
Original Article
Austin J Cancer Clin Res. 2022; 9(2): 1103.
In-Silico Modelling and Analysis of the Effect of Immusante on Immune System and Cancer Pathology
Garg A¹, Lomore K¹, Gangwar V¹, Korla K¹, Bhat SS², Rao RP², Rafiq M², Kumawat R², Babu UV², and Venkatesh KV1,3*
¹MetFlux Research Private Limited, Mumbai 400078, India
²Research and Development, Himalaya Wellness, Bengaluru 562162, India
³Indian Institute of Technology (I.I.T.) Bombay, Mumbai 400076, India
*Corresponding author: Venkatesh KV, Department of Chemical Engineering, IIT Bombay, Mumbai, Maharashtra, India
Received: June 29, 2022; Accepted: July 28, 2022; Published: August 04, 2022
Abstract
Cancer therapy has been at the centre stage for decades in the scientific fraternity. Several chemical and biochemical actives have been studied for their effect on cancer therapy. In the present study, we have studied the therapeutic effects of a herbal formulation, Immusante, containing bioactive compounds such as Apigenin, Quercetin, Betulinic Acid, and Oleanolic Acid on cancer cell proliferation, angiogenesis and survival. The immunomodulatory effects of Immusante were incorporated in a cancer-specific mathematical model to quantify the therapeutic effects. Cancer model simulation with Immusante showed a significant reduction of tumour proliferation, reduction of immunosuppressive species, and increase in the CD8 effector T cell function. The effect of the Immusante, along with a chemotherapy drug, 5-fluorouracil (5-FU), was simulated to assess the effect of combination therapy. The addition of 5-FU as a monotherapy showed a 28% increase in the population with no/ very low tumorigenesis. A combination of 5-FU and Immusante showed a 44% increase in the population with no/very low tumorigenesis. A similar trend was seen for population size with low immune suppression, where 5-FU monotherapy showed a 26% increase, Immusante showed 12%, and a combination of 5-FU and Immusante showed a 43% increase in the population with low immune suppression, indicating increased efficiency of effector species in the presence of Immusante. Similar results were obtained for other drugs such as Cyclophosphamide, Platinum-based drugs, Vincristine, Taxane, Irinotecan, and Anthracyclines combined with Immusante. It is observed that chemotherapy alone can bring clearance in the tumour, further augmented by incorporating Immusante as a herbal adjuvant. Simulations suggest that combination enhances effectiveness and reduces the side effects of chemotherapy. It was observed that Immusante could effectively counter side-effects and adaptive response of cancer cells to improve efficacy and normalize immune response. The immunomodulatory properties of Immusante have been shown to restore balance in immune response and reduce the cytotoxic effects of drugs on healthy organs. These characteristics make Immusante a promising adjuvant during cancer management and improve the quality of the treatment.
Keywords: Apigenin; Quercetin; Betulinic acid; Oleanolic acid; 5-Fluorouracil; cancer; Herbal adjuvant
Introduction
Cancer is a complex disease defined by uncontrolled cell growth, mutations, perturbed signalling cascades and metabolism, eventually escaping immune responses. This impairment leads to disturbed cellular growth, proliferation, angiogenesis, metastasis, and disabling of apoptosis and cell cycle checkpoint mechanisms, affecting the immune dynamics, and therefore is considered one of the leading factors for mortality and morbidity worldwide [1,2]. Studies related to immune cell responses and their interactions with tumours and perturbed signalling dynamics have been a centre of various theoretical and mathematical research. A recently published mathematical model discussed the interactions between the immune system dynamics and tumours and observed that tumour dynamics is tangled to two nodes, regulatory and effector cells, i.e., Tregs and CD8 T cells, respectively. It captured the dynamic features of tumour growth by associating three motifs from systems biology: negative feedback, in-coherent feedforward loops, and bistability. The model summarises observations of separate zones of tumour control and focuses on an intermediate region in which tumours can be eliminated [3].
Such deviations in the immune dynamics of the tumour environment have been correlated with perturbations in the intermediate signalling factors and pathways. Several studies have identified STAT3 as a significant molecule that mediates tumourinduced immune suppression and contributor to tumorigenic activity [4-6]. This activity occurs through intermediary steps involving Tumour Associated Macrophages (TAMs) and T regulatory cells (Tregs), mediating crosstalk between the two, generating immunosuppression concerning both innate and adaptive immunity [7]. TAMs within a tumour induce STAT3 activation and release cytokines like IL-10 and IL-6, indicating a correlative relationship between cytokines and tumour growth [8,9]. Tregs can accumulate within the tumour microenvironment, releasing immunosuppressive mediators (e.g., IL-10) and ultimately suppressing immune responses mediated by CD8 T cells [10]. This immunosuppression by antigenic stimulation triggers STAT3 to create an active feed forward mechanism to increase STAT3 activity both in tumour cells and associated immune cells. The cytokines increase STAT3 expression through a positive feedback loop [4].
This cascade of events leads to multiple immune suppressive effects. Various physiological effects can be correlated from previous studies, such as increased angiogenesis through amplified VEGF expression [11], inhibition of Dendritic Cells (DCs) maturation due to upregulation of STAT3, which impairs the immune system’s ability to induce and maintain an anti-tumour immune response [10,12], direct suppression of CD8 T cells function by inducing exhaustion due to induction of PD-L1 expression on tumour or APCs, and indirect suppression of CD8 T cells due to impaired DCs maturation [13,14]. In addition, STAT3 is also implicated in promoting metastasis and impairing p53 based checks on tumour proliferation [15]. Therefore, STAT3 mediated signalling and immunomodulation can cause enhanced proliferation of tumours (in resistance to apoptosis), triggering/activating immune suppressive effects and reducing CD8 T cells effector function. Apart from immunomodulation, such activities contribute to tumour cell migration/invasion, apoptosis/survival, and angiogenesis [16], thus making cancer a long-lasting disease and extending the survival of patients. These continuous changes in physiological conditions within the tumour microenvironment require therapeutic strategies that focus on the major pathways and nodal interacting species.
Current chemotherapeutic strategies for treating cancer include mechanisms like induction of apoptosis or autophagy, regulation of the cell cycle, inhibition of tumour cell migration and invasion, and stimulation of the immune response of patients. Various chemotherapy drugs used are 5-FU (5-Fluorouracil), Cyclophosphamide, Platinum derivatives, Vincristine, Taxane, Irinotecan and Anthracycline. Among these, 5-FU is a widely used chemotherapeutics in treating various cancers. 5-FU is catabolized into active metabolites, resulting in DNA damage [17]. DNA damage causes activation of p53, leading towards cell cycle arrest to repair the damaged DNA. In the absence of a repair process, apoptosis occurs. Another mechanism of apoptosis is through TNF-Rs and TRAIL-Rs and their binding to their respective ligands. 5-FU acts by depleting immature myeloid-derived suppressor cells (MDSCs) that accumulate with tumour progression and suppress T-cell activation, increasing IFN-γ production by tumour-specific CD8 T cells required for cancer management [18]. But the elimination of MDSCs also leads to the activation of NLRP3 inflammasome in dying MDSCs, causing secretion of IL-1β, elicitation of Th17 cells, IL-17 production, henceforth supporting tumour growth [19]. Another study exhibits the effect of 5-FU treatment upregulating PD-L1 expression, involved in negative regulation of the immune response, leading to systemic immunosuppression in gastrointestinal cancers [20,21]. These effects have been documented for various cancers, and their synergy with 5-FU has been observed in several cancers, including head and neck squamous cell carcinoma and colorectal cancer [22]. Such treatments have immense side effects, while surgical interventions are ineffective in preventing cancer metastasis, highlighting the need for combination therapy with chemotherapeutic drugs such as 5-FU and prevent tumour progression.
