Review Article
Austin J Cardiovasc Dis Atherosclerosis. 2014;1(2): 4.
Post-Pci Anemia, Bleeding and Transfusion: How are they related with Ischemic Outcomes?
Puddu PE*, Iannetta L, Torromeo C and Schiariti M
Department of Cardiovascular, Respiratory, Nephrological and Geriatrical Sciences, University of Rome, Italy
*Corresponding author: Puddu PE, Laboratory of Biotechnologies Applied to Cardiovascular Medicine, Department of Cardiovascular, Respiratory, Nephrological, Anesthesiological and Geriatrical Sciences, Sapienza, University of Rome, Viale del Policlinico, 155, Rome 00161, Italy.
Received: August 20, 2014; Accepted: September 19, 2014; Published: September 21, 2014
Abstract
Baseline and post-Percutaneous Coronary Intervention (PCI) anemia (PPA) were related to ischemic outcome. Several criteria were proposed to define post-PCI anemia which is strictly related to another two common problems: bleeding and transfusion. In the context of Acute Coronary Syndromes (ACS) and PPA in particular, it should be important to define anemia in comparative terms versus baseline levels: > 15% of red blood cell decrease should be a practical cut-off value. If one wishes to concentrate on Hemoglobin (Hb), a > 2 g/dl Hb decrease from baseline should be considered. We aimed at reviewing current literature and showing the importance of post-PCI anemia recognition in the setting of ACS and PPA. By taking into account all these parameters it is possible to recognize sub-populations exposed to short-term hemorrhagic and/ or long-term ischemic risks. Ischemic and hemorrhagic risks need be carefully evaluated along with thrombocytopenia and its prognostic significance in order to put all these blood and rheological parameters into a clinically oriented perspective. Therapeutic decisions should be oriented accordingly.
Keywords: Anemia; Ischemia; PCI; Bleeding; Transfusion
Introduction
In the setting of Acute Coronary Syndromes (ACS) laboratory parameters as anemia, impaired glucose metabolism and renal failure may be related to ischemic outcome or just considered as comorbidities [1] thus interfering with cardiac ischemia and impacting on patient’s prognosis [1,2]. Anemia, either primary or secondary, was related to increased risk of death and heart failure and a risk score proposed it as a covariate [3]. On the other hand, post- Percutaneous Coronary Intervention (PCI) anemia was associated not only to higher mortality but also to increased rate of Major Adverse Cardiac Events (MACE) [1] as was thrombocytopenia, intimately connected to ischemic outcomes [4-7]. However, anemia and/or thrombocytopenia may be secondary to the presence of truly primary causes impacting on long-term outcome such as Intra-Aortic Balloon Pump (IABP), a recently proposed reasonable primary cause of adverse ischemic outcome instead of post-PCI thrombocytopenia [8]. Thus, studies aimed at assessing or defining the role of blood and/ or rheological parameters are of clinical and predictive importance [8]. We aimed at defining post-PCI anemia and determining its relationship with adverse ischemic outcomes.
Definition of ACS Related Anemia
Both baseline [1,9-14] and post-PCI [3,11,15,16] anemia were related to ischemic outcomes. Actually there is no uniform definition of anemia in the setting of ACS. Several studies defined it according to World Health Organization’s (WHO) criteria: hemoglobin (Hb) levels ≤ 13 g/dl in men and ≤ 12 g/dl in women [10-13. Others proposed a hematocrit value < 39% in men and < 36% in women [1]. Sattur et al. first identified post-PCI anemia (PPA), defining it as a nadir of Hb levels ≤10 g/dl after angioplasty, according to common clinical practice of transfusing patients with these values [15]. A recent work analyzed prognostic implications not only of PPA, but also of longitudinal Hb levels following the first Acute Myocardial Infarction (AMI), showing that Hb drop was associated to worse outcome independently from an anemic state [16]. In the context of ACS and PPA in particular, it should be important to define anemia in comparative terms versus baseline levels: > 15% of Red Blood Cell (RBC) decrease should be a practical cut-off value. If one wishes to concentrate on Hb, a > 2 g/dl Hb decrease from baseline should be considered.
