
Research Article
Austin J Cardiovasc Dis Atherosclerosis. 2015; 2(2): 1016.
A Simple Combined Clinical and Echocardiographic Score Associated with Adverse Long Term Outcomes in Patients with Heart Failure: A Single Center Experience
Aggarwal A1, Khoury Abdulla R1, Mehta N2, Goldstein J1,2, Dixon SR1,2, Berman A1,2 and Abbas AE1,2*
1Department of Cardiovascular Medicine, Beaumont Health, Royal Oak, MI, USA
2Oakland University William Beaumont School of Medicine, Royal Oak, MI, USA
*Corresponding author: Amr E Abbas, Department of Cardiovascular Medicine, Beaumont Health, Royal Oak, MI, USA
Received: December 12, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
Background: In acute decompensated heart failure, it has been shown that the clinical risk factors (CRF) of hypotension and renal dysfunction can be used as an inpatient clinical risk prediction model. However, the long-term predictive value of CRF and the incremental benefit of echocardiographic variables have not been fully investigated.
Methods: We retrospectively identified all patients admitted with acute heart failure during the study period. We examined the clinical, echocardiographic variables and their association with Major Adverse Cardiac Event (MACE) at 18 months, logistic regression models and Kaplan- Meier curves were then derived. A combined echo and clinical score was developed to further risk stratify these patients according to MACE at 18 months.
Results: 120 patients were included. CRFs were associated with MACE at 18 months with C-statistic of 0.65. The echocardiographic indices of both Tricuspid Regurgitation Velocity (TRV) = 3.2 m/s and the ratio of mitral inflow E wave velocity to the mitral annular E’ velocity (E/E’) = 15 improved the C-statistic to 0.73. Adding age, further improved the C-statistic to 0.80 with an earlier onset of MACE in the presence of echocardiographic variables. A simple score (0-4) further stratified the incidence of MACE at 18 months.
Conclusion: In patients with acute heart failure, the strong prognostic value of hypotension and renal dysfunction of in-hospital mortality was not extended to MACE at 18 months. However, the addition of TRV and E/E’ together with age improved the late-outcome prognostic risk score model.
Abbreviations
ASE: American Society of Echocardiography; CRF: Clinical Risk Factors; E/E’: Ratio of Mitral Inflow E Wave Velocity to the Mitral Annular E’ Velocity; MACE: Major Adverse Cardiac Events; LVOT: Left Ventricular Outflow Tract Time Velocity; TRV: Tricuspid Regurgitation Velocity
Introduction
Heart failure is one of the leading causes of morbidity and mortality in the United States. With over one million annual hospital admissions and a one-year mortality of 30%, the annual health care costs exceed 17 billion dollars [1]. Approximately 60% of patients are readmitted to the hospital within six months of discharge [2]. Moreover, readmissions for HF have become a quality standard that is linked to incentive based reimbursement and quality metrics for health systems [1].
Accordingly, stratifying patients based on their risk profile for adverse outcomes may improve disease management, for example by identification of a subset of patients at higher risk for adverse outcomes that may require and benefit from closer monitoring. Several clinical and echocardiographic risk models to better establish long term prognosis have been proposed [2-10].
The large multicenter Acute Decompensated Heart Failure National Registry (ADHERE) registry demonstrated that the combination of the Clinical Risk Factors (CRFs) of systolic blood pressure <115 mmHg, blood urea nitrogen >43 mg/dl, and creatinine > 2.75 mg/dl were associated with in-hospital mortality in patients with acute HF with both reduced and preserved ejection fraction [11]. Furthermore, previously studied echocardiographic parameters (such as ventricular and atrial volumes) may independently identify patients with adverse outcome such as HF exacerbation and cardiac death [3,6]. The Echo Heart Failure Score identified several echo criteria (left ventricular end systolic volume index, mitral deceleration time, and left atrial volume index, pulmonary artery systolic pressure, and the tricuspid annular peak systolic excursion that predicted mortality in systolic heart failure patients at 34 months [3]. However, a simple combined clinical and echocardiographic risk score is not available.