In these circumstances, herbal medicines containing natural bioactive compounds prove suitable candidates for preventing and treating numerous diseases because of their therapeutic properties, multi-targeted efficacy, and low toxicity. Immusante is one such herbal formulation (by Himalaya Wellness Company) containing Symplocos recemosa Roxb. (Symplocaceae) and Prosopis glandulosa Torr (Fabaceae) extracts that exhibits immunomodulatory properties. Immusante consists of biologically active compounds such as Apigenin, Quercetin, Betulinic Acid, and Oleanolic Acid, considered significant and characterized as the key phytoconstituents of this formulation [23,24]. It is established as efficacious and safe (Srivastava, A. N., Singh, U., and Kolhapure, S. A. 2004. Evaluation of clinical efficacy and safety of IM-133N as an immunomodulator in various carcinomas: A prospective clinical trial. Indian J. Clin. Pract. 15:25–37). Immusante is considered a potential therapeutic entity because of pharmacological properties like antioxidant, antimutagenic, anti-inflammatory, and many others.
Immusante modulates biological processes such as cell proliferation, apoptosis, migration and differentiation, and oxidative balance at the biochemical level and plays a major role in controlling or modulating carcinogenesis [25]. Immusante compounds also inhibit NF-κB, MAPK, JAK/STAT, PI3K/AKT signalling pathways, consequently limiting inflammation. Various studies have demonstrated Immusante compounds effectiveness on different tumour types, altering multiple checkpoints, and interacting at several nodal points of various signalling pathways, thereby demonstrating immunomodulation. These nodal points are well-known target sites for several therapeutic drugs, and their effect on tumour proliferation is summarised extensively in our previous review [26]. These compounds inhibit cell cycle progression, differentiation, angiogenesis, cell survival, death receptor expression and altering the balance of apoptotic proteins. The net effect inhibits proliferation and diminishes tumour cell survival.
The major bioactive compounds of Immusante, such as Apigenin, Quercetin and Betulinic Acid, are known to possess immunomodulatory properties in cancer conditions on the abovementioned physiological effects and suppress STAT3 phosphorylation, nuclear localization and transcriptional activity [27-30]. Quercetin blocks the STAT3 activation pathway stimulated by IL-6, potentially preventing and treating cancer cells [31]. The deterrent activity of these compounds downregulates STAT3 target genes such as VEGF, which are involved in cell growth, proliferation, migration and invasion [32] and induce apoptosis through a p53-dependent pathway [33]. The bioactive compound Apigenin of Immusante also leads to declined expression of PD-L1 in DCs, resulting in improved CD8 T cells function to prevent tumour cell escape [34]. Also, as discussed in our previous review, these bioactive compounds can reduce tumour cell proliferation and migration, sensitizing the apoptotic pathways and cell cycle checkpoints [26]. Based on the observations mentioned above, the macro-level immunomodulatory effects of Immusante are shown through green arrows in (Figure 1).
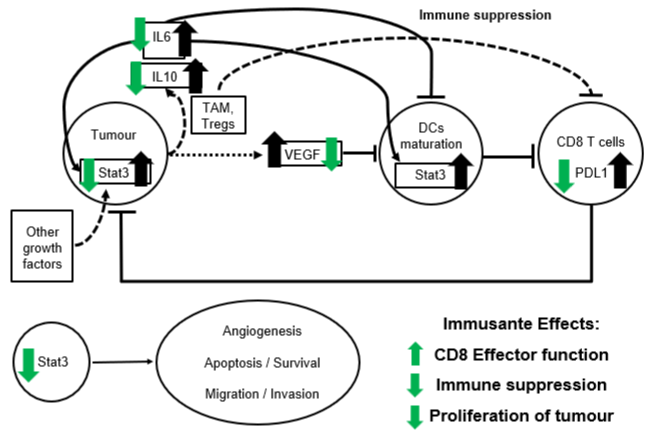
Figure 1: Immusante immunomodulation. The bold black arrows represent malfunction in the signalling network due to tumour cells, and the bold green arrows
represent the therapeutic effect of Immusante on the signalling network.
With a vast range of studies on the functioning of Immusante components involved in several signalling cascades and immune system responses, the current study focuses on analyzing the behaviour of Immusante immunomodulatory properties in the cancer microenvironment integrating it with a mathematical model reported by Sontag et al. [3]. The major signalling pathways are integrated with the immune dynamics to capture the cancer phenotype and how Immusante components can augment the region where tumours can be eliminated. The model is also used to evaluate the combined effect of Immusante and various chemotherapeutics. Overall, the current study represents the immunomodulatory, nonimmunomodulatory, and synergistic effects of Immusante on tumour growth and suppression and further extended to the population level.
Materials and Methods
The present study incorporates the important signalling factors and immunomodulation through STAT3 (Figure 1) to address tumour progression, immunosuppressants, and effector cells interplay. It creates a framework employing the mathematical model and its physiological parameter values discussed in Sontag et al. [3]. The tumour-specific immune model considering all the mechanisms mentioned above is implied with Immusante cancer management properties in this study, shown in Figure 2, and simulated for different scenarios at an individual and population level.