Incidence and etiology of ACS related anemia and bleeding
In the setting of ACS anemia, particularly PPA is strictly related to another two problems: bleeding (an important cause) and transfusion (a needed consequence). ACS patients with anemia were more vulnerable to bleeding than their counterparts without it [10]. Major bleeding was related to ischemic outcomes also [17-24.
A myriad of criteria were proposed to define major bleeding [17-24]. TIMI classification, a laboratory-based scale, defined major bleeding as any intracranial bleeding or an Hb reduction ≥ 5 g/dl (or a > 15% hematocrit decrease) [17]. These criteria included any blood transfusion with 1 unit of packed red blood cells [18]. During phase II studies this concept was expanded to any hemorrhagic death and cardiac tamponade [18]. However, it has not been widely recognized that in more recent TIMI trials bleeding criteria were further extended to include Hb level decrease ≥ 3 g/dl [18]. GUSTO classification, a clinically-based categorization, determined severe bleeding as intracranial or hemodinamically compromising blood loss requiring intervention [17]. Both TIMI and GUSTO classifications were used in different clinical trials but numerous other criteria were also created [18]. For example, STEMI-ASSENT 3 defined major bleeding as any need for transfusion or blood loss resulting in hemodynamic compromise. HERO-2 considered bleeding needing transfusion and hemodynamic compromise requiring blood or fluid replacement, inotropic support, surgical intervention or cardiopulmonary resuscitation. COMMIT study included cerebral and fatal bleeding and overt bleeding requiring transfusions. NSTEMI ACUITY trial defined major bleeding as intracranial or intraocular and reduction of Hb ≥ 4 g/dl (without overt source) or ≥ 3 g/dl (with overt source), any blood transfusion, hematoma with diameter ≥ 5 cm, re-operation for bleeding, access site hemorrhage requiring intervention. CURE trial included symptomatic intraocular or intracranial hemorrhages, reduction of Hb ≥ 5 g/dl, need for ≥ 2 units of blood and any fatal or disabling blood loss. OASIS-5 considered all symptomatic intracranial, intraocular or retroperitoneal bleeding, overt bleeding with reduction of Hb ≥ 3 g/dl, unconcealed bleeding requiring for ≥ 2 units of blood and any fatal clinically manifest blood loss. PCI REPLACE-2 trial such as STEEPLE trial included any intracranial, retroperitoneal and intraocular bleeding, but the first one defined major bleeding as overt blood loss resulting in reduction of Hb ≥ 3 g/ dl or any decrease ≥ 4 g/dl or any ≥ 2 units of blood transfused, while the second one defined them as clinically overt bleeding resulting in a decrease in Hb ≥ 3 g/dl or clinically overt bleeding requiring ≥ 1 units of blood. Finally, STEEPLE trial included any fatal bleeding or one requiring interventions or decompression of a closed space.
Management of ACS related anemia
It is quite clear that a strict relation not only between PPA and major bleeding exists but also between these variables and transfusions. In fact, as mentioned above, need for blood transfusion was a criterion for the definition of major bleeding [18]. Nevertheless, there was also a relation between transfusions and ischemic outcomes [25-27].
Baseline and post-PCI anemia were related to increased risk not only of death but also of MACE and therefore to myocardial ischemia [1,3,9-16]. A relationship between Hb levels and myocardial ischemia was demonstrated also through ECG modifications [9]. In fact, a recent work analyzing data from INTERACT trial determined an independent relationship between admission Hb levels and recurrent myocardial ischemia through continuous ECG monitoring during the first 48 hours of NSTEMI patients [9]. Several mechanisms were proposed to explain this correlation [9]: a) mismatch between oxygen supply and demand, b) activation of rennin-angiotensin-aldosterone and c) activation of the sympathetic nervous system. In fact, after hypoxia there is a lowering of systemic vascular resistances leading to the activation of the abovementioned systems those clues in fluid retention [9]. Also inflammatory response was singled out: chronic inflammation in patients with coronary artery disease leaded to anemia and decreased erythrocyte survival, promoting a subsequent erythropoietin release, therefore platelet activation and induction of pro-coagulant cytokines [9]. Reduced bioactivity of nitric oxide within coronary microcirculation was also proposed as a consequence of low Hb levels [28]. Finally, anemic patients were usually treated less aggressively [9] which might be a further cause of worse outcome.
