
Research Article
Austin J Cardiovasc Dis Atherosclerosis. 2015;2(1): 1010.
Human Diabetic Atherosclerotic Plaques are Associated with Increased Intra-Plaque Hemorrhage, Iron Deposition and Altered Heme Oxygenase-1 and Ferritin Protein Expression
K-Raman Purushothaman1,2*, Meerarani Purushothaman¹, Prakash Krishnan¹, Annapoorna Kini¹, Samin K Sharma¹, Valentin Fuster1,3 and Pedro R Moreno¹
¹The Zena and Michael A. Weiner Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York
²Department of Pathology, Icahn School of Medicine at Mount Sinai, New York
³Department of Cardiovascular, Centro Nacional de Investigaciones Cardiovasculares, Spain
*Corresponding author: K-Raman Purushothaman, Department of Medicine/Cardiology/Pathology, Icahn school of Medicine at Mount Sinai, Cardiovascular Institute, One Gustave Levy Place, Box 1030, NY 10029, New York,
Received: January 27, 2015; Accepted: February 22, 2015; Published: February 25, 2015
Abstract
Background: Heme Oxygenase-1 (HO-1), a rate limiting, antioxidant and atheroprotective enzyme is evolving as a key defense mechanism against Intra- Plaque Hemorrhage (IPH) and iron deposition in atherosclerosis. Extravasated erythrocytes liberate free heme within the plaque, which exerts toxic effect due to its iron component. HO-1 catalyzes the degradation of heme and the iron released is sequestered by ferritin. This study evaluated IPH derived iron deposition and HO-1/ ferritin pathway in diabetic atherosclerotic plaques.
Methods and Results: Two hundred and five aortic atherosclerotic plaques from 30 cadavers (16 DM and 14 non-DM) were evaluated for plaque characteristics, percentage of IPH (H&E stain), iron deposition score (Perl’s stain), HO-1 and ferritin protein expression grade (immunohistochemistry). Plaque characteristics were similar in both groups. Diabetic plaques had increased percentage of IPH (22.5% vs. 11.9%, P=0.03), iron deposition grade (0.68 ± 0.07 vs. 0.33 ± 0.05; P= 0.005), and HO-1 expression grade (1.93 ± 0.28vs.0.69 ± 0.29; P= 0.001) compared to non-DM plaques. However, DM plaques had decreased ferritin expression grade (0.57 ± 0.2 vs. 2.05 ± 0.28; P=0.001) compared to non-DM plaques.
Conclusion: This study documented an increase in IPH, iron deposition and HO-1 protein expression in diabetic plaques. Despite of these findings, a reduction in ferritin expression was detected in the presence of increased iron deposition, suggesting impairment in the catabolism of free heme in diabetic atherosclerotic plaques.
Keywords: Diabetes mellitus; Atherosclerosis; Intra-plaque hemorrhage; Hemeoxygenase-1; Iron; Ferritin
Abbreviations
HO-1: Hemeoxygenase-1; IPH: Intra-Plaque-Hemorrhage; DM: Diabetes Mellitus; CO: Carbon Monoxide; Fe2+: Ferrous; UPS: Ubiquitin-Proteasome System
Introduction
Advanced diabetic atherosclerosis is associated with increased plaque neovascularization, Intraplaque Hemorrhage (IPH), and inflammation [1-4]. Intraplaque hemorrhage is recognized as a key mechanism by which atherosclerotic plaque may expand rapidly resulting in plaque rupture leading to atherothrombosis [5-7]. Intra-Plaque Hemorrhage leads to extravasation of erythrocytes, which liberates Hemoglobin (Hb). Free Hb is now recognized as a pro-inflammatory mediator of vascular disease due to the toxic properties of iron-containing heme [8,9]. Accumulation of free heme induces lipid peroxidation, triggering downstream pro-inflammatory signaling pathway [8].
Free heme is catabolized by the enzyme Heme Oxygenase-1 (HOResearch 1) into Carbon Monoxide (CO), biliverdin, and ferrous (Fe2+) iron [10]. The ferrous iron is rapidly sequestered by ferritin, and thereby preventing oxidative injury [11]. Hence, both HO-1 and ferritin are effective anti-oxidant molecules that modulate the trafficking of iron as well as the potential oxidative injury mediated by iron in the plaque [10-13]. This has specific relevance in hyper oxidative states like Diabetes Mellitus (DM). We have previously reported an increase in iron deposition in diabetic atherosclerotic plaque [14], suggesting impairment in free heme catabolic pathway. In the present study, we sought to investigate the HO-1 and ferritin pathway in the plaque progression of Diabetic Mellitus (DM) associated atherosclerosis.
