
Research Article
Austin J Cardiovasc Dis Atherosclerosis. 2016;3(2): 1026.
Is Neutrophil-to-Lymphocyte Ratio useful in Predicting Drug-Eluting Stent Restenosis?
Sheikh AB¹*, Felzer JR², Daneshvar F³, Munir AB4, Bouchard M5 and Lafferty J3
¹Department of Cardiology, Ochsner Clinical Foundation / John Ochsner Heart and Vascular Institute, New Orleans, LA, USA
²Department of Medicine, Scripps Clinic/Green Hospital, La Jolla, CA, USA
³Department of Cardiology, Northwell Health / Staten Island University Hospital, Staten Island, NY, USA
4Department of Medicine, Northwell Health / Staten Island University Hospital, Staten Island, NY, USA
5Department of Pediatrics, Northwell Health / Staten Island University Hospital, Staten Island, NY, USA
*Corresponding author: Azfar B. Sheikh, M.D, Cardiology Fellow, John Ochsner Heart and Vascular Institute, New Orleans, LA 70130, USA
Received: June 13, 2016; Accepted: September 28, 2016; Published: September 30, 2016
Abstract
Objectives: This study aims to assess the usefulness of an elevated postprocedural Neutrophil-to-Lymphocyte Ratio (NLR > 4.5) as a prognostic tool in predicting in-stent restenosis (ISR) of coronary drug-eluting stents (DES). We will also assess if ISR can predict 10-year all-cause mortality.
Background: In patients undergoing percutaneous coronary intervention, ISR of DES is life threatening. NLR, a marker of subclinical inflammation, has been used to predict adverse outcomes in cardiovascular disease including ISR and mortality.
Methods: We conducted a case-control study on patients who underwent at least two cardiac catheterizations in Staten Island University Hospital between January 2004 and May 2015. We analyzed hematologic, angiographic and clinical data of 242 patients. A DES must have been implanted during initial cardiac catheterization and later followed by subsequent coronary angiogram which would reveal patency or ISR of DES. NLR was calculated from a blood sample taken within the first 24 hours after the initial procedure. Based on results, patients were divided into two groups, ISR group (≥50%) and non-ISR group (<50%). Propensity score matching (PSM) was used for direct comparison between the groups.
Results: NLR > 4.5 was not predictive of ISR [OR = 2.0, 95% CI (0.6, 6.6)]. Receiver operating curve analysis revealed a NLR value of 3.6 had 33% sensitivity and 76% specificity in predicting ISR. There was no difference 10- year all-cause mortality rate between the two groups. [OR = 1.3, 95% CI (0.5, 3.3)].
Conclusion: This is the first study that shows that an elevated postprocedural NLR has no significant value in predicting ISR of DES.
Keywords: Neutrophil lymphocyte ratio; Drug-eluting stents; In-stent restenosis; Mortality; Coronary artery disease
Abbreviations
ACS: Acute Coronary Syndrome; AUC: Area Under Curve; BMS: Bare Metal Stent; CAD: Coronary Artery Disease; CBC: Complete Blood Count; CRP: C-Reactive Protein; DES: Drug-Eluting Stent; DLC: Differential Leukocyte Count; ECP: Eosinophil Cationic Protein; EF: Ejection Fraction; EMR: Electronic Medical Records; GRACE: Global Registry of Acute Coronary Events; IL: Interleukin; IRB: Institutional Review Board; ISR: In-Stent Restenosis; IVUS: Intravascular Ultrasound; MCID: Minimal Clinically Important Difference; MMP: Matrix Metalloproteinase; NLR: Neutrophilto- Lymphocyte Ratio; NSTEMI: Non-ST Elevation Myocardial Infarction; OCT: Optical Coherence Tomography; PAI: Plasminogen Activator Inhibitor; PCI: Percutaneous Coronary Intervention; PSM: Propensity Score Matching; REDCap: Research Electronic Data Capture; ROC: Receiver Operating Characteristics; SD: Standard Deviation; SSDI: Social Security Death Index; ST: Stent Thrombosis; STEMI: ST Elevation Myocardial Infarction; TIMI: Thrombolysis In Myocardial Infarction; WBC: White Blood Cell
Introduction
With Coronary Artery Disease (CAD) as a leading cause of morbidity and mortality, there are numerous advances being made in the field of interventional cardiology to improve outcomes and minimize complications. Despite the widespread use of Drug- Eluting Stents (DES) during Percutaneous Coronary Interventions (PCI), patients have developed In-Stent Restenosis (ISR), a life threatening complication [1]. The primary mechanisms of ISR are acute inflammation causing neo-intimal proliferation, elastic recoil and negative arterial remodeling. Numerous inflammatory markers have been proposed to predict short and long-term cardiac mortality in patients with Acute Coronary Syndromes (ACS), Bare Metal Stent (BMS) ISR and Stent Thrombosis (ST). These include C-Reactive Protein (CRP), Eosinophil Cationic Protein (ECP), Matrix Metalloproteinase (MMP), and White Blood Cell (WBC) counts. Neutrophil-to-lymphocyte ratio (NLR), a marker of subclinical inflammation, has also been used to predict adverse outcomes in cardiovascular disease and cancer [2-10]. The objective of this study is to assess the usefulness of an elevated post-procedural NLR (> 4.5) as a prognostic tool in predicting DES ISR.