Methods
This study was compliant with the Health Insurance Portability and Accountability Act and the database was conducted under the auspices of the Human Investigation Committee of the Research Institute of William Beaumont Hospital. We retrospectively reviewed the medical records of consecutive patients admitted to Beaumont Health System, with the diagnosis of acute heart failure from January 1st 2011 to December 31st 2011. Diagnosis of heart failure was made by clinical evaluation and confirmed by both B-type natriuretic peptide and chest x-ray findings and noted on the discharge diagnosis. Our exclusion criteria included age >90 years, moderate or severe aortic or mitral valvular disease, technically difficult studies with inadequate Doppler signals, and patients with prosthetic valves.
Clinical characteristics such as age, sex, weight, systolic blood pressure, mean arterial pressure, history of hypertension, coronary artery disease, diabetes, and hyperlipidemia were recorded on the day of admission. Medications such as digoxin, β-blockers, aspirin, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics including aldosterone antagonists were recorded on the day of admission. Laboratory variables including blood urea nitrogen, creatinine, and sodium level were reported on day of admission. Risk model variables from the ADHERE registry (Systolic Blood pressure <115 mmHg, Creatinine > 2.75 mg/dl, and Blood Urea Nitrogen > 43 mg/dl) were termed traditional Clinical Risk Factors (CRF). Major Adverse Cardiovascular Events (MACE) were defined as death, myocardial infarction, stroke, cardiac arrest, and implantable cardiac defibrillator firing at 18 months.
Two experienced observers independently reviewed transthoracic 2-D Echocardiographic images obtained within the first 24 hours of hospitalization. All echocardiographic data were obtained and all calculations were performed as recommended by the American Society of Echocardiography (ASE) consensus documents [12].
The 2-D echocardiographic parameters include ejection fraction, inferior vena cava diameter and compressibility, and left ventricular end diastolic volume. The Doppler echocardiographic parameters include mitral valve E and A wave velocities, mitral valve deceleration time, tricuspid regurgitation velocity, Left Ventricular Outflow Tract time velocity (LVOT) integral, and E’ velocity. The calculated Doppler echocardiographic parameters include RV systolic pressure, E/A, E/E’, stroke volume, and cardiac output using the heart rate at the time of echocardiography.
After identifying patients who met the inclusion criteria, we divided the patients into two groups depending on whether or not they suffered a MACE. We then performed univariate and multivariate analyses for clinical and echocardiographic parameters between the two groups. Categorical variables were reported as counts and frequencies. They were examined using Pearson’s Chi-square tests where appropriate (expected frequency>5), and otherwise Fisher’s Exact tests were used. Continuous variables were tested for normality. Normally distributed variables were analyzed using twosample t-tests. Non-normally distributed variables were examined using Wilcox on rank tests. All continuous variables were reported as means and standard deviation followed by the median and 25th and 75th percentiles where needed.
We examined several separate logistic regression models to predict MACE at 18 months by including both clinical and echocardiographic variables. We then completed backward elimination logistic regression analysis of MACE at 18 months including all significant predictors of an event from previous analyses. Kaplan-Meier curves were completed to examine MACE at 18 months according to the presence of both, none, or either of TRV=3.2 and E/E’=15. The Jonckheere-Terpstra test for trend was utilized to test for a trend of incremental benefit of combined CRFs and echocardiographic variables that were associated with MACE. SAS® for Windows 9.3 Cary, NC was used for all analyses.
Results
During the period from January 1st, 2011, to December 28th 2011, 259 patients were admitted with the diagnosis of acute heart failure. Of those, 139 patients were excluded based on at least one of the exclusionary criteria; 2 patients were excluded based on age > 90, 115 patients were excluded due to moderate to severe left-sided valvular disease, and 22 patients were excluded due to inadequate Doppler signals, leaving 120 patients who were included in the analysis.
At 18 months, 33/120 (27 %) patients suffered MACE, while 87/120 (73 %) did not.