Materials and Methods
Full-thickness aortic wall histological sections from 205 plaques (120 DM and 85 non-DM) were taken sequentially at autopsy from 30 descendants (16 DM and 14 non-DM). The aorta was slit open longitudinally, and the intima was washed with saline and then examined visually. On gross examination, aortas had diffuse atherosclerotic lesions with variably distanced spaces between plaques. No aneurysms were observed. A 20-cm aortic segment from the lower thoracic aorta extending into the abdominal aorta above the renal arteries was selected. Individual atherosclerotic plaques raised above the surface with a long axis >0.75 cm were studied [15]. A 1.0- cm long by 0.5-cm wide sample with an edge of normal tissue was obtained for each plaque. The minimum distance between plaques was 0.5 cm. Plaques were characterized adopting the American Heart Association classification, early AHA plaques were excluded and only advanced AHA plaques were evaluated in the study [16]. Fibroatheromas were further characterized by fibrous cap thickness using oculometer in light microscopy. The study was approved by the investigational review board at Icahn School of Medical at Mount Sinai, New York.
Histochemistry for intra-plaque hemorrhage and plaque iron (Perl’s stain)
Intra-plaque hemorrhage was identified in the H&E stained slides by observing the presence of extravasated red blood cells and fibrin in the plaque as previously reported [16]. Plaque iron was identified and scored using Perl’s stain. In this method, ferric iron released from the attached proteins by treatment with dilute hydrochloric acid reacts with potassium ferrocyanide resulting in the formation of an insoluble ferric ferrocyanide (Prussian blue). Scoring of iron in the plaques was done as grade 0: with no iron stained area; grade 1: minimal amount of iron granules; grade 2: moderate amount of iron granules and grade 3: dense or severe iron granules under 40X high power field.
Immunohistochemistry for HO-1 and Ferritin
Formalin fixed paraffin embedded 4μm thick sections were deparaffinized and used to measure the expression of HO-1 and ferritin. Immunostaining was performed with specific primary rabbit polyclonal anti-human HO-1 antibody (Abcam, MA, Cat # Ab13243 at 1:100 dilutions) and with primary rabbit polyclonal anti-human ferritin (Novus biologicals, CO, cat # NB 600-920 at 1:100), by adopting avidin-biotin complex method using ABC kit (Vector Lab, CA, Cat # 6101) with biotinylated secondary antibody (Goat IgG). HO-1, and ferritin protein expressions were detected by developing brown color chromogen reaction with 3’3’Diaminobedzidine (DAB) reagent. Appropriate positive (lung tissue was used for HO-1 antibody; liver tissue was used for ferritin antibody) and rabbit negative controls (Dako, CA) were used in all immunochemistry protocols. The scoring of HO-1 and ferritin was performed semiquantitatively in 10 random high power fields under light microscopy in the 20X objective by measuring the intensity of immunostaining in each field and the distribution of the total number of cells stained and evaluated as grade 0: with no stained area; grade 1: mild intensity of HO1/ferritin positive cells; grade 2: moderate intensity of HO1/ ferritin positive cells and grade 3: dense or severe intensity of HO1/ ferritin positive cells.
Quantification of cellular co-localization of macrophage/ HO-1 and SMC/HO-1
Macrophage and SMC positive cells co-expressing HO-1 was detected by confocal microscopically. To detect this, primary antibodies against the following antigens were adopted, mouse monoclonal CD68 (M0814- DAKO, CA, 1:50 dilution), rabbit polyclonal HO1 (ab13423- Abcam, MA, 1: 100 dilution), mouse monoclonal a-smooth muscle actin (a-SMA-FITC, F3777-Sigma Aldrich, MO, 1:500 dilution). Secondary antibodies donkey antimouse Alexa Fluor 488 or anti-rabbit Alexa Fluor 594 (A-21202 and A-21207, respectively; Invitrogen, NY, 1:500 dilution) were used. Mounting medium containing DAPI (H-1200-Vector Lab, CA) was then applied. Screening of the co-expression of macrophage with HO-1 and SMC with HO-1 were performed in a blinded fashion. Three 20X magnification fields, randomly selected by the confocal Leica microscope, were identified. Images were acquired using Leica TCS SP5 DMI, inverted confocal laser scanning microscope at Mount Sinai’s Shared Resource Facility and analyzed using Leica LAS AF lite software system.