Methods
Study design
We conducted an observational case-control study on patients who underwent cardiac catheterizations at Staten Island University Hospital (Staten Island, New York) between January 2004 and May 2015. After the local Institutional Review Board (IRB) approved the study protocol, access was granted to the patient data using Electronic Medical Records (EMR).
The inclusion criteria included patients between the ages of 18 and 90, availability of a Complete Blood Count (CBC) drawn within the first 24 hours after the initial cardiac catheterization procedure in which a DES was implanted into native coronary vessels due to an abnormal stress test, stable angina refractory to medical management, or ACS. The procedure must have been considered a successful PCI, defined as luminal stenosis diameter < 20% with final Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow without any major complications [11]. This must be followed by a later coronary angiography allowing for assessment of previously stented vessels and determination of patency or ISR. After DES placement, the patient had to be compliant with their dual antiplatelet therapy for a minimum of 1 year, followed by one antiplatelet medication thereafter. Exclusion criteria included evidence of prior BMS placement in a native coronary vessel amenable for DES implantation, DES placement in graft vessel(s), End-Stage Renal Disease (ESRD) on dialysis, chronic inflammatory or autoimmune disease, active cancers, and conditions altering differential leukocyte counts such as HIV/AIDS, leukemia, lymphoma, chronic corticosteroid therapy (> 3 weeks), chemotherapy or other immunosuppressive medications. Patients that developed major or life-threatening bleeds in between the two cardiac catheterization procedures contraindicating the use of dual antiplatelet agents were also excluded.
Each patient had their demographic, hematologic and angiographic data collected and stored in Research Electronic Data Capture (REDCap) which included age, gender, medication use, history of hypertension, diabetes, hyperlipidemia, Chronic Kidney Disease (CKD), tobacco use, family history of CAD, left ventricular ejection fraction (EF), cell counts, post-procedural NLR, DES characteristics, time interval between cardiac catheterizations, and ISR percentage. The neutrophil, lymphocyte, monocyte, total WBC counts were collected from the first CBC obtained during the first 24 hours after the first cardiac catheterization procedure. Differential leukocyte counts (DLC) were obtained by the Coulter Counter apparatus (Coulter® Gen. S Hematology Analyzer, Beckman Coulter Corp., Hialeah, Florida). The NLR was calculated as the ratio of postprocedural neutrophil to lymphocyte counts from the same blood sample. Based on the collected data, patients were divided into two groups. Patients who developed significant ISR (≥50%) were labeled as part of the ISR group and patients who did not have significant ISR were labeled as part of the non-ISR group.
Assuming that the prevalence of an elevated pre-procedural NLR in patients with DES ISR was 19.5% compared to 6.6% in patients without ISR, as mentioned in a recent study [12], our sample size was estimated to detect a minimal clinically important difference (MCID) of 15% between the two groups. To detect this association with 5% type 1 error and 80% power required a minimum of 118 patients in each group.
The primary end points were prevalence of an elevated postprocedural NLR (> 4.5) in each group, and whether an elevated post-procedural NLR could predict DES ISR. Secondary endpoint included 10-year all-cause mortality assessed using EMR and/or Social Security Death Index (SSDI).
