
Review Article
Austin J Cardiovasc Dis Atherosclerosis. 2021; 8(1): 1042.
Cardiovascular Disease Prevention in the COVID-19 Era
Yokoyama M1, Kamide N1, Tojo T2 and Yamaoka-Tojo M1*
¹Kitasato University School of Allied Health Sciences, Sagamihara, Japan
²Sagamihara Kyodo Hospital, Sagamihara, Japan
*Corresponding author: Minako Yamaoka-Tojo, 1-15-1 Kitasato, Minami-ku, Sagamihara 252-0373, Japan
Received: March 01, 2021; Accepted: March 25, 2021; Published: April 01, 2021
Abstract
Although the coronavirus disease 2019 (COVID-19) pandemic is still ongoing, the path towards a better future is finally becoming clear as a result of the initiation of COVID-19 vaccination. While pneumonia was initially emphasized as the only complication of COVID-19, it has become clear that fatal complications, such as a thromboembolism, are also likely to occur. In the era of recurring coronavirus infections, it is important to identify the causes and risk factors regarding exacerbations in patients who may develop severe COVID-19. This review describes how to prevent COVID-19 exacerbations in the context of cardiovascular disease, especially exacerbations related to the vascular endothelium.
Keywords: Vascular endothelium; COVID-19; Systemic Inflammatory- Reactive Microvascular Endotheliopathy (SIRME); Vascular endothelial glycocalyx
The COVID-19 Pandemic
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARSCoV- 2), which causes coronavirus disease 2019 (COVID-19), has led to the emergence of a global pandemic. Three waves of the COVID-19 epidemic have already been observed in Japan (Figure 1) as well as in many other countries where the disease has killed many elderly people and patients with underlying diseases. Although the pandemic has not come to an end, progress is being made with COVID-19 vaccination.
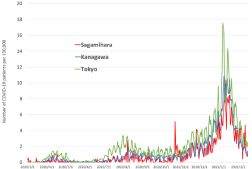
Figure 1: Changes in the number of COVID-19 patients in Tokyo and Kanagawa Prefecture. The changes in the number of COVID-19 patients per 100,000 in
Tokyo and neighboring Kanagawa Prefecture from March 1, 2020 to February 16, 2021 is shown. In Sagamihara City, a government-designated city located in
the northwestern part of Kanagawa Prefecture, the number of patients remained almost the same. On October 23, 2021, the number of COVID-19 patients in
Sagamihara temporarily increased due to the occurrence of clusters in hospitals and related facilities.
While pneumonia was initially emphasized as the only complication of COVID-19, serious cardiovascular complications resulting from COVID-19, such as systemic thromboembolism, acute myocardial infarction, myocarditis, severe arrhythmias, and long-term dysfunction of the heart, have also been reported [1- 3]. Moreover, studies have suggested that severe complications of COVID-19 are strongly related to a damaged vascular endothelium, the induced formation of thrombi, the release of inflammatory cytokines, and the production of excess Reactive Oxygen Species (ROS) [4,5]. Moreover, the vascular endothelium has been shown to be an important target of SARS-CoV-2 [6]. Here, we propose a new concept which we have termed the systemic inflammatory-reactive microvascular endotheliopathy (SIRME) [7] (Figure 2).
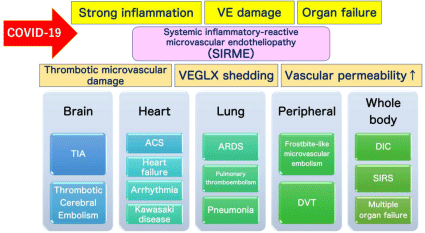
Figure 2: Definition of the Systemic Inflammatory-Reactive Microvascular Endotheliopathy (SIRME). SIRME results from damage to the Vascular Endothelial
Glycocalyx (VEGLX), which is impaired in an inflammatory response. SIRME is defined as: 1) The presence of a strong causative inflammation; 2) Vascular
endothelial damage with strong thrombogenic tendency and increased vascular permeability; 3) Organ failure. SIRME is presumed to be one of the major
mechanisms causing diverse COVID-19 complications. VE: Vascular Endothelial; VEGLX: Vascular Endothelial Glycocalyx; TIA: Transient Ischemic Attack; ACS:
Acute Coronary Syndrome; ARDS: Acute Respiratory Distress Syndrome; DVT: Deep Vein Thrombosis; DIC: Disseminated Intravascular Coagulation; SIRS:
Systemic Inflammatory Response Syndrome.
