
Case Report
Austin J Cardiovasc Dis Atherosclerosis. 2016; 3(3): 1029.
Diagnosis and Management of Acute Left Main Occlusion during Transcatheter Aortic Valve Replacement
Hafiz M A and Kakouros N*
Division of Cardiology, Department of Medicine, University of Massachusetts Medical School, Massachusetts
*Corresponding author: Nikolaos Kakouros, Division of Cardiology, Department of Medicine, University of Massachusetts Medical School, Massachusetts
Received: November 23, 2016; Accepted: December 22, 2016; Published: December 26, 2016
Case Presentation
History
Mr. C is a 55-year-old man with past medical history of Laennec’s cirrhosis complicated by coagulopathy, pulmonary effusions and ascites for which he underwent a trans-jugular intrahepatic portosystemic shunt procedure. He was subsequently found to have severe aortic stenosis during evaluation for liver transplant. On direct questioning, he reported being unable to walk even 100 yards due to dyspnea and had required multiple hospital admissions for heart failure. Following this he was referred for Transcatheter Aortic Valve Replacement (TAVR) to our quaternary care medical center. Pre-TAVR assessment revealed preserved left ventricular ejection fraction, absence of obstructive coronary artery disease, a bicuspid aortic valve with severe aortic stenosis (mean gradient 58mmHg) with heavily calcified leaflets and moderate aortic regurgitation.
Procedure
A TAVR-protocol cardiac CT was obtained and aortic annulus was found to be suitable for a SAPIEN 3 #29mm transcatheter heart valve (THV, Edwards Life sciences, Irvine, California) via transfemoral access. Morph metric analysis of the volumetric CT data also revealed bulky and heavily calcified native aortic leaflets with a low origin of the left main (Figure 1, Panel a). For further evaluation, pigtail catheters were placed in the non-coronary cusp and left coronary cusp and dual-injection aortography performed for proper delineation of the aortic annulus in relation to the left main (Figure 1, Panel b). This confirmed low origin of the LMS.
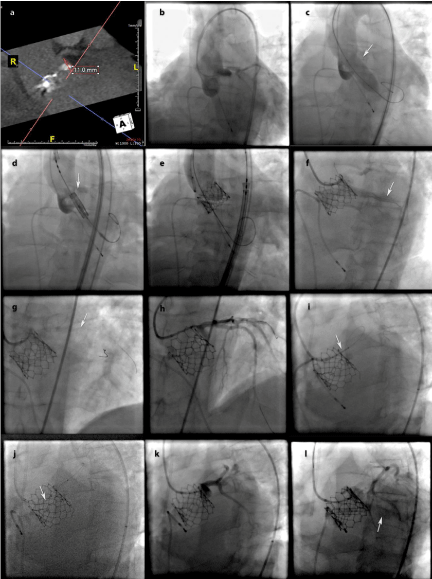
Figure 1: Computed tomography (CT) and Fluoroscopy images: a) CT depicting low left main height relative to the aortic annulus, b) dual pigtail catheter injection
to define the left main height relative to the aortic annulus during the transcatheter aortic valve replacement procedure, c) balloon aortic valvuloplasty, white arrow
points to the bulky leaflet in the left coronary cusp, d) positioning the valve prosthesis in the aortic annulus plane, white arrow points to bulky calcified leaflet in the
left coronary cusp, e) bioprosthetic valve deployed and aortic angiogram depicting no significant flow into the left main artery but moderate to severe paravalvular
aortic regurgitation, f) engaging the left main artery with a guide, white arrow points to the coronary guidewire in the left ventricle, an angiogram reveals minimal
flow into the left main artery and paravalvular leak, g) successful passage of a coronary guide wire into the left anterior descending artery (white arrow), h) coronary
angiography of the left coronary system confirms proper engagement with the guiding catheter, i) positioning the bare metal stent in the left main artery (white
arrow), j) maneuvering the stent in place, white arrow points to the Guideliner, k) angiogram post stent deployment reveals restoration of flow into the left main
artery, and l) contrast injection into the paravalvular gutter reveals reduction in the paravalvular regurgitation.
Transcutaneous access was obtained in the right femoral artery and an 18Fr sheath placed. We then proceeded with Balloon Aortic Valvuloplasty (BAV), performed during rapid ventricular pacingusing a 23mm balloon. During the BAV, the heavily calcified aortic leaflet abutting the left main was noted to move very close to the left main ostium (Figure 1, Panel c, white arrow). Despite the concerning leaflet displacement, there was reassuringly good flow of contrast into the left main during the BAV, so we decided to proceed directly to valve deployment (Figure 1, Panel d, white arrow depicts the heavily calcified leaflet in left coronary cusp).
Immediately after the transcatheter heart valve was fully deployed, monitoring EKG leads showed ST segment elevation and TEE revealed reduced LVEF with anterior wall motion abnormalities, along with concurrent low pulse pressure and hypotension (Figure 2, Panel a depicts pre TAVR central aortic pressure and Panel b black curve depicts post prosthesis deployment tachycardia and low pulse pressure). The THV delivery catheter “nose cone” was withdrawn from the left ventricle and an aortic root angiogram was obtained revealing no flow into the left main artery and significant paravalvular leak between the left valve leaflet and the prosthesis valve frame (Figure 1, Panel e).