Statistical analysis
Plaque characteristics including the prevalence of atheroma, fibroatheroma, calcific and fibrotic plaques, thin cap fibroatheroma, and intra-plaque hemorrhage were determined by DM status of the decedent. Next, the mean and 95% confidence intervals for HO-1 protein expression and ferritin protein expression were calculated by DM status. Standard errors, used in calculating the confidence intervals, and tests of significance (i.e., p-values) for differences across DM status were determined using generalized linear mixed models which accounted for the presence of multiple plaques within each decedent. Analyses were conducted using IBM SPSS/PASW Statistics 20 (SPSS Inc., Chicago, Illinois). Probability values < 0.05 were considered significant.
Results and Discussion
Demographics
Thirty descendants (16 DM and 14 non DM) were used in this study. All DM decedents in this analysis had Type II DM. Age and gender incidence showed no significant difference between DM and non-DM. A fasting glucose of greater than 125 mg/dl on two different occasions was used as the criteria for DM status. All DM had increased HbA1C level (Table 1). None of the decedents were insulin dependent. The prevalence of cardiac death was similar in DM and non-DM (33% vs. 23%; P=NS).
Clinical Profile
Diabetics
(n= 16)
Non-Diabetics
(n=14)
P Value
Age (years)
67.38 ± 3.0
66.71 ± 3.9
NS
Sex- Female (%)
42.6
31.3
NS
Glucose- F (mg/dl)
183.81 ± 22.4
102.43 ± 3.6
0.003
HbA1c (%)
7.39 ± 0.3
5.31 ± 0.1
0.0001
CAD (%)
62.5
21.5
0.03
CVD death (%)
33
23
0.55
Table 1: Clinical demographic characteristics by diabetes mellitus status.
Plaque characteristics
Advanced atherosclerotic plaques were studied as per AHA classification and included in the analysis. The distribution of each type of plaque was similar in both groups as shown in (Table 2). The incidence of thin-cap fibroatheroma was also similar in DM compared to non-DM plaques (25.4% versus 24.4%; P=NS).
Histopathological plaque morphology
Diabetics
Non- Diabetics
P-value†
Atheroma – AHA-IV
24 (20)
17 (20)
1.00
Fibroatheroma – AHA-VA
49 (41)
35 (41)
0.97
Calcific – AHA-VB
16 (13)
17 (20)
0.485
Fibrotic –AHA-VC
16 (13)
4 (5)
0.131
Thin cap fibroatheroma ( cap thickness = < 65μm)
30 (25)
20 (24)
0.899
† p-values are adjusted for within-person correlation of plaques
Table 2: Plaque characteristics by diabetes mellitus status.
Intra-plaque hemorrhage and plaque iron deposition
The incidence of IPH was higher in DM compared to non-DM plaques (DM- 22.5% vs. non-DM 11.9%; P= 0.03) as shown in the (Figure 1). The mean iron content was also increased in DM plaques (0.68 ± 0.07 vs. 0.33 ± 0.05; P=0.005), as shown in (Figure 2). We have previously documented an increase in neovascularization and IPH in DM plaques [2]. In the present study, an increase in Perl’s stain in the atherosclerotic plaques from patients with DM indicates impairment in the clearance of Hb derived iron in DM plaques. Increased iron deposition induces oxidative stress through Fenton reaction [17,18]. This could be due to impairment in the HO-1/ferritin pathway.
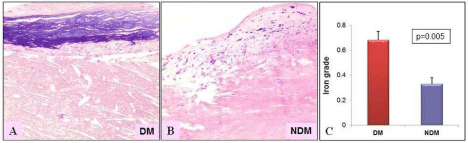
Figure 2: Human atherosclerotic plaques showing iron deposition in the
intimal plaque stained with Perl’s stain. (A) Diabetic plaques show a dense
iron deposition (grade-3) compared to (B) Non-diabetic plaques, mild iron
deposition (grade-1) (p=0.005). (C) The bar graph illustrating the significant
increase in mean Perl’s iron deposition grade in diabetic plaques compared
to non-diabetics. 20X magnification. (Non-DM: NDM).
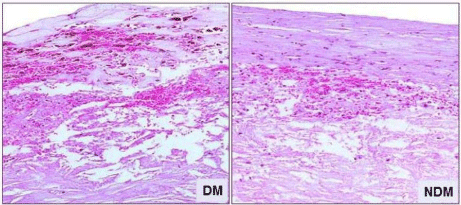
Figure 1: Intra-Plaque Hemorrhage (IPH) showing extravasation of Red
Blood Cells (RBC) and fibrin deposition in H&E stained section. Percentage
of IPH was higher in plaques from patients with DM compared to plaques
from non-DM patients. 20X magnification. (Non-DM: NDM).