SARS-CoV-2 and Angiotensin-Converting Enzyme 2
Coronaviruses have a spike receptor-binding superfamily domain, which binds to the Angiotensin-Converting Enzyme 2 (ACE2) [8]. As more progress related to COVID-19 is being made and various findings become published, the approximate identity of COVID-19 and its resulting severe complications are becoming more apparent [9,10]. Based on the genomic sequence of SARSCoV- 2, researchers have found that the virus has a very high affinity for cells that strongly express ACE2, causing severe damage to such cells [11,12]. In addition, studies have shown that patients with cardiovascular disease, diabetes, and hypertension are more likely to experience severe COVID-19 and show a higher mortality rate than other groups of patients [4,6]. Moreover, obese people, smokers, and patients with chronic kidney disease are also more likely to become severely ill. In fact, scientists have long known that the ACE2 is expressed in vascular endothelial cells in such patients [13]. Based on these findings, researchers have concluded that SARS-CoV-2 uses ACE2 to invade vascular endothelial cells [14].
Vascular Endothelial Function
Arteriosclerosis progression, plaque rupture [15], and microvascular dysfunction in chronic heart failure are closely related to vascular endothelial dysfunction. Therefore, the evaluation of vascular endothelial function can be used as a screening method for high-risk patients with cardiovascular diseases to stratify the risk of developing cardiovascular diseases in the future. In Japan, evaluation of vascular endothelial function is covered by health insurance. For this evaluation, two measurement methods may be used: Flow- Mediated Dilation (FMD), which is performed by an ultrasonic device that evaluates blood flow-dependent vasodilatory reactions, and Reactive Hyperemia Peripheral Arterial Tonometry (RH-PAT), measured using an Endo-PAT™, which is a device that uses fingertip pulse waves. Vascular endothelial function measurements are useful as an index for primary and secondary prevention of cardiovascular diseases. Decreased vascular endothelial function is also related to heart failure, which is divided into cardiac systolic dysfunction (Heart Failure with Reduced Ejection Fraction, HFrEF) and diastolic dysfunction (Heart Failure with Preserved Ejection Fraction, HFpEF). Of those, HFpEF is known to be more common in elderly patients. Furthermore, HFpEF is more likely to occur when cardiac microvascular endothelial function declines.
Vascular Endothelial Glycocalyx Damage
As vascular endothelial cells hold various functions that help maintain homeostasis in the body, the state of intravascular dysfunction can be seen as a state in which the balance of biological functions is disturbed. Lifestyle-related diseases induce vascular endothelial dysfunction and damage the Vascular Endothelial Glycocalyx (VEGLX), which covers vascular endothelial cells. In acute inflammatory diseases, the VEGLX is peeled off and floats in large quantities in the blood [16]. The VEGLX is also impaired in patients with hypertension [17] and diabetes [18-21] as well as in patients with chronic diseases, such as bronchial asthma and chronic obstructive pulmonary disease, heart failure [22,23], ischemic heart disease [24], microvascular angina [25], kidney disease [26], atherosclerosis [27-29], hyperuricacidemia [30], or obesity, and elderly people [21] and smokers. Large amounts of VEGLX fragments have also been detected in the blood of a variety of acute and serious diseases, such as severe infections [31,32], sepsis [33-37], trauma [38,39], acute coronary syndrome [40,41], stroke [42], and multiple organ failure. Although the VEGLX is known to cover the inside of blood vessels and functions as a barrier for vascular endothelial cells, studies have recently shown that it also controls intracellular signals [43]. When the VEGLX becomes damaged, the function of the vascular endothelial cells deteriorates, resulting in many inflammatory substances as well as substances that promote blood clots being released into the circulating blood from the vascular wall.
Severe Complications of COVID-19
Many studies have reported that COVID-19 patients may experience severe disease as a result of decreased vitamin D concentration in the blood [44,45]. The relationship between vitamin K deficiency and severe COVID-19 has also been reported [46,47]. As insufficient vitamin K levels will result in an impaired vascular endothelial function, the virus is more likely to invade vascular endothelial cells with overexpressed ACE2 and impaired VEGLX.
