/14651858.CD009069.pub3.
- Yan HJ, Su Z, Liu SJ, Fan JH, Qiao YL. Knowledge and attitude toward HPV vaccination promotion after COVID-19 epidemic in various populations in China. Chinese Journal of Public Health. 2021; 37(12): 1731-1736.
- Yan X, Shen L, Xiao Y, Wang Q, Li F, Qian Y. Prevalence, characteristics, and distribution of HPV genotypes in women from Zhejiang Province, 2016– 2020. Virology Journal. 2021; 18(1). doi:10.1186/s12985-021-01676-z.