HO-1 and Ferritin protein expression in Atherosclerotic plaques
Hemeoxygenase-1 protein expression was increased in DM compared to non-DM plaques (1.93 ± 0.28 vs. 0.69 ± 0.29; P=0.001) as shown in the (Figure 3). Nevertheless, ferritin protein expression was decreased in DM plaques compared to non-DM plaques (DM: 0.57 ± 0.2 vs. Non-DM: 2.05 ± 0.28; P=0.001) as shown in (Figure 4). In addition, confocal microscopy observation depicted macrophages and smooth muscle cells showing increased co-localization with HO-1 in DM plaques compared to non-DM plaques as shown in (Figure 5A & 5B).
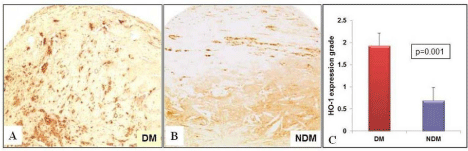
Figure 3: Heme oxygenase-1 expression measured in the fibrous cap
and lipid core of the plaques by immunohistochemistry. (A) DM plaques
show higher HO-1 expression (grade 3) compared to (B) Non-diabetic
atherosclerotic plaque (grade 1) (p=0.001). (C) The bar graph depicting the
significant increase in mean HO-1 grade in diabetic plaques compared to
non-diabetics plaques. 20X magnification. (Non-DM: NDM).
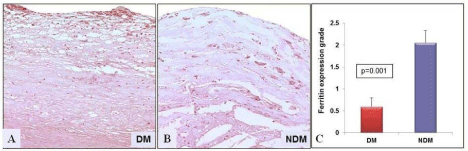
Figure 4: Ferritin expression measured in human atherosclerotic plaques
by immunohistochemistry. (A) Diabetic plaques show reduced ferritin
expression (grade 1) compared to (B) non-diabetic plaque, which show an
increased expression of ferritin labeled in brown color in the fibrous cap and
lipid core (grade-3) (p=0.001). (C) The bar graph illustrating a significant
decrease in ferritin expression in diabetic plaques compared to non-diabetics.
20X magnification. (Non-DM: NDM).
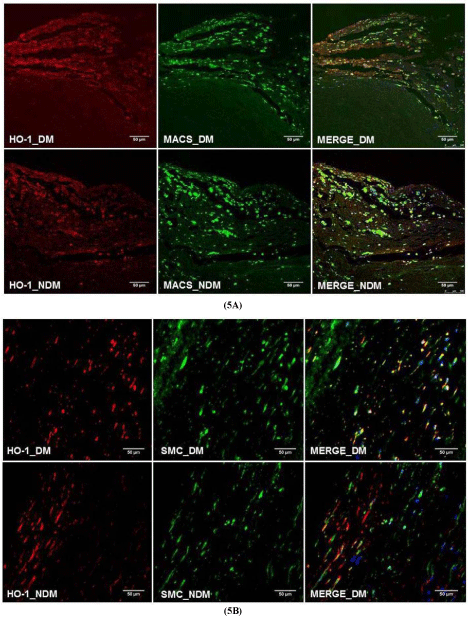
Figure 5: Immunoflourescence co-localization of (A) macrophages
with HO-1 (B) alpha actin with HO-1 protein show DM has increased colocalization
in macrophages and alpha-actin suggesting possible expression
of HO-1 protein from macrophages and smooth muscle cells. 20X
magnification. (Non-DM: NDM).
The main function of HO-1 in atherosclerotic plaques is the degradation of free heme liberated from Hb. HO-1 acts as a defense mechanism against the deleterious effects of free heme induced oxidative injury. The increased amount of HO-1 protein in DM plaque observed in this study may not be associated with an increase in HO-1 specific activity that catabolizes free heme with the liberation of antioxidant carbon monoxide and bilirubin in the plaque. Impairment in the HO-1 activity is associated with increased oxidative stress. This could lead to inactivation of HO-1 in the DM plaque [19]. The increase in HO-1 protein observed can also be explained by alterations in HO-1 degradation pathway within atherosclerotic plaques. The Ubiquitin-Proteasome System (UPS) is the principal degradation route of intracellular proteins in atherosclerosis [20]. Ubiquitin-proteasome system metabolizes HO-1 through the endoplasmic reticulum associated degradation pathway, which is dysfunctional in DM atherosclerosis [20-22]. Impaired degradation in DM plaques may contribute to HO-1 protein accumulation, as observed in this study.