Studies have highlighted that olfactory dysfunction, excessive hair loss, and prolonged general fatigue induced by COVID-19 are related to zinc deficiency [48-50]. Furthermore, in serious diseases, such as severe COVID-19, various minerals and vitamins are consumed in large amounts in the body [49]. The proper supplementation of these valuable nutrients may effectively prevent COVID-19 aggravation, thus accelerating recovery from various COVID-19 sequalae.
SIRME Could Explain Severe COVID-19 Complications
Various lifestyle-related diseases cause disorders of the VEGLX, arteriosclerosis progression, and other cardiovascular diseases. Contrastingly, serious COVID-19 complications, such as Acute Respiratory Distress Syndrome (ARDS), Disseminated Intravascular Coagulation Syndrome (DIC), Kawasaki disease shock syndrome [51-53], microvascular thrombosis, and arrhythmia, are known to be accompanied by VEGLX damage [54-56]. Based on their relationship with a damaged glycocalyx, we have termed these symptoms “Systemic Inflammatory-Reactive Microvascular Endotheliopathy” (SIRME) [5].
The concept of SIRME could explain the mechanism behind COVID-19 aggravation in a unified manner (Figure 2). The definition of SIRME is the presence of a causative inflammation, a strong thrombotic tendency, and organ damage occurring simultaneously. In more severe SIRME, the fragility of fragmented glycocalyx is high and ground-glass shadows may be frequently observed in both lungs. Together, these should be recognized as an emergency condition preceding sudden COVID-19 aggravation. By evaluating the state of the VEGLX, the severity of COVID-19 can be determined at a very early stage of the disease. VEGLX damage in SIRME is considered to occur at an earlier and milder stage than the previously proposed shock-induced endothelial disease (SHINE) [57] and Systemic Inflammatory Response Syndrome (SIRS). Using the concept of SIRME to explain COVID-19 aggravation, it can be predicted that SARS-CoV-2 and other viruses, which mainly target vascular endothelial cells, will cause the same pathological condition.
Various Pathological Conditions Caused by SIRME
The presence of inflammation, strong thrombotic tendency, and organ damage as defined by SIRME cause microvascular damage, VEGLX loss, and increased vascular permeability [58]. As shown in Figure 2, if SIRME occurs in the brain, the condition will result in cerebral embolism, whereas if it occurs in the heart, it will result in myocardial infarction, heart failure, arrhythmia, and Kawasaki disease shock syndrome [59]. Furthermore, if SIRME occurs in the lung, the condition will cause ARDS [60,61], pulmonary thromboembolism, and pneumonia. Finally, if systemic thrombosis is a consequence of SIRME, DIC, SIRS, and multi-organ failure will occur.
The Vascular Endothelial Glycocalyx and COVID-19
Recently, a number of studies on the aggravation of VEGLX and COVID-19 have been published [62-66]. Biomarkers related to the VEGLX and vascular endothelial damage are abnormally high in patients with severe COVID-19. Therefore, it is possible to identify patients who are at an increased risk of becoming severely ill and who may benefit greatly from prompt treatment. VEGLX is being increasingly considered as a new potential biomarker, especially in patients with severe COVID-19. Thus far, VEGLX damage has acted as an index for determining the effectiveness of screening for cardiovascular diseases, diabetes control, and cardiovascular protection measures. Proper disease management to prevent damage to the VEGLX will lead to the development of future atherosclerotic diseases and the prevention of cardiovascular events [67]. To prevent cardiovascular disease in the COVID-19 era, we should limit the intake of high amounts of sodium [68], oxidized lipoproteins [69], and sugar [18,19]. In addition, preventing obesity and smoking while encouraging increased physical activity is crucial in order to ensure the sufficient management of coronary risk factors. Whereas cardiovascular disease management is necessary to ensure a healthy VEGLX, a healthy VEGLX will in turn prevent the aggravation of COVID-19 and other emerging viral infections that target the vascular endothelium.
Acknowledgment
This work was partly supported by JSPS KAKENHI grant number JP19K11371.
References
- Giannis D, Ziogas IA and Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020; 127: 104362.
- Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med. 2020.
- Piccolo V, Neri I, Filippeschi C, Oranges T, Argenziano G, Battarra VC, et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020; 34: e291-e293.
- Zhang J, Tecson KM and McCullough PA. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. 2020; 21: 315-319.
- Yamaoka-Tojo M. Vascular Endothelial Glycocalyx Damage in COVID-19. Int J Mol Sci. 2020; 21: 9712.
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020; 383: 120-128.
