
Case Report
Austin Cardio & Cardiovasc Case Rep. 2025; 9(1): 1065.
Takotsubo Cardiomyopathy after Esophageal Surgery in a Patient with an Undiagnosed Coronary Artery Disease
Tan EFS*, Gharti SB, Ahmed M, Gelan YD and Adedayo AM
One-Brooklyn Health, Interfaith Medical Center, 1545 Atlantic Ave, Brooklyn, NY, 11213, USA
*Corresponding author: Ernestine Faye S Tan, One-Brooklyn Health, Interfaith Medical Center, 1545 Atlantic Ave, Brooklyn, NY, 11213, USA Email: drernestinefayetan@gmail.com
Received: June 19, 2025 Accepted: July 15, 2025 Published: July 18, 2025
Abstract
Takotsubo cardiomyopathy (TCM), also known as “broken heart syndrome” or stress cardiomyopathy, is a transient, non-ischemic form of heart failure characterized by left ventricular apical ballooning, elevated cardiac enzymes, and regional systolic dysfunction. It commonly presents with symptoms similar to acute coronary syndrome (ACS), leading to frequent misdiagnosis. TCM is often triggered by significant emotional or physical stress, with a notable predilection in postmenopausal women. Although typically reversible, the condition’s pathophysiology remains incompletely understood, with the most accepted theory linking it to excessive catecholamine release and myocardial dysfunction.
We present a case of a 73-year-old female who developed TCM following an elective right thoracotomy for esophageal cyst resection. The patient exhibited new onset severe systolic dysfunction, apical ballooning, elevated cardiac enzymes, and arrhythmias postoperatively. Despite an initial diagnosis of acute coronary syndrome (ACS), further cardiac work-up, including echocardiography and cardiac catheterization, confirmed TCM as well as coronary artery disease. This case highlights the importance of distinguishing between TCM and ACS, as they may present similarly but have distinct management strategies. The patient’s condition improved over the following months, with normalization of left ventricular function, supporting the reversible nature of TCM.
This case underscores the need for thorough evaluation when diagnosing heart failure with no clear ischemic cause, ensuring that ACS or CAD is not overlooked. While TCM can resolve with appropriate care, careful clinical judgment is essential to avoid misdiagnosis and optimize patient outcomes.
Introduction
Takotsubo cardiomyopathy (TCM), also called the “broken heart syndrome,” or “stress cardiomyopathy,” is a form of non-ischemic cardiomyopathy characterized by transient apical ballooning, elevated cardiac enzymes, and regional systolic dysfunction [1,2]. It is commonly misclassified as acute coronary syndrome due to the similar clinical and diagnostic presentation, although the former is a form of reversible, non-ischemic heart failure which resolves completely in one to six months [2].
Cases have been reported to predominantly affect postmenopausal women [3,4], and are often related to life events that cause significant physical or emotional strain, such as abuse, deaths of relatives, calamities, accidents, medical procedures or illnesses, and stimulant drugs [1,3,4]. A report by The International Takotsubo Registry showed that the rates of neurologic or psychiatric disorders were higher in patients with TCM than those with acute coronary syndrome (55.8% vs. 25.7%, with a p <0.001) [3].
Despite TCM commonly described as the “broken heart syndrome” in literature, it may also be triggered by significant positive life events, and in other cases, it may have no trigger at all [3]. Epidemiological reports reveal that TCM accounts for 1% to 3% of acute coronary syndromes [5] and 0.5% to 0.9% of ST-segment elevation myocardial infarcts [6], although cases remain to be underreported or misclassified as acute coronary syndrome due to the similarity in presentation.
Despite the rising cases of TCM after COVID-19, a consensus regarding the management remains to be developed. In hopes to further understand the condition, we present a case of Takotsubo cardiomyopathy in a patient who was admitted for elective right thoracotomy for esophageal cyst resection, but had a complicated postoperative course characterized by new onset severe systolic dysfunction, apical ballooning, elevated cardiac enzymes, global hypokinesis, and unstable atrial fibrillation.
Case Presentation
This is a case of a 73- year- old female who was admitted for elective right thoracotomy and resection of symptomatic esophageal cysts.
Prior to surgery, the patient underwent cardiac evaluation and clearance. 2D echocardiogram showed normal systolic and diastolic functions, with an ejection fraction (EF) of 60%. The LV had normal wall thickness (Figure 1a), and normal wall motion. No other significant findings were noted. A stress test was done, which showed normal results. The patient denied any symptoms and was stratified as intermediate risk to undergo the procedure. The patient underwent the procedure with no intraoperative complications.