Anemia and ischemic outcomes
Baseline and post-PCI anemia were widely related to increased mortality [1,3,9-16]. Rousseau et al. first demonstrated an independent relationship between Hb value at admission and recurrent ischemia through continuous ECG monitoring in NSTEMI patients, enforcing the existence of this causative link [9]. Sattur et al. evaluated the role of PPA and showed it as common, frequently not associated to bleeding or significant Hb drop and a potential marker of poor long-term outcome [15].
In a study among 868 consecutive patients in whom baseline and post-PCI platelet count were considered and the role of IABP was determined as a potential detrimental factor for 1-year cumulative ischemic outcome we observed [8] that thrombocytopenia (> 25% delta platelet count) might be a cryptic protagonist, although IABP itself might be a further cryptic protagonist of worse outcome. Although selected reasons for taking advantage of IABP certainly exist [29], the IABP-SHOCK II randomized trial pointed to the absence of either short [30] or long-term [31] survival benefits among post- AMI patients with cardiogenic shock treated with IABP. There were indeed few subgroups, such as <50-year aged or non-hypertensive patients in whom IABP was prognostically advantageous also at long term [30,31], although no real explanation for this was presented. Interestignly, patients with 1 mmol/l higher Hb or with > 10% higher hematocrit had a univariate advantage at long-term with hazard ratios 0.87 (p<0.0004) and 0.83 (p<0.01), respectively.
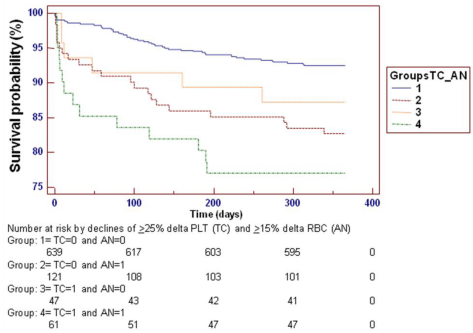
Based on the abovementioned univariate results in the IABP-SHOCK II Study and aimed at trying to find potential explanations for the apparently detrimental role of IABP, we looked at post PCI anemia among our patients [8]. In an ad-hoc analysis, we first defined relative anemia by > 15% RBC decrease from baseline values and an interaction with > 25% delta platelet count was considered (Figure 1). The results showed that anemia “absorbs” the significant predictive role of thrombocytopenia although in a forced Cox model where IABP was also fitted, the IABP detrimental role was still present. Second, we looked at absolute > 2 g/dl Hb decrease from baseline to post- PCI and the abovementioned results with delta RBC were also more clear-cut, both univariately and multivariately. In particular, when a stepwise forward Cox model was run in presence of both absolute Hb and absolute delta Hb values, not only were these covariates both significant (p<0.00001), also in presence of a still significant detrimental role of IABP (p<0.00001), but the detrimental role of delta platelet count (p<0.0085), still existing when only absolute baseline Hb values were considered (p<0.0002), was completely eliminated. These results point to the importance of both absolute basal Hb values and of absolute delta changes of Hb (both as a dychotomous variable with a cut-off value > 2 g/dl and as continuous delta absolute values). Moreover, when the dychotomous role of > 2 g/dl Hb decrease from baseline to post-PCI and the interaction with the dychotomous role of > 25% delta platelet count was considered there was also a clear difference in cumulative ischemic events at 1-year (data not published).
TC: Thrombocytopenia (> 25% delta platelet count, from baseline values); AN: anemia (> 15% red blood cell decrease, RBC, from baseline values).
Figure 1 : Kaplan-Meier curves for the incidence of composite ischemic events during 1 year following Primary Coronary Intervention (PCI) among 868 patients subdivided according to thrombocytopenic and anemic groups. Grouping for TC was performed in accordance with Schiariti et al. [8,32]. A globally significant difference exists among groups. However, versus group 1, only group 2 is individually significantly different (hazard ratio 2.47 with 95% confidence intervals 1.33-4.59). Numbers of patients at risk are presented at different time intervals in all 4 groups. Similar results were obtained by categorizing anemia using the dychotomous criterion of > 2 g/dl Hb decrease from baseline to post-PCI and the interaction with the dychotomous role of > 25% delta platelet count.TC: Thrombocytopenia (> 25% delta platelet count, from baseline values); AN: anemia (> 15% red blood cell decrease, RBC, from baseline values).