Furthermore, a reduction in ferritin expression was seen in DM plaques. Ferritin is an iron storage protein that exhibits cytoprotective activity by rapidly sequestering free iron, which is the key catalyst of oxygen radical generation in biological systems [23]. Due to its high capacity to store free iron [24], ferritin can effectively reduce iron-mediated reactive oxygen generation [25]. As a result, a decrease in ferritin expression in addition to impaired HO-1 activity in atherosclerotic plaques may clearly reduce the protective antioxidant activity in patients with DM.
Limitations of the study
The study was performed on formalin fixed paraffin embedded tissues, which limits quantification of HO-1 activity and molecular biology. As a surrogate to HO-1 activity, we quantified ferritin expression. Reduced levels of ferritin expression suggest reduced activity of HO-1 protein. Further studies using molecular biology tools may be needed to clarify this issue.
Conclusion
Our findings include increased intra-plaque hemorrhage with plaque iron deposition associated with increased HO-1 protein expression and concomitant decreased ferritin protein expression. This illustrates impairment in the regulation of HO-1/ferritin pathway in DM atherosclerotic plaques. Furthermore, molecular mechanism underlying the HO-1/ferritin pathway may address the specific link between HO-1 and ferritin associated with increased iron induced oxidative stress.
References
- Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006; 113: 2245-2252.
- Purushothaman KR, Meerarani P, Muntner P, Lento PA, O'Connor WN, Sharma SK, et al. Inflammation, neovascularization and intra-plaque hemorrhage are associated with increased reparative collagen content: Implication for plaque progression in diabetic atherosclerosis. Vasc Med. 2011; 16: 103-108.
- Virmani R, Narula J, Farb A. When neoangiogenesis ricochets. Am Heart J. 1998; 136: 937-939.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque part I: evolving concepts. J Am Coll Cardiol. 2005; 46: 937-954.
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005; 25: 2054-2061.
- Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. The American journal of pathology. 2009; 174: 1097-1108.
- Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003; 349: 2316-2325.
- Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005; 293: 1653-1662.
- Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002; 100: 879-887.
- Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008; 117: 231-241.
- Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, et al. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003; 17: 1724-1726.
- Hoekstra KA, Godin DV, Cheng KM. Protective role of heme oxygenase in the blood vessel wall during atherogenesis. Biochem Cell Biol. 2004; 82: 351-359.
- Ishikawa K, Sugawara D, Wang Xp, Suzuki K, Itabe H, Maruyama Y, et al. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001; 88: 506-512.
- Moreno PR, Purushothaman KR, Purushothaman M, Muntner P, Levy NS, Fuster V, et al. Haptoglobin genotype is a major determinant of the amount of iron in the human atherosclerotic plaque. J Am Coll Cardiol. 2008; 52: 1049-1051.
- Moreno PR, Lodder RA, Purushothaman KR, Charash WE, O'Connor WN, Muller JE. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation. 2002; 105: 923-927.
- Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004; 110: 2032-2038.
- Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003; 92: 1193-1200.
- Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton JW. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984; 259: 14354-14356.
- Kinobe R, Ji Y, Nakatsu K. Peroxynitrite-mediated inactivation of heme oxygenases. BMC Pharmacol. 2004; 4: 26.
- Versari D, Herrmann J, Gossl M, Mannheim D, Sattler K, Meyer FB, et al. Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2006; 26: 2132-2139.
- Lin PH, Chiang MT, Chau LY. Ubiquitin-proteasome system mediates heme oxygenase-1 degradation through endoplasmic reticulum-associated degradation pathway. Biochim Biophys Acta. 2008; 1783: 1826-1834.
- Marfella R, Di Filippo C, D'Amico M, Paolisso G. Diabetes, ubiquitin proteasome system and atherosclerotic plaque rupture. Circ Res. 2007; 100: e84-85.
- Eisenstein RS, Garcia-Mayol D, Pettingell W, Munro HN. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci U S A. 1991; 88: 688-692.
- Galvez N, Fernandez B, Sanchez P, Cuesta R, Ceolin M, Clemente-Leon M, et al. Comparative structural and chemical studies of ferritin cores with gradual removal of their iron contents. J Am Chem Soc. 2008; 130: 8062-8068.
- MacKenzie EL, Iwasaki K, Tsuji Y. Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008; 10: 997-1030.
Citation: Purushothaman KR, Purushothaman M, Krishnan P, Kini A, Sharma SK, et al. Human Diabetic Atherosclerotic Plaques are Associated with Increased Intra-Plaque Hemorrhage, Iron Deposition and Altered Heme Oxygenase-1 and Ferritin Protein Expression. Austin J Cardiovasc Dis Atherosclerosis. 2015;2(1): 1010. ISSN:2472-3568