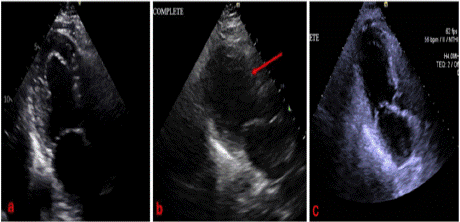
Figure 1: Comparison of left ventricular size before (a) and after (b)
surgery, and 2 months post-surgery (c). A red arrow depicts new onset
ballooning of the mid and apical segments of the left ventricle, raising
concern for Takotsubo cardiomyopathy.
During the postoperative course, the patient was referred for hypotension and new onset atrial fibrillation with slow ventricular response (47 beats per minute) noted on cardiac monitor, for which Atropine was given. The patient was eventually placed on Norepinephrine and Epinephrine drips for persistent hypotension and bradycardia. An electrocardiogram (EKG) was done after treatment, showing improvement of the bradycardia, although patient remained hypotensive and in atrial fibrillation (Figure 2).

Figure 2: Electrocardiogram showing atrial fibrillation with controlled
ventricular response after Atropine administration.
Troponin I was serially monitored, which increased from 1052 pg/ ml to >25,000 pg/ml in less than 24 hours (normal value <11.8pg/ml). Brain Natriuretic Peptide (BNP) was 162 pg/ml (normal value <100 pg/ml). Electrocardiograms were serially monitored, which showed atrial fibrillation with no ST elevations or depressions. Repeat 2D echocardiogram showed a dilated LV with severely decreased systolic function and an EF of 28%. Global hypokinesis, with ballooning of mid and apical segments, were consistent with Takotsubo cardiomyopathy (see Figure 1b). The hypotension and bradycardia improved, and vasopressors were discontinued within 24 hours.
Diagnostic cardiac catheterization was done, which showed severe single vessel CAD with 100% thrombotic occlusion of large OM3 branch, severe LV dysfunction with an EF of <30%, and a severely elevated LV end-diastolic pressure of 40 mm Hg. Left ventriculogram was consistent with Takotsubo cardiomyopathy. As the patient was post-thoracotomy, percutaneous coronary intervention was deferred and anticoagulation/ antiplatelets were held until surgical clearance. The patient continued to be monitored at the Intensive Care Unit (ICU) and dual antiplatelet therapy (DAPT) was planned once the patient was cleared for anticoagulation by surgery service. The patient eventually developed rapid ventricular response with the atrial fibrillation, and amiodarone drip was eventually started. The patient was transferred to a more advanced cardiac center for further monitoring, after which she improved, and was discharged stable, with reversion of her atrial fibrillation back to sinus. Follow up 2D echocardiogram after 2 months revealed a result similar to preoperative findings, and the left ventricular dilatation and ballooning were no longer appreciated (see Figure 1c). The patient’s ejection fraction reverted back to normal at 56%.
Discussion
Takotsubo cardiomyopathy is described as a reversible type of heart failure where there are transient regional left ventricular wall motion abnormalities that extend beyond a single epicardial coronary artery territory [7]. It mimics acute coronary syndrome in presentation, with patients usually complaining of chest pain, shortness of breath, or arrhythmias [8]. In severe cases, patients may also present with shock, similar to our case. EKG may show findings such as ST segment elevations or depressions, T wave changes, and prolonged QTc intervals [9,10]. Cardiac markers like troponins and pro-BNPs are also commonly elevated, like in our patient.
2D echocardiography shows a decreased systolic function and ejection fraction, with regional wall motion abnormalities not restricted to one coronary artery territory [7]. In our case, the EF decreased from 60% in the preoperative study, to 28% in the postoperative course. The patient was also found to have new onset mid to apical ballooning, consistent with TCM, as well as global hypokinesia.
The underlying cause and pathophysiology of TCM is not fully understood, but the most widely accepted theory is that it results from sympathetic overstimulation, leading to catecholamine- induced reversible myocardial dysfunction [11]. Vijiiac and associates (2020) lists various proposed mechanisms on how the enhanced sympathetic stimulation can induce left ventricular (LV) dysfunction, such as: direct cardiomyocyte toxicity, microvascular dysfunction, multivessel coronary spasm, plaque rupture and thrombosis, and activation of myocardial survival pathways [8].
Surgery is one of the triggers of TCM, which occurred in our patient after she underwent esophageal cyst surgery. Immediate postoperative symptoms of heart failure, accompanied by new troponin elevations and echocardiographic findings, strongly allude to the relationship of the stressful event to her cardiomyopathy.