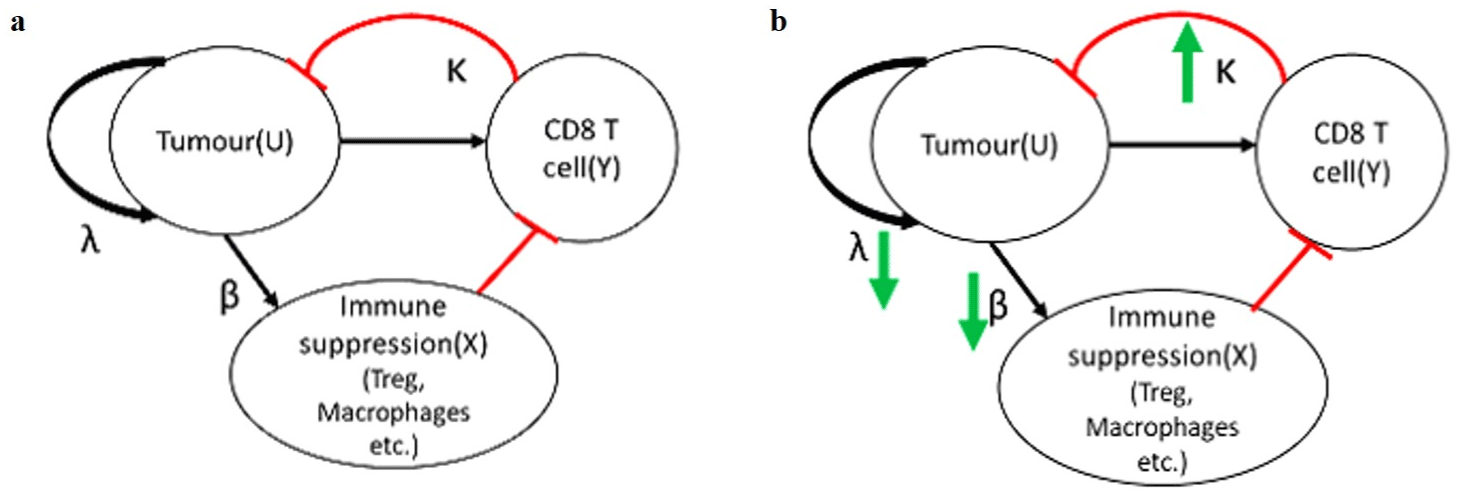
Figure 2: Mathematical model framework. The model accounts for three key variables: U represents the tumour cell count, X is the approximation for a level of
immune suppression, and Y is the CD8 effector T cell count. (a) Cancer microenvironment: U has an intrinsic proliferation rate (rate λ). U upregulates X via antigenic
stimulation and upregulates X (rate β) via upregulating suppressive cytokines, Tregs etc. X inhibits Y through multiple mechanisms. Y inhibits U (rate κ) via CD8
mediated cytotoxicity; (b) Immusante effect on the cancer microenvironment: The bold green arrows represent the effects of the four compounds of Immusante as
discussed in the previous section on relevant rate constants of the reduced model.
The model accounts for three key variables, U, X, and Y, where the variable U signifies an immune challenge, such as the number of cells in a tumour. The variable X represents an “intermediate regulatory node” because U drives it and, in turn, regulates Y. This node has an explicit biological correlate, immune-suppressing factors such as the number of T regulatory cells (Tregs) in a defined tumour microenvironment. The variable Y represents an agent that can eliminate challenge U, such as the number of tumour-specific effector T cells, i.e., CD8 T cells in the same environment.
Effect of the Varying Growth Rates of Tumour Cells on Tumour Growth Dynamics
The effect of varying growth rates on tumour growth dynamics was simulated, and the changes in the number of tumour cells (U), immune-suppressing species (X) and CD8 T cells (Y) were observed. The tumour cells proliferate with positive autoregulation having a rate constant of λ ranging from 10-4 to 100. The effect of varying tumour growth dynamics was introduced using different levels of λ in each simulation. The tumour cells activate both the CD8 T Cells and immune-suppressing factors. The rate constant for the formation of immune-suppressing factors is β. CD8 T cells inhibit tumour growth with a rate constant κ CD8 T cells mediated cytotoxic effects. The unit of time was considered as days in the model. Cell populations (U, X, Y) are in units of 106 cells. The parameters used and corresponding units are as follows: κ = 10-5 (106 cells)–1 day–1 and β = 1 day–1.
Effect of Immusante on the Tumour Growth Dynamics
The effect of Immusante was observed on the tumour growth dynamics by incorporating the major immunomodulatory effects of Immusante into the mathematical model. The effect was simulated using increased CD8 effector function (κ), reduced tumour suppression (β) and keeping the tumour proliferation rate (λ) constant, as shown by bold green arrows in Figure 2.
Steady-State Dynamics in the Absence and Presence of Immusante
The effect on the steady-state of tumour growth dynamics with and without the Immusante effect was simulated by increasing κ, reducing β and decreasing λ to observe the changes in the biphasic behaviour of tumour cells. The parameter values are presented in (Table 1).
Drug effect
λ
κ
β
Individual dynamics:
Tumour growth dynamics
10-4 to 100
10-5
1
Immusante effect on Tumour growth dynamics
10-4 to 100
10-3
0.5
Immusante effect on Steady-state dynamics
10-4 to 100
10-6
0.785
Population dynamics (range):
Drug-free
10-3, 100
10-6, 10-4
0.8, 1.2
Immusante
10-3.5, 10-0.5
10-6, 10-4
0.8, 1.2
Chemotherapy (5-FU)
10-4, 10-2
10-6, 10-4
0.8, 1.2
Chemotherapy (5-FU) + Immusante
10-5, 10-2
10-5, 10-3
0.7, 1.1
(λ = Tumour proliferation rate, κ = CD8 effector function, β = Immune suppression)
Table 1: The parameters perturbed to simulate the therapeutic effect of the adjuvant with respect to the tumour growth conditions. The adjuvant effect is simulated in different scenarios at individual and population levels and combined with chemotherapeutics to analyze the synergistic effects.
Population-Level Effect of Chemotherapy and Immusante
The population-level simulations were carried out to observe the effect of chemotherapy with and without the adjuvant Immusante effect on the tumour growth dynamics. The model included the mechanisms through which chemotherapeutics like 5-FU acts and has side effects, as shown in (Figure 3).
The effect of Immusante was simulated by decreasing λ and keeping β and κ constant with respect to the drug-free condition. The Chemotherapy drug effect was simulated by decreasing λ and keeping β and κ constant. The effect of combinatorial therapy with chemotherapy and Immusante was simulated by decreasing λ and β and increasing κ. The effect of varied combination therapy administration profiles was studied using varying parameter sets under four different scenarios: Drug-free, Immusante, chemotherapy (5-FU effect), and chemotherapy (5-FU) + Immusante environments.
For population dynamics, a set of 10000 random combinations was created using varying growth rate constant (λ), immune suppression rate constant (β), and CD8 T cell inhibition rate constant (κ), and initial conditions. These combinations represent an in-silico population of 10000 individuals with varying inherent immune capability and immune suppression states. The parameter changes are mentioned in (Table 1).
Results and Discussion
Tumour growth dynamics in different scenarios were analyzed using the system physiology-based mathematical model of immunomodulation and signalling pathways at an individual and population level.
Effect of Varying Growth Rates on Tumour Growth Dynamics
Simulations were performed to assess the tumour growth dynamics under different conditions using the mathematical model shown in Figure 2. Tumour growth dynamics were simulated for different growth rates for tumour cells (λ) ranging from 10-4 to 100. The dynamics of Tumour cells (U), Immune suppressing species (X), and CD8 effector T cell response (Y) are shown in (Figure 4).