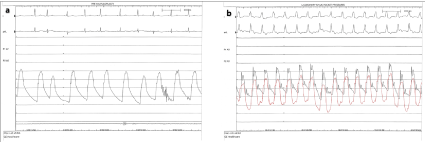
Figure 2: Hemodynamic tracings: a) central aortic pressure before and b) after valve deployment. The red tracing in (b) shows dampened pressure in the left
coronary cusp when trying to engage the left main artery. Electrocardiographic tracings in figure (b) reveal tachycardia and widening of the QRS complexes with
ST segment deviation and T wave inversions consistent with ischemia.
The Edwards delivery sheath was immediately removed and replaced with a JL-5.0 Launcher 6 French guiding catheter (Medtronic, Minneapolis, Minnesota) (Figure 1, Panel f) but the left main artery could not be directly engaged due to interference by the THV metal frame. Delivery of a short ASAHI PROWATER coronary guide wire (Abbott Laboratories. Abbott Park, Illinois, U.S.A) into the left main was also unsuccessful. The wire was instead advanced behind the prosthetic valve frame into the paravalvular gutter and then into the left ventricle (Figure 1, Panel f, white arrow). Using this wire as a “rail”, the guiding catheter was stabilized and brought closer to the sino-tubular junction. With this “stabilized” rail-guiding catheter arrangement, a WHISPER J 190cm coronary guide wire (Abbott Laboratories. Abbott Park, Illinois) was advanced (Figure 1, Panel f, white arrow depicts the coronary guide wire) through the paravalvular space above the valve frame to the distal left anterior descending artery. A Guideliner catheter (Vascular Solutions, Minneapolis, Minnesota) was used to extend the guidecatheter tip into the sinus space for further support (Figure 1, Panel h). A ventricular zed hemodynamic tracing was observed in the left coronary cusp space (between the left main artery and the prosthesis outer frame) confirming severe reduction of blood flow to the left main artery (Figure 2, Panel b, red hemodynamic tracing). Following this, the first guide wire was removed from the left ventricle and redirected into the distalramus intermediusartery (Figure 1, Panel i). A4.0x18mm bare metal stent was positioned and deployed at the ostial left main artery (Figure 1, Panel i-j, white arrow depicts Guideliner). The proximal part of the stent extended to the aorta, above the sinotubularjunction, in a “snorkel” arrangement, restoring flow to the left coronary system (Figure 1, Panel k-l). This stent was post-dilated using a 4.5mm non-compliant balloon.
Final angiography revealed good blood flow into the left main and improved, mild-moderate, paravalvular aortic regurgitation (Figure 1, Panel l). The EKG changes resolved and TEE showed recovery of left ventricular function with reversal of wall motion abnormalities and left ventricular ejection fraction of 55%. The patient was transferred to acute care post procedure and was discharged from the hospital with good recovery. Two months later, the patient received an orthotropic liver transplant. A year after the procedure, he has moderate paravalvular aortic regurgitation and is doing well.
Conclusion
Coronary occlusion is a serious complication of TAVR, which, in case of left main artery occlusion, can prove rapidly fatal if uncorrected [1]. Mechanisms leading to coronary ostial occlusion include displacement of a native aortic valve leaflet, or a stentless surgical bioprosthesis, narrow sinus of valsalva, deployment of an over-sized valve prosthesis or even deployment of a paravalvular leak closure device [2,3]. The common risk factor in most cases, however, is a low coronary origin (typically defined as less than 10 mm from the aortic annulus plane). Anticipation and prevention of this potentially fatal complication requires careful planning via volumetric pre-procedural imaging using cardiac CT or 3D TEE, intra-procedural surveillance of high-risk cases and immediate recognition of compromised coronary circulation using all ancillary data like hemodynamic, electrocardiography and echocardiography. Cases identified prior to the procedure as being at increased risk of potential coronary ostial occlusion should have an appropriate plan with debriefing of the multi-disciplinary staff (invasive imaging, anesthesia, scrub technicians and nurses) in addition to ensuring the immediate availability of appropriate equipment in procedure area to facilitate the rapid execution of the “rescue” plan. This plan can include, depending on the degree of perceived risk: a) precautionary engagement of the coronary artery with guiding catheter, b) placement of guide wires, balloons or stents [4] in the coronary at risk prior to THV deployment, c) wiring the vessel once vascular compromise has occurred with or without mechanical circulatory support or even d) resort to conversion to open heart surgery with coronary artery bypass grafting [5]. Once recognized, hemodynamic deterioration can occur rapidly but with above steps an adverse outcome can potentially be averted as demonstrated by in our patient’s case. Of additional instructional note, as demonstrated in this case, evidence of flow in the coronary vessel at risk during BAV may provide false reassurance that the vessel will not be compromised after THV deployment. In high-risk cases, a more precautionary approach is often warranted.
References
- Tuzcu EM, Kapadia SR. Left main occlusion with transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2011; 78: 660-661.
- Okuyama K, Jilaihawi H, Makkar RR. Leaflet length and left main coronary artery occlusion following transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2013; 82: 754-759.
- Price MJ, Shah MG, Burke RF, Riley RD, Rizik DG. Impingement of left main ostium after device occlusion of paravalvular leak post-transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015; 8: 1-2.
- Winther S, Christiansen EH, Thuesen L. Stenting of acute left main coronary artery occlusion using balloon anchoring technique after transcatheter aortic valve implantation. J Interv Cardiol. 2011; 24: 470-473.
- Watson D, Yakubov S. 159-IREPAIR OF LEFT MAIN CORONARY OCCLUSION AND ANNULAR DISRUPTION FOLLOWING TRANSCATHETER AORTIC VALVE REPLACEMENT. Interactive CardioVascular and Thoracic Surgery. 2013; 17: 108.