Statistical analysis
The Kolmogorov-Smirnov test was used to test the distribution pattern. Categorical variables were summarized as frequencies and percentages. They were compared using chi square or Fisher’s exact test depending on sample size. Continuous variables are summarized as mean ± standard deviation (SD) and compared using two-sample t-test (or Wilcoxon Rank Sum Test). Analysis of the primary endpoint was carried out initially using univariate logistic regression analysis. Variables for which the unadjusted p value was < 0.1 in univariate logistic regression analysis were included in propensity score matching (PSM) analysis to account for confounders. The odds ratio (OR) for primary and secondary endpoints was calculated along with the associated 95% confidence intervals (CI) using propensity score matched data. Additionally, we explored cut-points using receiver operating characteristics (ROC) curves to assess how well postprocedural NLR can predict ISR. (Figure 1). All statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, North Carolina). A p-value ≤ 0.05 was considered statistically significant.
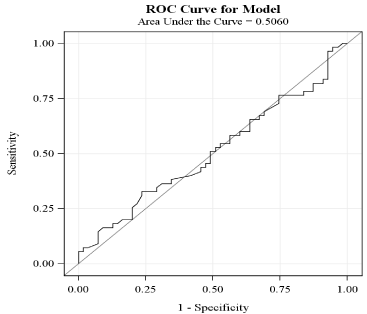
Figure 1: Receiver operator characteristics curve for NLR after propensity
score matching.
NLR: Neutrophil-to-lymphocyte ratio; ROC: Receiver operator characteristics.
Results
After screening through 2500 EMRs, the first 242 patients that met our criteria were included in the statistical analysis. Baseline demographic, hematologic and angiographic data collected from univariate logistic regression analysis is shown in Table 1. Due to large variation in baseline characteristics, PSM was utilized to adjust for confounding variables by grouping patients with similar baseline characteristics together, making our total sample size 110 with 55 patients in each group. Table 2 shows results after adjusting for propensity score in which covariates were found to be balanced between the two groups. Results of primary and secondary outcomes are based on this matched data. Figure 2 and Figure 3 depict percent distribution of indications for DES implantation and type of DES implanted, respectively.
Variable
No ISR (n=118)
ISR
(n=124)
P-value
Age
61±9.2*
58±10.4
0.64
Gender
Male
87 (73.7)**
74 (59.7)
0.021
Race
0.124
White
104 (88.1)
105 (84.7)
African American
4 (3.4)
5 (4.0)
Hispanic
4 (3.4)
0 (0.0)
Asian
3 (2.5)
6 (4.8)
Middle Eastern
3 (2.5)
8 (6.5)
Smokers
71 (60.2)
66 (53.2)
0.276
Family history of CAD
59 (50.0)
79 (63.7)
0.031
Diabetes
34 (28.8)
79 (63.7)
0.001
Hypertension
90 (76.3)
106 (85.5)
0.068
CKD
2 (1.7)
3 (2.4)
1.0***
Hypercholesterolemia
97 (82.2)
102 (82.3)
0.991
Obesity (BMI >30)
42 (35.6)
68 (54.8)
0.003
Medications
Aspirin
118 (100.0)
124 (100.0)