The baseline demographics between these two groups are illustrated in (Table 1A). The echocardiographic characteristics in the two groups are shown in (Table 1B). Of those patients who experienced MACE, the mean age was higher, the estimated glomerular filtration rate was lower, and a history of coronary artery disease was more frequent compared to those patients who did not have MACE at 18 months (Table 1A). The distribution of major adverse cardiac events is shown in (Table 2). The majority of events were death followed by cardiac arrest and myocardial infarction (Table 2).
MACE
No Event
Any Event
p value
(N=87)
(N=33)
Male
54 (62.1%)
22 (66.7%)
0.64
Age mean+/-SD (median)
66+/-15 (68)
76+/-10 (81)
0.001
Weight-Kg mean+/-SD (median)
91+/-26 (85)
90+/-32 (88)
0.61
Risk 1: Blood urea Nitrogen (BUN)>43
9 (10.3%)
9 (27.3%)
0.041
Risk 2: Creatinine >2.75/ ESRD†
6 (6.9%)
5 (15.2%)
0.17
Risk 3: Systolic blood pressure<115 mmHg
17 (19.8%)
5 (15.2%)
0.56
High risk score >0
0
57 (65.5%)
20 (60.6%)
7 (21.2%)
0.006
1
28 (32.2%)
3-Feb
2 (2.3%)
6 (18.2%)
GFR‡ Median (25th, 75th)
71 (52, 99)
40 (28, 79)
0.0002
Systolic blood pressure Mean+/-SD (median)
135+/-25 (135)
133+/-22 (132)
0.76*
Diastolic blood pressure Mean+/-SD (median)
79+/-17 (77)
76+/-16 (79)
0.34*
Mean arterial pressure Mean+/-SD (median)
97+/-18 (95)
95+/-17 (99)
0.77
BNP§Median (25th, 75th)
900 (463, 1404)
912 (486, 2070)
0.47
Sodium Mean+/-SD (median)
139+/-4.2 (139)
139+/-4.0 (140)
0.49
Beta blockers
73 (83.9%)
29 (87.9%)
0.78
Aspirin
69 (79.3%)
23 (69.7%)
0.27
Angiotensin-Converting Enzyme Inhibitor
46 (52.9%)
12 (36.4%)
0.11
Angiotensin Receptor Blockers
16 (18.4%)
7 (21.2%)
0.73
Diuretic
57 (65.5%)
26 (78.8%)
0.16
Digoxin
13 (14.9%)
6 (18.2%)
0.66
Aldosterone Antagonist
17 (19.5%)
4 (12.1%)
0.34
Hypertension
69 (79.3%)
28 (84.9%)
0.49
Diabetes
39 (45.9%)
12 (36.4%)
0.35
Coronary artery disease
43 (49.4%)
23 (69.7%)
0.046
Hyperlipidemia
58 (66.7%)
23 (69.7%)
0.75
*Indicates normally distributed variables that were analyzed using two-sample t-tests.
† End stage renal disease ‡Glomerular filtration rate §Brain natriuretic peptide.
Table 1A: Univariate analysis of baseline clinical and laboratory variables.