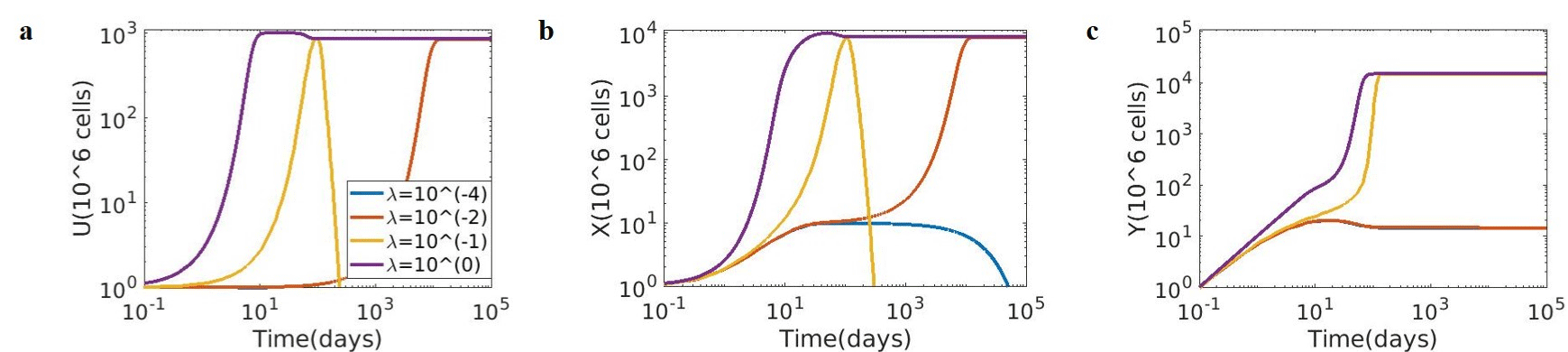
Figure 4: Model simulation of tumour growth dynamics at various growth rates (λ). (a) Tumour cells (U); (b) Immune suppressing species (X); (c) CD8 T cells
(Y). (λ = tumour growth rates ranging from 10-4 to 100, CD8 effector function к = 10-5 (106 cells)–1 day–1, and Immune suppression β = 1/day).
As discussed by Sontag and group [3], different thresholds are observed for a range of growth rates. As shown in Figure 4a, if the growth rate λ of the tumour is less than λ = 10-4 (Blue), it is eventually eliminated by the immune system. When the tumour is more aggressive, λ > 10-4 but λ < 10-2 (Red), it cannot be eliminated (it is “tolerated” by the immune system) and saturates later. For an even more aggressive challenge, with λ > 10-2 but λ < 10-1 (Yellow), again the tumour is eliminated; and if λ > 10-1 (Purple), again there is no elimination (the tumour “escapes”). Therefore, the clearance of tumours is observed for λ value less than 10-4 followed by saturation, and then clearance at intermediate, i.e., λ = 10-1 value, followed by saturation at higher λ values.
The immune-suppressing species (X) also exhibit a similar trend, i.e., at a high tumour proliferation rate (λ > 10-1), the number of immune suppression species also increase and saturate whereas, at intermediate λ = 10-1 value, immune suppression species increase and are cleared off by the system (Figure 4b). Interestingly, the tumour saturates both above and below these intermediate values. This is in accordance with observations wherein tumours with a very low growth rate are naturally cleared by the innate immune response through CD8 T cells activity. There are more CD8 T cells at very high tumour proliferation, but the CD8 T cells fail to respond due to high immune suppression. As expected at a very low tumour proliferation rate, the low concentration of CD8 T cells can clear the tumour cells (Figure 4c). CD8 T cells (Y) inhibit tumour proliferation, whereas immune-suppressing species (X) inhibit the production of CD8 T cells (Y).
Effect of Immusante on Tumour Growth Dynamics
The immunomodulatory effect of Immusante was introduced by reducing tumour proliferation and immunosuppressive species and increasing CD8 effector function on the tumour growth dynamics. The altered tumour growth dynamics of Tumour cells (U), Immune suppressing species (X), and CD8 effector T cell response (Y), due to the incorporation of Immusante, for different growth rates for tumour cells (λ) ranging from 10-4 to 100 is shown in (Figure 5). The effect of Immusante was simulated by increasing the rate parameters for CD8 effector function (κ) by 100-fold and decreasing immune suppression (β) by 0.5-fold, relative to the tumour condition.
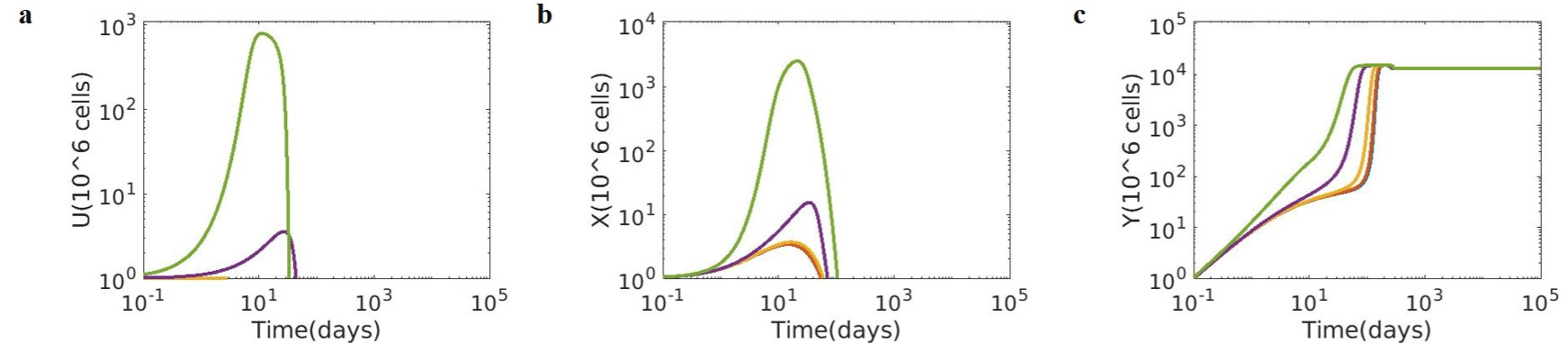
Figure 5: Effect of Immusante on the altered tumour growth dynamics. (a) Tumour cells (U); (b) Immune suppressing species (X); (c) CD8 T cells (Y). For all
λ values, tumour cells are cleared with a high CD8 T cell activity and lowered immune suppression effect. (λ = tumour growth rates ranging from 10-4 to 100, CD8
effector function к = 10-3 (106 cells)–1 day-1 and Immune suppression β = 0.5/day).
Varying levels of enhanced κ and decreased β represents the significant effects of Immusante immunomodulatory activity: increased tumour clearance, reduced immune suppression, and increased CD8 effector response, as seen in Figure 5. It was observed that for all λ values, tumour cells are cleared with a high CD8 T cell activity and lowered immune suppression effect. This analysis establishes the efficiency of the Immusante effect on the tumour proliferation rate. It facilitates faster clearance as different rate parameters fluctuate, and the preventive factors are retained even at very low parameter values. It indicates that with the introduction of Immusante, tumour clearance is enhanced with a concomitant reduction in immune suppression and heightened effecter cell activity for a wide range of tumour proliferation rates. The biological effect of Immusante on tumour growth by immunomodulation and altering the signalling pathway status is corroborated by the simulation results obtained using the mathematical model.
Steady-State Dynamics
When the tumour cells were simulated for different growth rates (λ) ranging from 10-4 to 100, the biphasic behaviour observed explains that tumours can sneak through from the immune responses and can only be eliminated within the intermediate region (Figure 6a), as described in Sontag et al. [3]. Thereby indicating that tumours with a moderate growth rate escape the inherent immune defence; these are transitory to the actual capability of the immune response, as discussed earlier in (Figures 4 and 5), while simulating the tumour growth dynamics. The immune response also clears tumour cells with growth rates immediately higher than the moderate.