Bleeding seems to add prognostic value to laboratory parameters [17]. Blood losses were associated with myocardial infarction, stroke risk and mortality. Hence their assessment and implementation of all measures to reduce these complications should be an integral part of ischemic patients undergoing PCI [17]. Judicious balance of antithrombotic effect and bleeding risk should be a clue in this setting [17].
Notably in anemic ACS patients undergoing PCI, aggressive antithrombotic and antiplatelet therapies may contribute to increase hemorrhagic complication rates. Increased mortality associated with both anemia and major bleeding underlines the need for maximization of pharmacological safety in this high-risk population [10]. Clinical evidence suggests overt bleeding, therefore transfusions, are more relevant than laboratory parameters, as Hb levels [19]. There should be a relation between bleeding entity and clinical presentation [20]. Notably anemic patients are more likely transfused and the presence of transfusion seems related to worse outcomes, particularly at short-term [26]. Although bleeding and recurrent AMI affect mortality it seems that the formers have a greater impact [21]. Evidence suggests that transfused patients, particularly the youngest, have worse outcome [26].
Some studies demonstrated that transfusions in anemic ACS patients with PCI were associated to increased rate of ischemic events [25,27] although others showed that transfusions in elderly patients were associated to reduced mortality when hematocrit was ≤ 33% and to increased mortality when it was ≥ 36% [10]. However, Sabatine et al. demonstrated that transfusions reduced cardiovascular mortality in STEMI patients with Hb < 12 g/dl, while in NSTEMI they were anyway associated to increased ischemic risk [14].
Conclusion
When ACS patients develop an anemic state, a high risk population subset should be defined in whom ischemic and hemorrhagic risks are carefully evaluated along with thrombocytopenia and its prognostic significance [32], in order to put all these blood and rheological parameters into a clinically oriented perspective. Therapeutic decisions should be oriented accordingly.
References
- Kruk M, Przyluski J, Kalinczuk L, Pregowski J, Kaczmarska E, Petryka J, et al. Clustering of admission hyperglycemia, impaired renal function and anemia and its impact on in-hospital outcomes in patients with ST-elevation myocardial infarction. Atherosclerosis. 2010; 209: 558-564.
- Puddu PE, Schiariti M, Cuturello D Iannetta L, Saladini A, Bugiardini R. Kidney dysfunction and long-term outcome in post-PCI acute coronary syndrome patients treated by high-dose tirofiban: the role of creatinine clearance. Br J Med Med Res. 2013; 3: 897-913.
- Plakht Y, Shiyovich A, Weitzman S, Fraser D, Zahger D, Gilutz H. A new risk score predicting 1- and 5-year mortality following acute myocardial infarction Soroka Acute Myocardial Infarction (SAMI) Project. Int J Cardiol. 2012; 154: 173-179.
- Nikolsky E, Sadeghi HM, Effron MB, Mehran R, Lansky AJ, Na Y, et al. Impact of in- hospital acquired thrombocytopenia in patients undergoing primary angioplasty for acute myocardial infarction. Am J Cardiol. 2005; 96: 474–481.
- Wang TY, Ou FS, Roe MT, Harrington RA, Ohman EM, Gibler WB, et al. Incidence and prognostic significance of thrombocytopenia developed during acute coronary syn- drome in contemporary clinical practice. Circulation. 2009; 119: 2454–2462.
- Caixeta A, Dangas GD, Mehran R, Feit F, Nikolsky E, Lansky AJ, et al. Incidence and clinical consequences of acquired thrombocytopenia after antithrombotic therapies in pa- tients with acute coronary syndromes: results from the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Am Heart J. 2011; 161: 298–306.
- De Labriolle A, Bonello L, Lemesle G, Roy P, Steinberg DH, Xue Z, et al. Decline in plate- let count in patients treated by percutaneous coronary intervention: definition, inci- dence, prognostic importance, and predictive factors. Eur Heart J. 2010; 31: 1079–1087.
- Schiariti M, Saladini P, Cuturello D, Iannetta L, Torromeo C, Puddu PE. Decline in platelet count and long-term post- PCI ischemic events: implication of the intra-aortic balloon pump. Vascular Pharmacol. 2014; 60: 25-31.
