
Editorial
Austin J Cardiovasc Dis Atherosclerosis. 2015; 2(1): 1011.
Atherosclerosis in Apolipoprotein E-knockout Mice as a Model of Human Disease
Shibata MA¹*, Shibata E², Fujioka S¹ and Harada- Shiba M²
¹Department of Anatomy & Histopathology, Graduate School of Health Sciences, Osaka Health Science University, Japan
²Department of Molecular Innovation in Lipidology, National Cerebral & Cardiovascular Center Research Institute, Japan
*Corresponding author: Masa-Aki Shibata, Department of Anatomy & Histopathology, Graduate School of Health Sciences, Osaka Health Science University, 1-9-27, Temma, Kita-ku, Osaka, 530-0043, Japan
Received: September 07, 2015; Accepted: September 22, 2015; Published: September 25, 2015
Editorial
Cardiovascular disease was the top cause of death in the world in 2000 and 2012 according to the World Health Organization fact sheet [1]. In 2012, an estimated 56 million people died worldwide, with cardiovascular diseases responsible for 17.5 million of those deaths; this translates to 3 in every 10 fatalities. Ischemic heart disease alone killed 7.4 million people [1]. In Japan, vascular disease of the heart and of the brain are the 2nd and 4th most common cause of death, respectively, according to the 2012 annual report of the Ministry of Health, Labour and Welfare [2]. When considered together, they come close to malignant neoplasm as the most lethal disease of man. The primary etiologic lesion of cardiovascular disease is atherosclerosis, which causes vessel stenosis, embolus via thrombus formation, and ischemia of heart and brain.
Establishment and availability of a relevant atherosclerotic animal model is an extremely crucial tool in translational research to study and understand the disease process. A gene-targeted mouse model of atherosclerosis was created by the Jackson Laboratory in Bar Harbor, Maine, USA; C57BL/6J mice homozygous for the ApoEtm1Unc mutation results in functional knockout of the anti-atherogenic apolipoprotein E (ApoE) gene involved in cholesterol metabolism [3,4]. This ApoEknockout (ApoE-KO) mouse has been and remains to be the most popular animal model for atherosclerosis research because of its propensity to spontaneously develop hypercholesterolemia and atherosclerotic lesions that are similar to those found in humans [5,6].
Macroscopic findings of the atherosclerotic lesions developed by ApoE-KO mice are presented in (Figure 1). Atherosclerotic lesions are seen in aortic arch and in the bifurcations of the descending aorta (Figure 1). Histopathological findings of the atherosclerotic lesions are shown in (Figure 2). These lesions were classified into 3 categories: i) Early, ii) Progressive and iii) Combined. Early lesions show fatty streaks which involve foamy cell accumulations (Figure 2A & 2B). Progressive lesions are composed of foamy cell accumulations, a fibrous cap and lipid cores (Figure 2C & 2D). Complicated lesions are characterized by accompanying ossification and/or occlusion of the lumen (Figure 2E-2G).
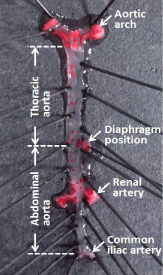
Figure 1: Macroscopic appearance of the atherosclerotic lesions in a 28-weekold
female ApoE-KO mouse fed basal diet. As illustrated, the entire aorta,
from the aortic arch through to branching of the iliac arteries, was opened
lengthwise, pinned flat on a wax dissection board and stained with Oil-red O
(Wako Pure Chemical Industries, Inc., Osaka, Japan). Atherosclerotic lesions
are observed as reddish are as particularly in the aortic arch and in the
bifurcations of the descending aorta, as well as sporadically along its length.