MACE
No event
(N=87)
Any event
(N=33)
p value
EF† category
= 30
31-44
= 45
45 (51.7%)
19 (21.8%)
23 (26.4%)
19 (57.6%)
8 (24.2%)
6 (18.2%)
0.64
Left atrium diameter (cm)
Mean+/-SD (median)
4.6+/-0.7 (4.6)
4.7+/-0.6 (4.7)
0.59*
MV‡E/A ratio
Mean+/-SD
Median (25th, 75th)
N=72
2.16+/-1.13
1.90 (1.28, 2.90)
N=27
2.04+/-0.80
1.94 (1.40, 2.80)
0.98
MV‡ Deceleration Time (ms)
Mean+/-SD (median)
168+/-60 (165)
187+/-63 (173)
0.15
LVOT-TVI §
Mean+/-SD (median)
13.2+/-4.5 (12.5)
13.9+/-3.9 (12.5)
0.25
Stroke Volume (ml)
Mean+/-SD (median)
41+/-15 (39)
46+/-18 (40)
0.38
E:E’ Mean+/-SD (median)
21+/-10 (19)
24+/-7.9 (21)
0.10
E:E’= 15
62 (71.3%)
31 (93.9%)
0.008
TRV ǁ = 3.2 or E:E’= 15
Neither
One of them
Both of them
20 (23.0%)
50 (57.5%)
17 (19.5%)
2 (6.1%)
17 (51.5%)
14 (42.4%)
0.013
TRV ǁ = 3.2
22 (25.3%)
14 (42.4%)
0.067
IVC¶ diameter
Mean+/-S D (median)
N=72
2.1+/-0.6 (2.1)
N=27
1.9+/-0.5 (2.0)
0.14*
IVC Compressibility
37/72 (51.4%)
14/27 (51.9%)
0.97
LVEDV#
Median (25th, 75th)
N=76
145 (99, 201)
N=31
143 (101, 192)
0.86
*Indicates normally distributed variables that were analyzed using two-sample t-tests.
† Ejection fraction, ‡ Mitral Valve, § Left ventricle outflow tract-Time velocity integral.
ǁ Tricuspid valve regurgitation, ¶ Inferior vena cava, # Left ventricle end diastolic volume.
Table 1B: Univariate analysis of baseline echocardiographic variables.
MACE type
Number of events at 18 months
Death
23
Cardiac arrest
8
Myocardial infarction
8
ICD firing*
3
Stroke
2
*Implantable cardiac defibrillator.
Table 2: Different types and numbers of MACE.
In the total cohort of 120 patients, MACE occurred within 18 months in 13 /43 (30%) with = 1 CRF, compared to20/77 (26%) without CRFs (p =0.006) (Figure 1). A logistic regression model to determine if the 3 traditional CRFs were associated with MACE at 18 months showed a C-statistic = 0.65 (Figure 2).
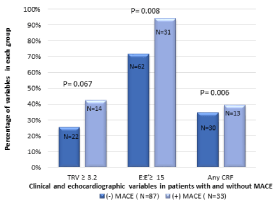
Figure 1: Comparison of main variables between the two groups with and
without MACE: Compared to patients without MACE, those who had MACE
were more likely to have CRF, had higher pulmonary artery pressure and
higher left atrial filling pressure. CRF: clinical risk factor, MACE: Major
adverse cardiac events. TRV: Tricuspid regurgitation velocity. E/E’: Ratio of
mitral inflow E wave velocity to the mitral annular E’ velocity.
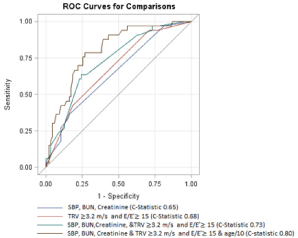
Figure 2: Comparison of ROC curves and C-statistic for the different clinical,
echocardiographic, and combined models: CRF alone did not predict
MACE, whereas the best models achieved using the combination of CRF,
echocardiographic variables of TRV, E/E’ and additional use of age as clinical
variable. CRF: clinical risk factor, TRV: Tricuspid valve regurgitation, E/E’:
The ratio of mitral valve inflow E wave velocity to mitral annular valve E’
velocity. MACE: Major adverse cardiac events
A total of 36/120 (30%) patients exhibited TRV =3.2 m/s, whereas 84/120 (70%) had TRV = 3.2 m/s. Of patients who had MACE, 14/33 (42%) had TRV = 3.2 m/s, whereas in those who had no MACE at 18 months, 22/87(25%) had TRV = 3.2 m/s (p = 0.067) (Figure 1).
In the total cohort, 93/120 (77.5%) patients showed E/E’ = 15, whereas 27/120 (22.5%) had E/E’ < 15. Specifically, 31/33 (94%) patients who experienced a MACE had an E/E’ of =15 whereas 62/87 (71%) without MACE had an E/E’ =15 (p = 0.008) (Figure 1).