- Rousseau M, Yan RT, Tan M, Lefkowitz CJ, Casanova A, Fitchett D, et al. Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) Trial Investigators. Relation between hemoglobin level and recurrent myocardial ischemia in acute coronary syndromes detected by continuous electrocardiographic monitoring. Am J Cardiol. 2010; 106: 1417-1422.
- Willis P, Voeltz MD. Anemia, hemorrhage, and transfusion in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. Am J Cardiol. 2009; 104: 34C-8C.
- Valente S, Lazzeri C, Chiostri M, Sori A, Giglioli C, Gensini GF. Prior and new onset anemia in ST-elevation myocardial infarction: a different prognostic role? Intern Emerg Med. 2011; 6: 329-336.
- Pilgrim T, Vetterli F, Kalesan B, Stefanini GG, Räber L, Stortecky S, et al. The impact of anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circ Cardiovasc Interv. 2012; 5: 202-210.
- Meneveau N, Schiele F, Seronde MF, Descotes-Genon V, Oettinger J, Chopard R, et al. Anemia for risk assessment of patients with acute coronary syndromes. Am J Cardiol. 2009; 103: 442-447.
- Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005; 111: 2042-2049.
- Sattur S, Harjai KJ, Narula A, Devarakonda S, Orshaw P, Yaeger K. The influence of anemia after percutaneous coronary intervention on clinical outcomes. Clin Cardiol. 2009; 32: 373-379.
- Leshem-Rubinow E, Steinvil A, Rogowski O, Zeltser D, Berliner S, Weitzman D, et al. Hemoglobin nonrecovery following acute myocardial infarction is a biomarker of poor outcome: a retrospective database study. Int J Cardiol. 2013; 169: 349-353.
- Pham PA, Pham PT, Pham PC, Miller JM, Pham PM, Pham SV. Implications of bleeding in acute coronary syndrome and percutaneous coronary intervention. Vasc Health Risk Manag. 2011; 7: 551-567.
- Steinhubl SR, Kastrati A, Berger PB. Variation in the definitions of bleeding in clinical trials of patients with acute coronary syndromes and undergoing percutaneous coronary interventions and its impact on the apparent safety of antithrombotic drugs. Am Heart J. 2007; 154: 3-11.
- Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Mahaffey KW, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol. 2006; 47: 809-816.
- Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007; 49: 1362-1368.
- Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011; 4: 654-664.
- Applegate RJ. Long-term impact of periprocedural bleeding when does it end?. J Am Coll Cardiol. 2011; 58: 1757-1759.
- Suh JW, Mehran R, Claessen BE, Xu K, Baber U, Dangas G, et al. Impact of in-hospital major bleeding on late clinical outcomes after primary percutaneous coronary intervention in acute myocardial infarction the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol. 2011; 58: 1750-1756.
- Manoukian SV. Predictors and impact of bleeding complications in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. Am J Cardiol. 2009; 104: 9C-15C.
- Yang X, Alexander KP, Chen AY, Roe MT, Brindis RG, Rao SV, et al. CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol. 2005; 46: 1490-1495.
- Singla I, Zahid M, Good CB, Macioce A, Sonel AF. Impact of blood transfusions in patients presenting with anemia and suspected acute coronary syndrome. Am J Cardiol. 2007; 99: 1119-1121.
- Jolicoeur EM, O'Neill WW, Hellkamp A, Hamm CW, Holmes DR, Al-Khalidi HR, et al. APEX-AMI Investigators. Transfusion and mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J. 2009; 30: 2575-2583.
- Andreotti F, Pafundi T, Coluzzi G, Maseri A. Hemoglobin levels, nitric oxide bioavailability and cardiovascular outcomes. Am J Cardiol. 2011; 107: 1099.
- Schiariti M, Puddu PE, Nesta C, Grillo P, Missiroli B, Cassese M. Prophylactic intra-aortic balloon pump is useful to facilitate percutaneous coronary interventions; images from rescue cases. Heart Surg Forum. 2007; 10: E411-E414.
- Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367: 1287-1296.
- Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): ?nal 12 month results of a randomised, open-label trial. Lancet. 2013; 382: 1638-1645.
- Schiariti M, Iannetta L, Torromeo C, De Gregorio M, Puddu PE. Prognostic significance of post-PCI thrombocytopenia. World J Metaanal. 2014; 2: 24-28.