While Takotsubo cardiomyopathy is described as a non-occlusive type of cardiomyopathy, co-existent coronary artery disease does not exclude the diagnosis [12]. Some studies revealed a high prevalence of vulnerable plaques among patients with TCM [13,14]. A cohort study done by Y-Hassan in 2015 mentioned that acute coronary syndrome (ACS) can also be a triggering factor in the development of TCM [14], which shows that the coexistence of coronary artery disease (CAD) and TCM is not unlikely. A different study proposes that acute plaque rupture leading to transient ischemia and myocardial stunning was the pathogenic mechanism underlying TCM [13]. Although literature reveals that 15% of patients with TCM have significant atherosclerotic plaques [12], a direct causal association between plaque rupture and TCM requires further investigation. In cardiomyopathies, cardiac manifestations, malignant arrhythmias and sudden cardiac death dramatically affects prognosis, emphasizing the importance of early diagnosis and a high index of suspicion [15].
Clinical judgment must always be exercised in the approach to TCM and ACS must always be ruled out, as both cases can present similarly and coexist at the same time. In our patient, cardiac catheterization was done revealing severe single vessel CAD with 100% thrombotic occlusion of the large OM3 branch, severe LV dysfunction with LVEF <30%, severely elevated LVEDP of 40 mm Hg, and a left ventriculogram consistent with TCM. Due to the extensive surgery done, cardiothoracic surgery advised against dual antiplatelet therapy temporarily. Losartan, as well as Atorvastatin, were started. The patient was discharged stable, and follow-up revealed improvement of the ejection fraction, suggesting that the cardiomyopathy was reversible and further confirming Takotsubo cardiomyopathy as the cause of transient heart failure. Dual antiplatelet was initiated for the coronary artery disease and the patient improved back to her presurgical condition.
Conclusions
Careful consideration must always be exercised in ruling out ACS or CAD in patients presenting with TCM, to prevent missing lifethreatening complications requiring emergent intervention. Despite strong suspicions of TCM, and the knowledge that TCM is a reversible, non-ischemic cardiac event where full recovery is expected, CAD or ACS must be ruled out, as both entities can coexist, and the diagnosis of one does not rule out the other.
References
- Ahmad SA, Brito D, Khalid N, et al. Takotsubo cardiomyopathy. [Updated 2023 May 22]. In. StatPearls [Internet, Treasure Island (FL): StatPearls Publishing; 2023.
- Singh T, Khan H, Gamble DT, Scally C, Newby DE, Dawson D. Takotsubo syndrome: pathophysiology, emerging concepts, and clinical implications. Circulation. 2022; 145: 1002-1019.
- Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015; 373: 929-938.
- Assad J, Femia G, Pender P, et al. Takotsubo syndrome: a review of presentation, diagnosis and management. Clin Med Insights Cardiol. 2022; 16: 11795468211065782.
- Matta A, Delmas C, Campelo-Parada F, et al. Takotsubo cardiomyopathy. Rev Cardiovasc Med. 2022; 23: 38.
- Sattar Y, Woei Siew KS, Connerney M, et al. Management of takotsubo syndrome: a comprehensive review. Cureus. 2020; 12: 6556.
- Gupta S, Gupta MM. Takotsubo syndrome. Indian Heart J. 2018; 70: 165-174.
- Vijiiac A, Ploscaru V, Vatasescu RG. The great myocardial mimic—takotsubo syndrome. Maedica (Bucur. 2020; 15: 111-121.
- Duran-Cambra A, Sutil-Vega M, Fiol M, et al. Systematic review of the electrocardiographic changes in takotsubo syndrome. Ann Noninvasive Electrocardiol. 2014; 0: 1-6.
- Bennett J, Ferdinande B, Kayaert P, et al. Time course of electrocardiographic changes in transient left ventricular ballooning syndrome. Int J Cardiol. 2013; 169: 276-280.
- Lyon A, Citro R, Schneider B, et al. Pathophysiology of takotsubo syndrome. JACC state-of-the-art review. J Am Coll Cardiol. 2021; 77: 902-921.
- Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus on takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018; 39: 2032-2046.
- Ibanez B, Choi B, Navarro F, Farre J. Tako-tsubo syndrome: a form of spontaneous aborted myocardial infarction? Eur Heart J. 2006; 27: 1509- 1510.
- Y-Hassan S. Takotsubo syndrome triggered by acute coronary syndrome in a cohort of 20 patients: an often missed diagnosis. Int J Cardiol Res. 2015; 2: 28-33.
- Tan ES, Gharti SB, Schmidt M, et al. A fatal case of cardiac sarcoidosis presenting as refractory ascites. Cureus. 2025; 17: 85718.