-
Clopidogrel
109 (92.4)
109 (87.9)
0.245
Ticagrelor
0 (0.0)
0 (0.0)
-
Prasugrel
11 (9.3)
11 (8.9)
0.903
Ticlodipine
0 (0.0)
4 (3.2)
0.122***
Heparin
22 (18.6)
2 (1.6)
< 0.0001
Warfarin
3 (2.5)
2 (1.6)
0.677***
ACEI or ARB
67 (56.8)
85 (68.6)
0.058
Beta blocker
84 (71.2)
98 (79.0)
0.158
CCB
25 (21.2)
32 (25.8)
0.397
Statin
111 (94.1)
106 (85.5)
0.028
Insulin
5 (4.2)
20 (16.1)
0.002
Oral anti-diabetics
22 (18.6)
63 (50.8)
< 0.001
Other subcutaneous anti-diabetics
0 (0.0)
5 (4.0)
0.060***
Diuretics
24 (20.3)
26 (21.0)
0.904
WBC Count (×10 9/L)
7.5±2.3
7.5±2.2
0.775
Neutrophil Count (×10 9/L)
5.0±1.9
4.9±1.9
0.531
Lymphocyte Count (×10 9/L)
1.8±0.7
1.9±0.7
0.669
Monocyte Count (×10 9/L)
0.6±0.2
0.5±0.2
0.383
Platelet Count (×10 9/L)
216.9±61.3
229.5±52.1
0.086
Neutrophil (%)
65.8±9.1
64.2±9.3
0.186
Lymphocyte (%)
24.4±7.7
25.6±8.1
0.271
Monocyte (%)
7.3±2.4
7.4±2.2
0.603
Interval Between Cardiac Catheterizations (months)
27.4±26.5
24.1±24.3
0.311
Type of DES Implanted
0.017
Zotarolimus (Endeavor, Resolute)
32 (27.1)
20 (16.1)
Sirolimus (Cypher)
12 (10.2)
29 (23.4)
Paclitaxel (Taxus)
48 (40.7)
53 (42.7)
Everolimus (Xience, Promus)
26 (22.0)
22 (17.7)
Indication for DES Implantation
0.046
Abnormal stress test
37 (31.4)
27 (21.8)
Stable angina
17 (14.4)
15 (12.1)
Unstable angina
36 (30.5)
57 (46.0)
NSTEMI
11 (9.3)
16 (12.9)
STEMI
17 (14.4)
9 (7.3)
DES Diameter (mm)
3.0±0.4
3.0±0.4
0.368
DES Length (mm)
17.5±5.4
19.0±6.6
0.046
LVEF (%)
54±9
53±10
0.455
*Results for continuous variables are expressed as: Mean ± SD.
**Results for categorical variables are expressed as: Number of patients (% of patients).
***p value obtained from Fisher’s Exact Test.
ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker; BMI: Body mass index; CAD: Coronary artery disease; CCB: Calcium channel blocker; CKD: Chronic Kidney Disease; DES: Drug-eluting stent; DM: Diabetes mellitus; ISR: In-stent restenosis; LVEF: Left ventricular ejection fraction; NLR: Neutrophil-to-Lymphocyte ratio; NSTEMI: Non-ST elevation myocardial infarction; SD: Standard deviation; STEMI: ST elevation myocardial infarction; WBC: White blood cell.
Table 1: Baseline demographic, clinical, hematologic, and procedural characteristics with respect to presence of in-stent restenosis.
Age
60±8.6*
61±10.5
0.71
Gender
Male
36 (65.5)**
37 (67.3)
0.842
Race
0.947
White
46 (83.6)
46 (83.6)
African American
3 (5.5)
2 (3.6)
Hispanic
2 (3.6)
0 (0.0)
Asian
1 (1.8)
2 (3.6)
Middle Eastern
3 (5.5)
5 (9.1)
Smokers
31 (56.4)
31 (56.4)
1.0
Family history of CAD
32 (58.2)
26 (47.3)
0.257
Diabetes
17 (30.9)
16 (29.1)
0.796
Hypertension
40 (72.7)
44 (80.0)
0.346
CKD
1 (1.8)
0 (0.0)
0.317
Hypercholesterolemia
44 (80.0)
41 (74.6)
0.467
Obesity (BMI >30)
21 (38.2)
20 (36.4)