Figure 6: Biphasic behaviour in tumour saturation based on varying growth rates (λ). (a) Cancer micro-environment; (b) Immusante effect on the Tumour
cells (U) in the cancer microenvironment, for which the rate parameters for CD8 effector function, i.e., к and β, are increased (0.000025) and decreased (0.785),
respectively, relative to the tumour condition. Tumour clearance was observed at λ <10-4 and at intermediary λ value 10-1. Tumours show saturating growth for two
ranges of λ with clearance otherwise. The intermediate region was broadened by the effect of Immusante com-pounds correlating to greater elimination of tumour
cells.
The effect of Immusante was simulated by modulating the rate parameters for CD8 effector function, i.e., κ is kept approximately constant, but the immune suppression effect, i.e., β, is decreased by 0.2-fold, relative to tumour condition. The altered tumour growth dynamics of Tumour cells (U) due to the incorporation of Immusante is shown in (Figure 6). The intermediate region is broadened as an effect of Immusante compounds, correlating greater the elimination of tumour cells than the system’s innate immune response (Figure 6b). Therefore, highly aggressive tumours that are not cleared by innate immune capability and escape immune responses can be managed with Immusante immunomodulatory effects.
Population-Level Effect on Tumour Growth Dynamics
The signalling model with the immune response was integrated with the specific side-effects of chemotherapeutics like 5-FU to quantify the combined effects of the drug with Immusante. Other drugs categories include Cyclophosphamide, Platinum derivatives, Vincristine, Taxane, Irinotecan and Anthracycline. The effects of 5-FU and other chemotherapeutics and Immusante effect on various nodal points (explained in detail within the Supplementary section) were incorporated into the tumour-immune model. Immusante, with its varied response at the major nodal points in the pathway and immune cells, was seen to counter and reduce the possible side effects of chemotherapeutic treatments. It acts as an adjuvant and provides additional efficacy to alleviate the ill effects of immunosuppression in cancer.
The simulations were performed by creating 10,000 random combinations of tumour growth rate constant (λ), immune suppression rate constant (β), and CD8 T cell inhibition rate constant (κ), and initial conditions. These distinct parameters and initial condition values represent an in-silico population with varying inherent immune capability and immune suppression states. The effect on the population is shown in four different scenarios: Drug-free, Immusante, chemotherapy (5-FU effect), and chemotherapy (5-FU) + Immusante environments. The effect of Immusante was simulated by decreasing λ and β and increasing κ. The effect of chemotherapy (5-FU effect) was simulated by decreasing λ and keeping β and κ constant. In comparison, the effect of 5-FU + Immusante was simulated by decreasing λ and β and increasing κ.
Effect of Drugs on the Tumour Cells (U) and Immune Suppressing Species (X)
The log uniform distribution of tumour cells in a population within a normal physiological range and when the drugs are introduced with and without the herbal adjuvant is shown in (Figures 7 and 8). The first blue bar corresponding to the left on the x-axis represents the normal condition, and the following bars represent varying degrees of tumour growth rates in a population.
About 50% of the population demonstrate a tendency of tumorigenesis (Figure 7a). On introducing the effect of Immusante only considering its effect on the signalling pathway, the Drug-free and Immusante graphs show a 13% increase in population with no/very low tumorigenesis (Figure 7b). Figure 7c shows a 28% increase in population with no/very low tumorigenesis under only Chemotherapy (5-FU) condition. The comparison between Drugfree and Chemotherapy (5-FU) + Immusante graphs shows a 44% increase in population with no/very low tumorigenesis (Figure 7d).
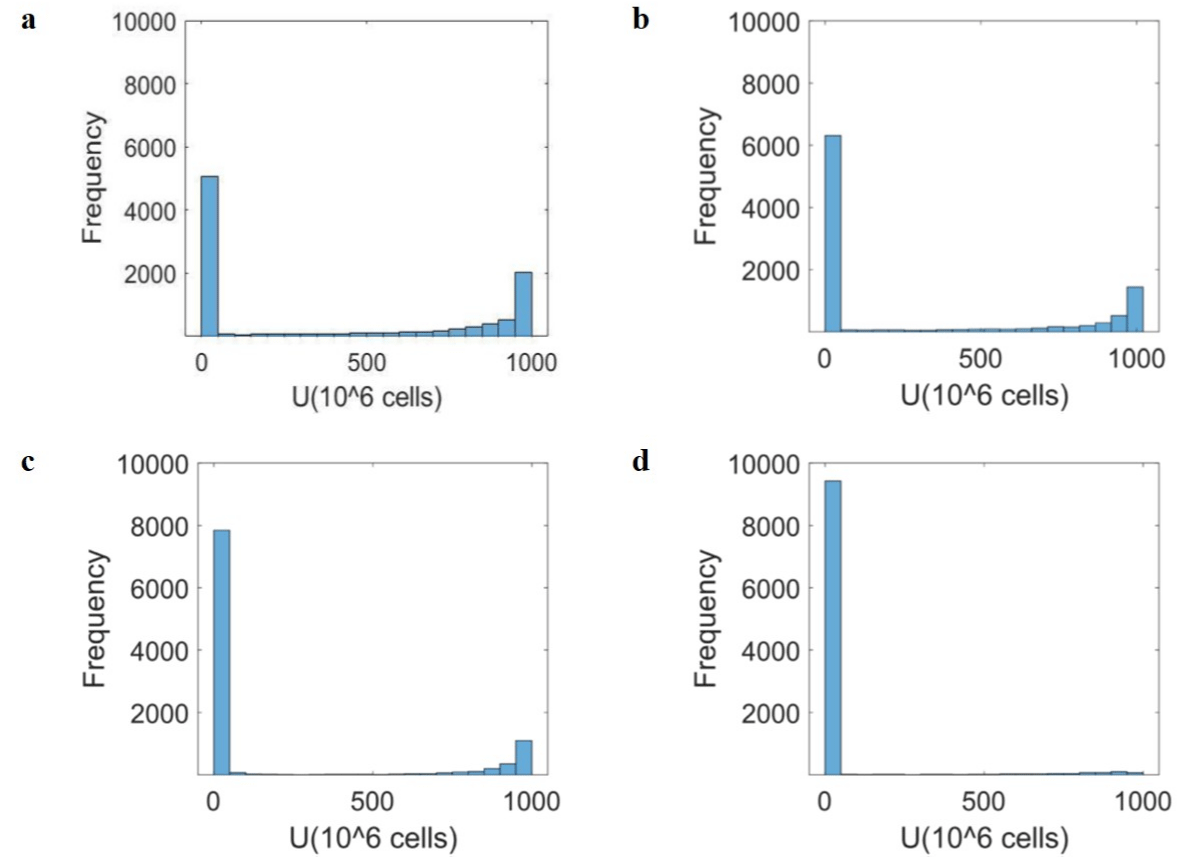
Figure 7: Drug effect on the Tumour cells (U). (a) Drug-free shows log uniform distribution of tumour saturation levels in 10,000 individuals’ population for tumour
cell number (U). The simulations are performed by randomly choosing 10,000 combinations of tumour growth rate constant (λ), immune suppression rate constant
(β), and CD8 T cell inhibition rate constant (κ). The values are taken for λ ԑ (10-3, 100), β ԑ (0.8, 1.2), Κ ԑ (10-6, 10-4) in the given range; (b) The effect of Immusante
is simulated by decreasing λ (10-3.5, 10-0.5) and keeping β and Κ constant. The comparison between Drug-free and Immusante graphs shows a 13% increase
in population with no/very low tumorigenesis; (c) The Chemotherapy drug (5-FU) effect is simulated by decreasing λ (10-4, 10-2) and keeping β and Κ constant.