Logistic regression modeling performed using both TRV =3.2 m/s and E/E’ =15 yielded a C-statistic of 0.68 for MACE at 18 months. Combining the presence of the 3 CRFs together with a TRV = 3.2m/s and E/E’ =15 improved the C-statistic slightly (C-statistic 0.73, Figure 2). No other echocardiographic variables measured as listed above appeared to be associated with MACE in this population.
The Kaplan–Meier curves for the time to the first MACE is shown in (Figure 3). These curves showed that the more echocardiographic variables (TRV =3.2 m/s, E: E =15) the patients had on admission, the earlier the incident of the first MACE occurred.
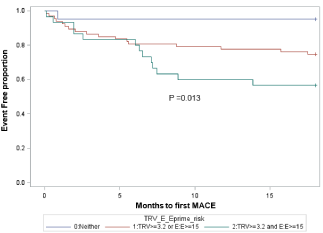
Figure 3: Kaplan–Meier curves for the echocardiographic variables (TRV and
E: E’) and MACE. The more echocardiographic variables (TRV =3.2 m/s, E:
E =15) the patients had on admission, the sooner the incident of the first
MACE occurred.TRV: Tricuspid valve regurgitation. E/E’: The ratio of mitral
valve inflow E wave velocity to mitral annular valve E’ velocity. MACE: Major
adverse cardiac events
Moreover, a combined echocardiographic and clinical score was created to develop a stepwise risk model for MACE depending on the number of CRFs and/or echocardiographic variables present (Figure 4, Table 3).
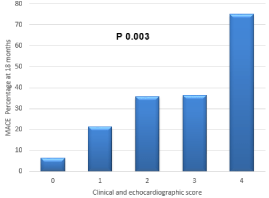
Figure 4: Combined echocardiographic and clinical score: A scoring system
(0-4) was created to develop a stepwise risk model for MACE depending
on the number of CRFs and/or echocardiographic variables present. MACE:
Major adverse cardiac events. CRF: clinical risk factors.
Score
Criteria
Percentage of MACE* at 18 months.
0
Patients with no CRFs† or Echocardiographic variables
6.3%
1
Either 1 CRF and no Echocardiographic variables or one echocardiographic variable and no CRFs
21.3%
2
Patients with 1 CRF and 1 Echocardiographic variable or Echocardiographic variables and no CRFs
35.7%
3
Patients with > 1 CRF and 1 Echocardiographic variable or 1 CRFs and 2 Echocardiographic variables
36.4%
4
Patients with > 1 CRF and both Echocardiographic variables
75%
*MACE: Major adverse cardiac events. † CRF: clinical risk factors (systolic blood pressure (SBP) <115 mmHg, blood urea nitrogen (BUN) >43 mg/dl, and creatinine > 2.75 mg/dl).
Table 3: Simple Clinical and Echocardiographic Score for MACE at 18 months.
- A score of zero (patients with no CRFs or Echocardiographic variables (n=16)): 1/16 (6.3%) had a MACE.
- A score of 1 (patients with either 1 CRF or no Echocardiographic variables (n=6), or one echocardiographic variable and no CRFs (n=41)): 10/47 (21.3%) had a MACE.
- A score of 2 (patients with 1 CRF and 1 Echocardiographic variable (n=22) or 2 Echocardiographic variables and no CRFs (n=20)): 15/42 (35.7%) had a MACE.
- A score of 3 (patients with > 1 CRF and 1 Echocardiographic variable (n=4) or 1 CRFs and 2 Echocardiographic variables (n=7)): 4/11 (36.4%) had a MACE.
- A score of 4 (patients with > 1 CRF and both Echocardiographic variables (n=4)): 3/4 (75%) had a MACE.
The Jonckheere-Terpstra test for trend which takes the increasing values of the scores into account, resulted in p value of 0.003.