0.847
Medications
Aspirin
55 (100.0)
55 (100.0)
-
Clopidogrel
49 (89.1)
50 (90.9)
0.706
Ticagrelor
0 (0.0)
0 (0.0)
-
Prasugrel
7 (12.7)
4 (7.3)
0.317
Ticlodipine
0 (0.0)
1 (1.8)
0.317
Heparin
3 (5.5)
2 (3.6)
0.564
Warfarin
3 (5.5)
1 (1.8)
0.317
ACEI or ARB
31 (56.4)
34 (61.8)
0.513
Beta blocker
37 (67.3)
40 (72.7)
0.532
CCB
13 (23.6)
17 (30.9)
0.346
Statin
51 (92.7)
52 (94.6)
0.706
Insulin
4 (7.3)
2 (3.6)
0.414
Oral anti-diabetics
10 (18.2)
14 (25.5)
0.285
Other subcutaneous anti-diabetics
0 (0.0)
0 (0.0)
-
Diuretics
10 (18.2)
12 (21.8)
0.617
WBC Count (×10 9/L)
7.4±2.0
7.7±1.6
0.414
Neutrophil Count (×10 9/L)
4.8±1.6
5.0±1.5
0.432
Lymphocyte Count (×10 9/L)
1.8±0.7
1.9±0.7
0.877
Monocyte Count (×10 9/L)
0.6±0.2
0.6±0.2
0.962
Platelet Count (×10 9/L)
220.8±65.9
219.8±45.8
0.918
Neutrophil (%)
64.9±8.4
65.1±9.5
0.924
Lymphocyte (%)
25.0±7.2
24.8±8.2
0.868
Monocyte (%)
7.5±2.4
7.5±2.3
0.827
Interval Between Cardiac Catheterizations (months)
28.5±25.9
25.5±27.1
0.526
Type of DES Implanted
0.873
Zotarolimus (Endeavor, Resolute)
14 (25.5)
12 (21.8)
Sirolimus (Cypher)
7 (12.7)
7 (12.7)
Paclitaxel (Taxus)
23 (41.8)
24 (43.6)
Everolimus (Xience, Promus)
11 (20.0)
12 (21.8)
Indication for DES Implantation
0.323
Abnormal stress test
18 (32.7)
15 (27.3)
Stable angina
11 (20.0)
7 (12.7)
Unstable angina
17 (30.9)
21 (38.2)
NSTEMI
4 (7.3)
7 (12.7)
STEMI
5 (9.1)
5 (9.1)
DES Diameter (mm)
3.0±0.4
3.0±0.4
0.738
DES Length (mm)
19.0±6.0
18.1±6.2
0.456
LVEF (%)
56±8
56±8
0.771
*Results for continuous variables are expressed as: Mean ± SD.
**Results for categorical variables are expressed as: Number of patients (% of patients).
ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker; BMI: Body mass index; CAD: Coronary artery disease; CCB: Calcium channel blocker; CKD: Chronic Kidney Disease; DES: Drug-eluting stent; DM: Diabetes mellitus; ISR: In-stent restenosis; LVEF: Left ventricular ejection fraction; NLR: Neutrophil-to-Lymphocyte ratio; NSTEMI: Non-ST elevation myocardial infarction; SD: Standard deviation; STEMI: ST elevation myocardial infarction; WBC: White blood cell.
Table 2: Baseline demographic, clinical, hematologic, and procedural characteristics after propensity score matching.
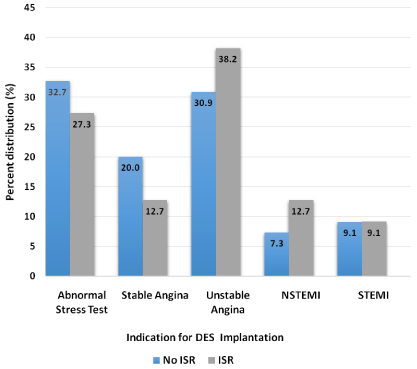
Figure 2: Percent distribution of indications for DES implantation in ISR and
non-ISR groups after propensity score matching (p = 0.323).
DES: Drug-eluting stent; ISR: In-stent restenosis; NSTEMI: Non-ST elevation
myocardial infarction; STEMI: ST elevation myocardial infarction.
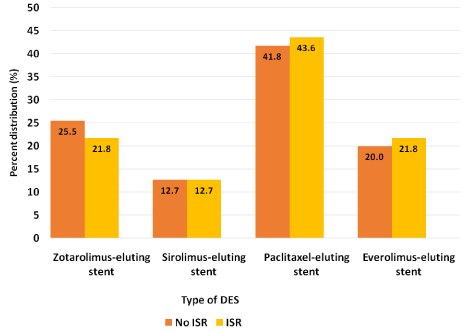
Figure 3: Percent distribution of DES implanted in ISR and non-ISR groups
after propensity score matching (p = 0.873).
DES: Drug-eluting stent; ISR: In-stent restenosis.