The comparison between Drug-free and Chemotherapy (5-FU) graphs shows a 28% increase in population with no/very low tumorigenesis; (d) The rightmost
Chemotherapy (5-FU) + Immusante effect is simulated by decreasing λ (10-5, 10-2) and β (0.7, 1.1) and increasing Κ (10-5, 10-3). The comparison between Drug-free
and Chemotherapy (5-FU) + Immusante graphs show a 44% increase in population with no/very low tumorigenesis.
A similar effect can be observed on the immune-suppressing species. As shown in (Figures 7a and 8a), about 50% of the population under the Drug-free condition shows high tumour occurrence. A 12 % increase in the population with low immune suppression was seen as an effect of Immusante administration (Figure 8b). A 26% increase with low immune suppression under only Chemotherapy (5-FU) conditions is seen in (Figure 8c). The comparison between Drug-free and Chemotherapy (5-FU) + Immusante graphs show a 43% increase in population with low immune suppression, indicating more functionality of effector species in the presence of Immusante (Figure 8d).
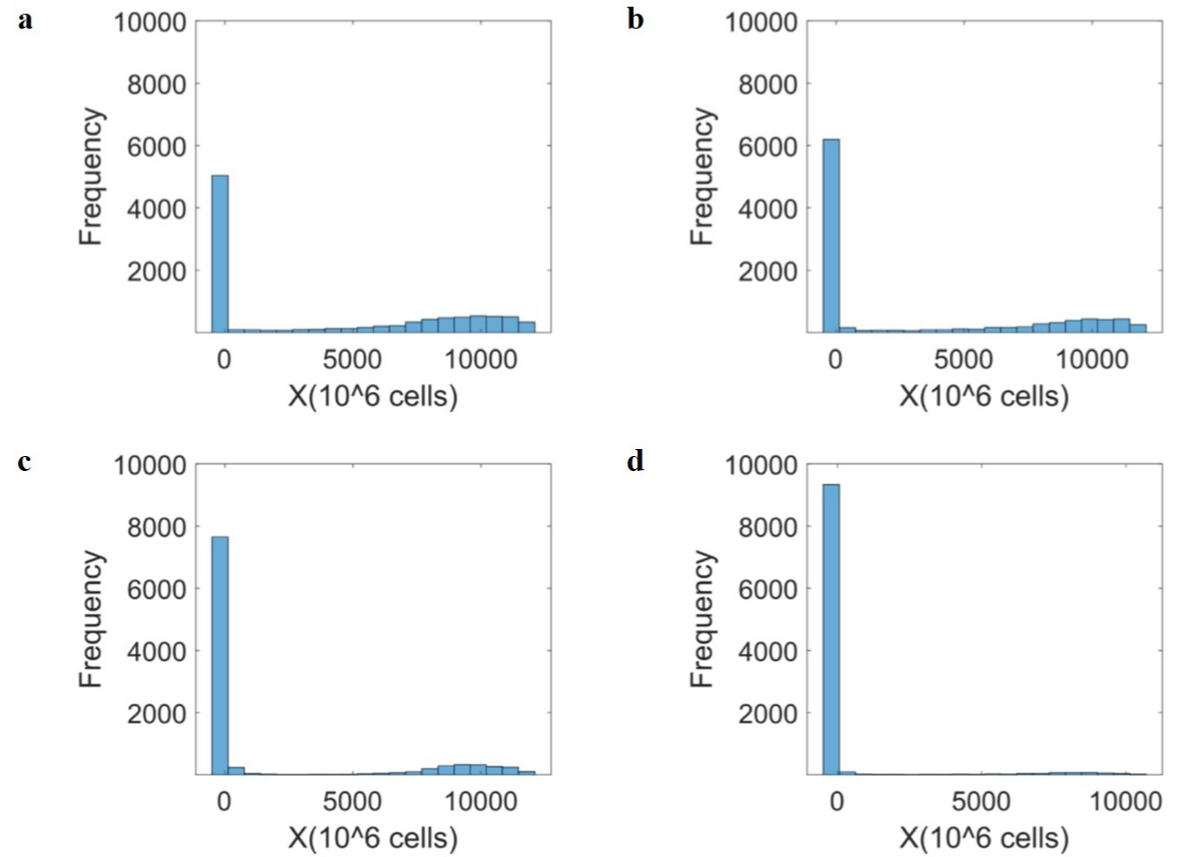
Figure 8: Drug effect on the Immune suppressing species (X). (a) Drug-free shows log uniform distribution of tumour saturation levels in 10,000 individuals’
population for Immune suppressing species (X). The simulations are performed by randomly choosing 10,000 combinations of tumour growth rate constant (λ),
immune suppression rate constant (β), and CD8 T cell inhibition rate constant (κ). The values are taken for λ ԑ (10-3, 100), β ԑ (0.8, 1.2), Κ ԑ (10-6, 10-4) in the given
range; (b) The effect of Immusante is simulated by decreasing λ (10-3.5, 10-0.5) and keeping β and Κ constant. The comparison between Drug-free and Immusante
graphs shows a 12% increase in population with low immune suppression; (c) The Chemotherapy drug (5-FU) effect is simulated by decreasing λ (10-4, 10-2)
and keeping β and Κ constant. The comparison between Drug-free and Chemotherapy (5-FU) graphs shows a 26% increase in population with low immune
suppression; (d) The rightmost Chemotherapy (5-FU) + Immusante effect is simulated by decreasing λ (10-5, 10-2) and β (0.7, 1.1) and increasing Κ (10-5, 10-3). The
comparison between Drug-free and Chemotherapy (5-FU) + Immusante graphs show a 43% increase in population with low immune suppression.
The mathematical simulations indicate that chemotherapy drugs mediate tumour clearance, further augmented by Immusante administration. The population-level analysis demonstrates the therapeutic effect of Immusante compounds and shows an enhanced effect of Immusante in a population and greater tumour clearance at a steady state.
Combination Therapy Effects of Immusante with Chemotherapeutics with Varied Administration Profile
The signalling model with the immune response was integrated with the specific side-effects of chemotherapeutics to quantitate the combined effects of the drug and Immusante. Simulations showed the effects of 5-FU, long-duration 5-FU (periodic), Immusante, and different combinations of 5-FU and Immusante, as seen in Figure 3. The simulation results show an indicative synergistic effect of 5-FU and Immusante. Seven different conditions were simulated to assess the effect of the adjuvant comprehensively, viz., Untreated, 5-FU alone as a therapeutic, Immusante alone, a combination of 5-FU and Immusante, multiple dosages of 5-FU treatment (5-FU periodic), multiple combination dosages 5-FU and Immusante (5-FU + Immusante periodic) and intermediary dose of Immusante during continuous 5-FU treatment.