Discussion
The results of this study suggest that in patients with acute HF, the strong prognostic value of the ADHERE registry CRFs (Systolic blood pressure < 115mm Hg, blood urea nitrogen > 43 mg/dl, Creatinine > 2.75mg/dl) that predicted in-hospital mortality in the registry, was not extended to MACE at 18 months. However, the addition of TRV (an index of pulmonary artery systolic blood pressure) and E/E’ (an index of left atrial pressure) together with age improved the late-outcome prognostic risk model and suggested an earlier onset of MACE with a stepwise increase in MACE according to the simple risk score.
As the number of CRFs and echocardiographic indices increased and as the age of patients increase, there was a stepwise increase in MACE at 18 months. This study is unique as it studies the longterm prognostic value of the ADHERE registry CRFs, and provides a simple combined clinical and echocardiographic long-term risk score model associated with MACE in patients with HF. Simply stated, elderly patients who present with acute HF and have low blood pressure, abnormal kidney function, and echocardiographic evidence of elevated left atrial and right ventricular systolic pressures suffer a high incidence of MACE at 18 months.
The present findings are consistent with and extend those of Lee et al. who showed in 4031 patients admitted for HF in multiple centers in Canada that age, systolic blood pressure, and blood urea nitrogen were important variables in predicting mortality at one year [13]. The present data is also consistent with prior findings documenting that the presence of both pulmonary hypertension and elevated left atrial pressure have negative long-term prognostic value in patients with acute heart failure [14,3]. Moreover, they extend the validity of the ADHERE CRFs to long term outcomes when combined with simple echocardiographic parameters.
In contrast to prior studies [3,6], the present results did not find a predictive benefit of other previously described echocardiographic variables such as left ventricular end diastolic and end systolic volume index, mitral deceleration time. The left atrial volume index and the tricuspid annular peak systolic excursion were not studied as in the Echo Heart Failure Score [3]. The goal of our study was to provide a simple combined clinical and echocardiographic score, rather than a pure clinical or a pure echocardiographic score, that can evaluate the long-term outcome of patients at 18 months. In this study, the mortality ranged from 6.3% to 75% according to the number of CRFs and echo parameters noted. We can only speculate whether our inclusion of both clinical and echocardiographic variables may have confounded or influenced the contribution of other echocardiographic variables noted in the Echo Heart Failure Score.
It is well established that renal dysfunction and hypotension are negative prognostic signs in patients with acute heart failure [15,2], undoubtedly related in part to the fact that hypotension and impaired kidney function on admission represent a group of patients with poor end organ perfusion and a far advanced stage of heart failure. The mechanisms and etiology of morbidity and mortality in patients with hypotension and renal dysfunction are multifactorial. A major mechanism is a cascade of neurohormonal activation, including activation of the sympathetic system, renin-angiotensin system, and increased release of vasopressin [16-20]. Activation of these factors leads to vasoconstriction to maintain perfusion of vital organs but consequently increases after load, which leads to increased myocardial wall tension and oxygen requirement [21]. Neurohormonal activation has been demonstrated to be associated with cardiovascular morbidity and mortality [16,21].
In addition, patients admitted with heart failure with elevated left sided filling pressures and pulmonary hypertension was more likely to have MACE at 18 months. This might be explained by the fact that pulmonary hypertension and elevated left atrial pressure are likely to represent patients with advanced diastolic and/or systolic dysfunction and thus reflects a higher incidence of morbidity and mortality [22].
Our study provides a simple combined clinical and echocardiographic risk score model that correlates with hard endpoints in patients with heart failure. The clinical utilization of this study may have implications for risk stratification of late MACE, prognosis, and being able to provide clinicians with tools that can guide therapy, monitoring, and follow-up. The role of these models in selecting patients that will benefit from specific therapies is yet to be determined.
Our study was a single-center retrospective analysis of relatively small number of patients; it is subject to inherent limitations of such studies. Out of 259 patients screened, 139 patients were excluded because of strict echocardiographic and Doppler criteria required for inclusion which may impact the applicability of the results to patients with significant valvular disease. Even the absolute number of MACE was low, the relative percent of patients suffering MACE was high (>25%). Moreover, there was no validation cohort studied, given the relatively small number of patients. However, our study appeared to be in congruence with previously published studies and provided a combined model and risk score to predict long term MACE in these patients. Further studies may be required to validate the results in a larger group of patients and in more extended time period for follow up.