The results of primary and secondary outcomes are shown in Table 3. The prevalence of an elevated post-procedural NLR (> 4.5) was 16.4% in the ISR group compared to 9.1% in the non-ISR group. This difference was not statistically significant indicating that an elevated post-procedural NLR is not a significant predictor of ISR [OR = 2.0, 95% CI (0.6, 6.6)]. ROC curve analysis (Figure 1) was used to explore the association between post-procedural NLR and ISR. Area under curve (AUC) was 0.506. ROC analysis demonstrated an optimum cut-off value of 3.6, with a sensitivity of 33% and specificity of 76% for predicting ISR. The 10-year all-cause mortality rate was 7.3% in ISR group compared to 9.1% in non-ISR group [OR = 1.3, 95% CI (0.5, 3.3)].
Variable
No ISR
(n=55)
ISR
(n=55)
Difference
OR (95%CI)
P-value
NLR*
High (> 4.5)
5 (9.1)
9 (16.4)
7.3***
2.0 (0.6 , 6.6)
0.248
NLR**
2.9±1.2
3.3±2.8
0.4
-
0.3
10-year all-cause mortality
5 (9.1)
4 (7.3)
1.8***
1.3 (0.5, 3.3)
0.655
* NLR as a categorical variable, expressed as: Number of patients (% of patients).
**NLR as a continuous variable, expressed as: Mean ± SD.
***Differences are expressed as percentages.
CI: Confidence interval; ISR: In-stent restenosis; NLR: Neutrophil-to-Lymphocyte ratio; OR: Odds ratio.
Table 3: Primary and secondary outcomes after propensity score matching.
Discussion
ISR is a major complication amongst patients who undergo PCI. The use of post-procedural NLR in our study is based on pathogenesis of DES ISR. The pathogenesis involves the metal stent strut, an essential part of all coronary stents, which promotes a “foreign body” reaction leading to migration of inflammatory cells into the intima followed by vascular smooth muscle proliferation, a process known as neointimal proliferation [13,14]. In addition, allergymediated inflammatory reaction to the polymer employed in DES, via eosinophils, is also implicated [15]. Several markers of inflammation have been proposed to predict ISR of DES including NLR [12], CRP, ECP, IL-6, PAI-1, MMP and complement components C3a and C5a [5]. The goal of our study was to assess the usefulness of an elevated post-procedural NLR (> 4.5) as a prognostic tool in predicting ISR of DES.
Multiple studies have previously shown the benefit of NLR in its ability to predict adverse outcomes in CAD [3,6,7,16]. However, this is the first study to explore the predictive value of post-procedural NLR for ISR. Moreover, a set value of NLR has not been included in any risk scoring system (e.g. GRACE). Azab et al. [7] explained that there are two obstacles hindering the use of a set NLR value as part of a risk scoring system. Firstly, the brief steady kinetic state and short life of neutrophils (7 hours) make it difficult to pick which neutrophil count to use to calculate NLR. Secondly different NLR cut-off values used throughout the literature make it difficult to conduct metaanalyses. In our study the normal value of NLR (0.5 – 4.5) was derived from dividing the highest normal neutrophil count (6.8 ×10 9/L) and lowest normal lymphocyte count (1.5 ×109/L) to get the upper limit of NLR (4.5) and dividing the lowest normal neutrophil count (2.05 ×109/L) and highest normal lymphocyte count (4.0 ×109/L) to the lower limit of NLR (0.5). Since a majority of the patients included in this study were American Caucasian adults (86.3%), the neutrophil and lymphocyte values were based on a normal range seen in this population [17].
In our study, there was no significant difference in prevalence of an elevated post-procedural NLR in the ISR vs. non-ISR groups. NLR was not found to be a predictor of ISR in patients who underwent successful DES implantation. This finding is contrary to a similar case control study conducted by Chavarria [12], who also used NLR > 4.5 as a cut-off value to predict ISR. His study revealed that in patients who developed ISR, 19.5% had an elevated NLR compared to 6.6% in patients who did not develop ISR (p = 0.041). However, there were important differences in our studies. Our NLR values were calculated post-procedurally as opposed to pre-procedurally. We included patients who underwent cardiac catheterizations for numerous indications in addition to the previously studied stable and unstable angina, such as an abnormal stress test, NSTEMI, and STEMI. Our results were based on matched groups after propensity score matching. Even testing multiple cut-points, our ROC curve analysis revealed that the optimum NLR cut-off value of 3.6 had poor sensitivity and specificity in predicting ISR.