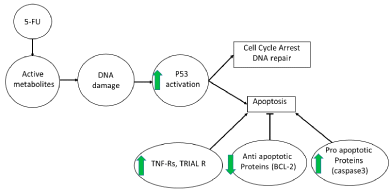
Figure 3: Adjuvant effect - Synergy with 5-FU. 5-FU is broken down to active metabolites, resulting in DNA damage. The DNA damage activates p53 which
can either cause cell cycle arrest and trigger DNA repair or cause apoptosis. The later fate is preferred when the cellular stress is high or the cell is sensitized to
apoptosis. With the Immusante effect shown in green arrows, the cell is subjected to apoptotic pressure by TNF-Rs and TRAIL R due to the skewed balance of
apoptotic proteins favouring apoptosis. Therefore, Immusante works synergistically with 5-FU to trigger apoptosis in tumour cells.
The simulation results indicate that only 5-FU reduces tumours up to 49% compared to only Immusante, which only causes a 17% reduction. When the effect of Immusante with 5-FU was simulated, it showed a significant reduction in tumour growth up to 99%. The model also indicates an adaptive response of the tumour on continuous 5-FU administration, which reduces the effect of 5-FU over time (5-FU periodic). But the adaptive response can significantly be overcome by Immusante with 5-FU. Immusante has also been shown to help reduce the side effects of continuous treatment of 5-FU. The results are shown in (Figure 9).
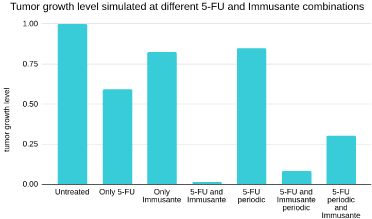
Figure 9: Combination therapy effects. The leftmost bar shows normalized level of tumour growth under the untreated condition. The 5-FU reduces tumour up
to 49% compared to only Immusante, which alone only causes 17% reduction shown in the figure. The effect of Immusante with 5-FU showed significant reduction
in tumour growth up to 99%. The adaptive response of tumour on continuous 5-FU administration, which reduces the effect of 5-FU from 49% to 15% over time.
Immusante with 5-FU helps in inhibiting adaptive response and inhibits tumour growth in con-tinuous administration of the drug. Immusante has also shown to help
with the side-effects of continuous treatment of 5-FU. The values tumour proliferation rate constant (α), suppressor cells rate constant (β), and CD8 T cells rate
constant (κ) for simulating all results are given as follows: Untreated: α = 1, β = 1, κ = 0; only 5-FU: α = 0.3, β = 1, κ = 0; only Immusante: α = 0.75, β = 0.75, κ =
0; 5-FU and Immusante: α = 0.23, β = 0.75, κ = 0; 5-FU periodic: α = 0.4, β = 1, κ = 0; 5-FU and Immusante periodic: α = 0.24, β = 0.75, κ = 0; 5-FU periodic and
Immusante: α = 0.3, β = 0.75, κ = 0.
It should be noted that the results shown in Figure 9 are indicative of the effect shown in individuals, and combination therapy may not represent the exact quantitative effect. It provides meaningful insights around expected results observed in experimental studies. The effect of Immusante was captured by a 25% decrease in tumour growth rate, 25% enhancement in immune activity and a 25% lowering of immune suppression. In addition, Immusante also reduced the side effect of 5-FU by reducing the effect of MDSC on tumour cells and lowering PD-L1 levels in the T-cells. Simulations suggest that combination enhances effectiveness and reduces the side effects of 5-FU. It was observed that Immusante could effectively counter side-effects and adaptive response of cancer cells to improve efficacy and normalize immune response.
Simulations were also conducted for the effect of other drugs such as Cyclophosphamide, Platinum-based drugs, Vincristine, Taxane, Irinotecan, and Anthracyclines, based on available literature data and their corresponding target action mechanism. The individual simulation results for other drugs showed a 39%, 30%, 24%, 28%, 52%, and 17% reduction in tumour growth compared to untreated, respectively. The combined effect of these drugs and Immusante further decrease the tumour level to 66%, 65%, 62%, 64%, 68%, and 60% compared to untreated growth, respectively. The reduction in the tumour growth is indicative and broadly observes the effect of the individual drug and combination. The actual percentage reduction may vary in experimental studies. The results for these drugs are provided in the Supplementary data.
Conclusion
Several medicinal herbs are known to contain bioactive compounds in varying proportions. Herbal medicines with immunomodulatory properties have been used to activate host defence systems that can then be used to support the host’s health during conventional chemotherapy when the immune system is impaired. Many herbal adjuvants can be administered along with regular therapeutics to elicit faster, stronger immune responses. The analysis of the herbal supplements positioned for immuno-modulatory properties in this study indicates that Immusante (a herbal formulation developed and recommended as immunotherapeutic support in conditions where immunity is compromised) contains major bioactive compounds, namely Apigenin, Quercetin, Betulinic Acid and Oleanolic Acid [23,24]. Our previous review refers to the altering mechanisms of these bioactive compounds [26]. Herbal products such as Immusante may contain additional phytoconstituents that act synergistically. In a cancer microenvironment, the present study discusses the quantitative analysis of a well-defined static signalling network detailing the major immunomodulatory effects of Immusante key components on cell proliferation, angiogenesis, and survival.
Based on the mathematical model in Sontag et al. [3], a curated set of simulations were carried out to assess and quantify the immunomodulatory effects of Immusante. The Immusante effect was introduced by suppressing of STAT3/IL-6/IL-10 signalling axis, reducing tumour proliferation, diminishing the formation of immunosuppressive species, and enhancing CD8 effector T cell function. Despite the exact roles of IL-6 and IL-10 being somewhat conflicted, there is considerable evidence for their tumour-promoting and immune suppressive effects in the context of STAT3 mediated signalling cascades. The effects on several key pathways indicate considerable evidence supporting the relevance of this network in several cancer types. Thus, the STAT3/IL-6/IL-10 signalling cascade is a target of significant interest in cancer management. Considering these effects on a macro level, a framework was constructed to capture the effects of Immusante. The biphasic behaviour is observed in the tumour growth dynamics, which explains that tumours escape from the immune responses and can only be eliminated within the intermediate region. The intermediate region was broadened by the effect of Immusante compounds correlating greater the elimination of tumour cells. Greater tumour clearance and enhanced immune function were observed in scenarios with Immusante effects for a wide range of parameters. The analysis above indicates the probable mechanisms to establish Immusante as an effective immunomodulator.