We did not include rehospitalization as an end point in this study, a known important end point in CHF patients. Our goal was to determine hard endpoints as defined above in addition to ICD firing regardless of the analysis. However, we believe the simplicity of the score renders it a readily available tool for further study regarding rehospitalization as well as validating the study in a large population.
As with any Doppler technique, measurements are subject to measurement error related to the Doppler angle. However, care was taken to provide accurate measurements by following the ASE guidelines and two independent reviewers were used to minimize error.
Our study included patients with preserved and reduced ejection fraction, which likely represents two populations with acute heart failure. However, we elected to combine both groups in similar fashion as the ADHERE registry.
In conclusion, in this study, a simple and clinically risk score model was able to correlate with long term MACE in patients presenting with acute heart failure. In patients with acute HF, the strong prognostic value of the ADHERE registry CRFs of hypotension and renal dysfunction that predicted in-hospital mortality in the study, was not extended to MACE at 18 months. However, the addition of TRV and E/E’ together with age improved the late-outcome prognostic risk score model.
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014; 129: e28-e292.
- O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010; 55: 872-878.
- Carluccio E, Dini FL, Biagioli P, Lauciello R, Simioniuc A, Zuchi C, et al. The 'Echo Heart Failure Score': an echocardiographic risk prediction score of mortality in systolic heart failure. Eur J Heart Fail. 2013; 15: 868-876.
- Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am CollCardiol. 2012; 59: 673-680.
- Giannuzzi P, Temporelli PL, Bosimini E, Silva P, Imparato A, Corra U, et al. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am CollCardiol. 1996; 28: 383-390.
- Grayburn PA, Appleton CP, DeMaria AN, Greenberg B, Lowes B, Oh J, et al. Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: the Beta-blocker Evaluation of Survival Trial (BEST). J Am CollCardiol. 2005; 45: 1064-1071.
- Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, et al. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am CollCardiol. 2012; 60: 2074-2081.
- Olson J, Samad BA, Alam M. The prognostic significance of right ventricular tissue Doppler parameters in patients with left ventricular systolic heart failure: an observational cohort study. Heart. 2012; 98: 1142-1145.
- Olson JM, Samad BA, Alam M. Prognostic value of pulse-wave tissue Doppler parameters in patients with systolic heart failure. Am J Cardiol. 2008; 102: 722-725.
- Sallach JA, Tang WH, Borowski AG, Tong W, Porter T, Martin MG, et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009; 2: 527-534.
- Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. Jama. 2005; 293: 572-80.
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am SocEchocardiogr. 2010; 23: 685-713.
- Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. Jama. 2003; 290: 2581-2587.
- Ristow B, Ali S, Ren X, Whooley MA, Schiller NB. Elevated pulmonary artery pressure by Doppler echocardiography predicts hospitalization for heart failure and mortality in ambulatory stable coronary artery disease: the Heart and Soul Study. J Am CollCardiol. 2007; 49: 43-49.
- Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr., et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004; 10: 460-466.
- Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol. 2005; 95: 8b-13b.
- Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. J Am CollCardiol. 1993; 22: 72a-84a.
- Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am CollCardiol. 2009; 54: 375-385.
- Mark AL. Sympathetic dysregulation in heart failure: mechanisms and therapy. ClinCardiol. 1995; 18: 13-18.
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. 2009; 54: 1747-1762.
- Pool PE. Neurohormonal activation in the treatment of congestive heart failure: basis for new treatments? Cardiology. 1998; 90: 1-7.
- Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail. 2013; 1: 290-299.
Citation: Aggarwal A, Khoury Abdulla R, Mehta N, Goldstein J, Dixon SR, Berman A, et al. A Simple Combined Clinical and Echocardiographic Score Associated with Adverse Long Term Outcomes in Patients with Heart Failure: A Single Center Experience. Austin J Cardiovasc Dis Atherosclerosis. 2015; 2(2): 1016. ISSN: 2472-3568