Routine cardiac catheterization with coronary angiography after 6 months of ISR remains controversial. According to the 2012 appropriate use criteria for diagnostic coronary angiography, unless there were signs or symptoms of ischemia, routine control angiography after stent implantation was unnecessary [18]. However, in a recent study conducted by Cassese et al. following a large cohort of approximately 10,000 patients [19], it was shown that presence of restenosis on follow-up angiography predicted 4-year all-cause mortality, and its prognostic value remained the same regardless whether patients were symptomatic or asymptomatic. In our study, all patients underwent a follow-up coronary angiography if they had signs or symptoms of ischemia, however there were no differences seen in 10-year all-cause mortality between the ISR and non-ISR groups. These discrepancies have made it difficult to conclude that presence of ISR has prognostic value without additional studies to conduct meta-analyses. Pre-procedural NLR, on the other hand, has been shown in studies to predict mortality in patients undergoing PCI for NSTEMI and STEMI [3,6,7,16]. In our study, there was no difference in 10-year all-cause mortality.
The limitations of this study are (a) the possibility of selection bias, (b) its retrospective design preventing us from inferring causality, (c) being conducted in a single center affecting study generalizability, and (d) diagnosis of ISR being based on visual inspection under fluoroscopy without use of intravascular ultrasound (IVUS) or optical coherence tomography (OCT) to illustrate morphology of stented segment. Despite the above mentioned limitations, this is the first study to demonstrate limitations of post-procedural NLR in predicting DES ISR in patients undergoing cardiac catheterization for all indications.
Conclusion
An elevated post-procedural NLR (> 4.5) has no significant ability to predict DES ISR in patients who underwent cardiac catheterization for an abnormal stress test, stable angina refractory to medical management, or acute coronary syndromes.
References
- Dangas GD, Claessen BE, Caixeta A, et al. In-stent restenosis in the drugeluting stent era. J Am Coll Cardiol. 2010; 56: 1897-1907.
- Papa A, Emdin M, Passino C, et al. Predictive value of elevated neutrophillymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008; 395: 27-31.
- Duffy BK, Gurm HS, Rajagopal V, et al. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006; 97: 993-996.
- Turak O, Ozcan F, Isleyen A, et al. Usefulness of the neutrophil-to-lymphocyte ratio to predict bare-metal stent restenosis. Am J Cardiol. 2012; 110: 1405- 1410.
- Niccoli G, Montone RA, Ferrante G, et al. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J Am Coll Cardiol. 2010; 56: 1783-1793.
- Nunez J, Nunez E, Bodi V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008; 101: 747-752.
- Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010; 106: 470-476.
- Kibos A, Campeanu A, Tintoiu I. Pathophysiology of coronary artery in-stent restenosis. Acute Card Care. 2007; 9: 111-119.
- Virmani R, Farb A. Pathology of in-stent restenosis. Curr Opin Lipidol. 1999; 10: 499-506.
- Bennett MR. In-stent stenosis: Pathology and implications for the development of drug eluting stents. Heart. 2003: 218-224.
- Smith SC Jr, Dove JT, Jacobs AK, et al. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)-executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation. 2001; 103: 3019-3041.
- Chavarria N. Elevated neutrophil-to-lymphocyte ratio is associated with drug eluting stent restenosis. J Am Coll Cardiol. 2013; 61: 182-185.
- Kornowski R, Hong MK, Tio FO, et al. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998; 31: 224-230.
- Chung IM, Gold HK, Schwartz SM, et al. Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. J Am Coll Cardiol. 2002; 40: 2072-2081.
- Niccoli G, Schiavino D, Belloni F, et al. Pre-intervention eosinophil cationic protein serum levels predict clinical outcomes following implantation of drugeluting stents. Eur Heart J. 2009; 30: 1340-1347.
- Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008; 102: 653-657.
- Skubitz KM. Neutrophilic leukocytes. In: Greer JP, Foerester J, Rodgers GM. Wintrobe’s Clinical Hematology. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health. 2014; 125-158.
- Patel MR, Bailey SR, Bonow RO, et al. CCF/SCAI/AATS/AHA/ASE/ASNC/ HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2012; 144: 39-71.
- Cassese S, Byrne RA, Schulz S, et al. Prognostic role of restenosis in 10,004 patients undergoing routine control angiography after coronary stenting. Eur Heart J. 2015; 36: 94-99.