The formulation appears to have considerable potential as an adjuvant during cancer management. It targets multiple nodal points cutting across signalling pathways and with few side effects. Hence, based on the available literature, the STAT3/IL-6/IL-10 signalling cascade and major immune markers were considered crucial in mediating the immunomodulatory activity of Immusante. The study also exhibits the synergistic effect of Immusante with chemotherapeutic drugs, such as 5-FU, which targets the apoptosis pathway and factors such as STAT3, VEGF, PD-L1, to cause increased tumour clearance. The effect of the Immusante, along with a chemotherapy drug, 5-fluorouracil (5-FU), was simulated to assess the effect of combination therapy. The addition of 5-FU as a monotherapy showed a 28% increase in the population with no/very low tumorigenesis. A combination of 5-FU and Immusante showed a 44% increase in the population with no/very low tumorigenesis. A similar trend was seen for population size with low immune suppression, where 5-FU monotherapy showed a 26% increase, Immusante showed 12%, and a combination of 5-FU and Immusante showed a 43% increase in the population with low immune suppression, indicating increased efficiency of effector species in the presence of Immusante. The adjuvant effect may help to increase the effectiveness of the administered drugs. Similar results were obtained for other drugs such as Cyclophosphamide, Platinum-based drugs, Vincristine, Taxane, Irinotecan, and Anthracyclines combined with Immusante. In the case of some of the drugs mentioned above, Immusante may also help overcome tumour adaptation which happens with continuous administration of the drug for long periods. The continuous use of these chemo drugs also causes dysregulation of immune response in patients. The immunomodulatory properties of Immusante have been shown to restore balance in immune response and reduce the cytotoxic effects of drugs on healthy organs. These characteristics make Immusante a promising adjuvant during cancer management in cancer therapeutics and improve the quality of the treatment.
Data Availability
The data used to support the findings of this study are available within the Supplementary information file.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors acknowledge the support from the Himalaya Wellness Company for providing resources, including the previous studies, and review and editing process.
References
- Madunić J, Madunić IV, Gajski G, Popić J, Garaj-Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer letters. 2018; 413: 11-22.
- Ponte LGS, Pavan ICB, Mancini MCS, Silva LGSD, Morelli AP, Severino MB, et al. The Hallmarks of Flavonoids in Cancer. Molecules. 2021; 26: 2029.
- Sontag ED. A Dynamic Model of Immune Responses to Antigen Presentation Predicts Different Regions of Tumor or Pathogen Elimination. Cell systems. 2017; 4: 231-241.e11.
- Yu H, Kortylewski M, Pardoll DM. Tumour immunology: Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature Reviews Immunology. 2007; 7: 41-51.
- Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Briefings in Functional Genomics. 2013; 12: 489-498.
- Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013; 15: 848-IN45.
- Abramo JM, Reynolds A, Crisp GT, Weurlander M, Söderberg M, et al. Individuality in music performance. Assess Eval High Educ. 2012; 37: 435.
- Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer letters. 2015; 367: 103-107.
- Huynh J, Etemadi N, Hollande F, Ernst M, Buchert M. The JAK/STAT3 axis: A comprehensive drug target for solid malignancies. Seminars in cancer biology. 2017; 45: 13-22.
- Ferguson SD, Srinivasan VM, Heimberger AB. The role of STAT3 in tumormediated immune suppression. Journal of Neuro-Oncology. 2015; 123: 385- 394.
- Adam D, Friedman SEC. and RL. Gene Alterations NIH Public Access. Bone. 2013; 23: 1–7.
- Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunologic Research. 2001; 23: 263-272.
- Bu LL, Yu GT, Wu L, Mao L, Deng WW, Liu JF, et al. STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC. Journal of Dental Research. 2017; 96: 1027-1034.
- Zhang N, Zeng Y, Du W, Zhu J, Shen D, Liu Z, et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. International journal of oncology. 2016; 49: 1360-1368.
- Niu G, Wright KL, Ma Y, Wright GM, Huang M, Irby R, et al. Role of Stat3 in Regulating p53 Expression and Function. Molecular and Cellular Biology. 2005; 25: 7432-7440.
- Huang S. Regulation of Metastases by Signal Transducer and Activator of Transcription 3 Signaling Pathway: Clinical Implications. Clinical Cancer Research. 2007; 13: 1362-1366.
- Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer. 2003; 3: 330-338.
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer research. 2010; 70: 3052-3061.
- Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nature Medicine. 2013; 19: 57-64.
- Zhang M, Fan Y, Che X, Hou K, Zhang C, et al. 5-FU-Induced Up-regulation of Exosomal PD-L1 Causes Immunosuppression in Advanced Gastric Cancer Patients. Front Oncol. 2020; 10: 495.
- Kraak LVD, Goel G, Ramanan K, Kaltenmeier C, Zhang L, Normolle DP, et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. Journal for Immunotherapy of Cancer. 2016; 4.
- Chan L, Chou T, Ding H, Chen P, Chiang F, Kuo P, et al. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochimica et biophysica acta. 2012; 1820: 1081- 1091.
- Varma RS, Guruprasad KP, Satyamoorthy K, Kumar LMS, Babu UV, Patki SP. IM-133N modulates cytokine secretion by RAW264.7 and THP-1 cells. Journal of Immunotoxicology. 2016; 13: 217-225.
- Firashathulla S, Inamdar MN, Rafiq M, Viswanatha GL, Kumar LMS, Babu UV, et al. IM-133N - A Useful Herbal Combination for Eradicating Diseasetriggering Pathogens in Mice via Immunotherapeutic Mechanisms. Journal of Pharmacopuncture. 2016; 19: 21-27.
- Gibellini L, Pinti M, Nasi M, Montagna JP, Biasi SD, Roat E, et al. Quercetin and Cancer Chemoprevention. Evidence-based Complementary and Alternative Medicine: eCAM. 2011; 2011: 1-15.
- Gangwar V, Garg A, Lomore K, Korla K, Bhat SS, Rao RP, et al. Immunomodulatory Effects of a Concoction of Natural Bioactive Compounds— Mechanistic Insights. Biomedicines. 2021; 9: 1522.
- Yan X, Qi M, Li P, Zhan Y, Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell & Bioscience. 2017; 7.
- Coombs MRP, Harrison ME, Hoskin DW. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer letters. 2016; 380: 424-433.
- Cao H, Tse AK, Kwan H, Yu H, Cheng C, Su T, et al. Quercetin exerts antimelanoma activities and inhibits STAT3 signaling. Biochemical pharmacology. 2014; 87: 424-434.
- Shin J, Lee H, Jung D, Jung JH, Lee H, Lee E, et al. Suppression of STAT3 and HIF-1 Alpha Mediates Anti-Angiogenic Activity of Betulinic Acid in Hypoxic PC-3 Prostate Cancer Cells. PLoS ONE. 2011; 6: e21492.
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Béliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glio-blastoma cell line growth and migration. Exp Cell Res. 2012; 318: 925–935.
- Cao H, Chu J, Kwan H, Su T, Yu H, Cheng C, et al. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Scientific Reports. 2016; 6.
- Seo HS, Choi HS, Kim SR, Choi YK, Woo SM, et al. Apigenin in-duces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol Cell Biochem. 2012; 366: 319–334.
- Xu L, Zhang Y, Tian K, Chen X, Zhang R, Mu X, et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. Journal of Experimental & Clinical Cancer Research: CR. 2018; 37.