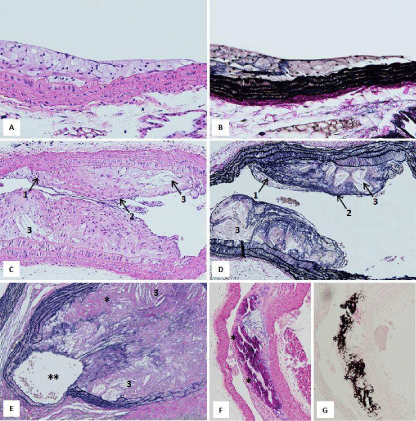
Figure 2: Histopathologic findings of the atherosclerotic lesions in ApoEKO
mice. A&B: Early lesion, C&D: Progressive lesion (1: foamy cell
accumulations, 2: fibrous cap, 3: lipid core), E:Complicated lesion (3: lipid
core, *: ossification, **: narrowing of the vascular lumen). F&G: Calcium
deposition (*) is demonstrated by H&E in F and by von Kossa stain in G(dark
brown area indicate positive). [A-D, plaques from a 15-week-old male ApoEKO
mouse fed basal diet; E-G, plaques from 23-week-old male ApoE-KO
mice fed 1.25% cholesterol diet. A,C,F=HE stain; B,D,E=Elastica van Gieson
stain; G: von Kossa stain. A&B: magnification 200 X, C-G: magnification100
X].
Despite the overall similarities of lesions, there are 2 notable differences between mouse and human disease. One is that, even on a long-term high-fat diet, cardiovascular infarction does not present in mice as it does in humans. Although severe atherosclerotic lesions are seen in the aortas of ApoE-KO mice it is not accompanied by arterio scleros is in the cardiac coronary arteries [5]. The second is that while the majority of studies on human atherosclerosis report less disease in pre-menopausal women than in adult men, postmenopausal women have an equal risk of developing cardiovascular disease [6,7], suggesting a protective effect of estrogen. In ApoE-KO mice, however, differences in incidence by sex are more equivocal; some studies report males have less plaque development than females [8,9] while other studies report no clear sex differences [5,10]. Nevertheless, since the atherosclerotic lesions in ApoE-KO mice are so strikingly analogous to atherosclerotic lesions in humans in their molecular expression as well as in their histopathological progression, the ApoE-KO mouse is currently considered the most relevant model for translational studies of atherosclerosis [5,6,11].
This work was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Health, Labor and Welfare (H23- seisakutansaku-ippan-004. A member of this research: Dr. Shibata, MA. The Research representative: Dr. Harada-Shiba M).
References
- World Health Organization. The top 10 causes of death in the world, 2000 and 2012. Fact sheet No. 310; updated May 2014.
- MinistryofHealth, LabourandWelfareinJapan. Annual Report. The top 10 causes of death in Japan 2012.
- Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl AcadSci USA. 1992; 89: 4471-4475.
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992; 71: 343-353.
- Coleman R, Hayek T, Keidar S, Aviram M. A mouse model for human atherosclerosis: long-term histopathological study of lesion development in the aortic arch of apolipoprotein E-deficient (E0) mice. ActaHistochem. 2006; 108: 415-424.
- Meyrelles SS, Peotta VA, Pereira TM, Vasquez EC. Endothelial dysfunction in the apolipoprotein E-deficient mouse: insights into the influence of diet, gender and aging. Lipids Health Dis. 2011; 10: 211.
- Perez-Lopez FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci. 2010; 17: 511-531.
- Smith DD, Tan X, Tawfik O, Milne G, Stechschulte DJ, et al. Increased aortic atherosclerotic plaque development in female apolipoprotein E-null mice is associated with elevated thromboxane A2 and decreased prostacyclin production. J PhysiolPharmacol. 2010; 61: 309-316.
- Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995; 36: 2320-2328.
- Elhage R, Arnal JF, Pieraggi MT, Duverger N, Fievet C, Faye JC, et al. 17 beta-estradiol prevents fatty streak formation in apolipoprotein E-deficient mice. ArteriosclerThrombVasc Biol. 1997; 17: 2679-2684.
- Shibata MA, Shibata E, Harada-Shiba M. Pathological and molecular biological analyses of atherosclerosis in ApoE-deficient mice as a human disease model for evaluation of toxicologic pathology study. The 47th Ann SciMtg of the Jpn Atherosclerosis Society, Proceedings. 2015; 236.