- Yamaoka-Tojo M. A note on systemic inflammatory-reactive microvascular endotheliopathy (SIRME): Prevention of cardiovascular disease and COVID-19. JJCDP 2020; 55: 1-14.
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020; 11: 1620.
- Kazory A, Ronco C and McCullough PA. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proc (Bayl Univ Med Cent). 2020; 0: 1-6.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX and China Medical Treatment Expert Group for C. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020; 382: 1708-1720.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395: 1417-1418.
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020; 181: 271-280, e8.
- Lee AC, Chakladar J, Li WT, Chen C, Chang EY, Wang-Rodriguez J, et al. Tobacco, but Not Nicotine and Flavor-Less Electronic Cigarettes, Induces ACE2 and Immune Dysregulation. Int J Mol Sci. 2020; 21: 5513.
- Yamaoka-Tojo M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed J. 2020; 43: 399-413.
- Bar A, Targosz-Korecka M, Suraj J, Proniewski B, Jasztal A, Marczyk B, et al. Degradation of Glycocalyx and Multiple Manifestations of Endothelial Dysfunction Coincide in the Early Phase of Endothelial Dysfunction Before Atherosclerotic Plaque Development in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice. J Am Heart Assoc. 2019; 8: e011171.
- Ostrowski SR, Gaini S, Pedersen C and Johansson PI. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: an observational study. J Crit Care. 2015; 30: 90-96.
- Oberleithner H and Wilhelmi M. Vascular glycocalyx sodium store - determinant of salt sensitivity? Blood Purif. 2015; 39: 7-10.
- Zuurbier CJ, Demirci C, Koeman A, Vink H and Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 1985; 2005; 99: 1471-1476.
- Pahwa R, Nallasamy P and Jialal I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J Diabetes Complications. 2016; 30: 563-572.
- Long DS, Hou W, Taylor RS and McCowan LM. Serum levels of endothelial glycocalyx constituents in women at 20 weeks’ gestation who later develop gestational diabetes mellitus compared to matched controls: a pilot study. BMJ Open. 2016; 6: e011244.
- Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, et al. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985). 2014; 116: 998-1005.
- Wadowski PP, Hulsmann M, Schorgenhofer C, Lang IM, Wurm R, Gremmel T, et al. Sublingual functional capillary rarefaction in chronic heart failure. Eur J Clin Invest. 2018; 48.
- Ikonomidis I, Pavlidis G, Lambadiari V, Kousathana F, Varoudi M, Spanoudi F, et al. Early detection of left ventricular dysfunction in first-degree relatives of diabetic patients by myocardial deformation imaging: The role of endothelial glycocalyx damage. Int J Cardiol. 2017; 233: 105-112.
- Gorshkov AY, Klimushina MV, Boytsov SA, Kots AY and Gumanova NG. Increase in perfused boundary region of endothelial glycocalyx is associated with higher prevalence of ischemic heart disease and lesions of microcirculation and vascular wall. Microcirculation. 2018; 25: e12454.
- Jaarsma C, Vink H, van Haare J, Bekkers S, van Rooijen BD, Backes WH, et al. Non-invasive assessment of microvascular dysfunction in patients with microvascular angina. Int J Cardiol. 2017; 248: 433-439.
- Butler MJ, Ramnath R, Kadoya H, Desposito D, Riquier-Brison A, Ferguson JK, et al. Aldosterone induces albuminuria via matrix metalloproteinasedependent damage of the endothelial glycocalyx. Kidney Int. 2019; 95: 94- 107.
- Koo A, Dewey CF, Jr. and Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol. 2013; 304: C137-146.
- Nieuwdorp M, Meuwese MC, Mooij HL, van Lieshout MH, Hayden A, Levi M, et al. Tumor necrosis factor-alpha inhibition protects against endotoxininduced endothelial glycocalyx perturbation. Atherosclerosis. 2009; 202: 296- 303.
- Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010; 53: 2646-2655.
- Ko J, Kang HJ, Kim DA, Kim MJ, Ryu ES, Lee S, et al. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. 2019; 33: 13334-13345.
- Tang TH, Alonso S, Ng LF, Thein TL, Pang VJ, Leo YS, et al. Increased Serum Hyaluronic Acid and Heparan Sulfate in Dengue Fever: Association with Plasma Leakage and Disease Severity. Sci Rep. 2017; 7: 46191.
- Suwarto S, Sasmono RT, Sinto R, Ibrahim E and Suryamin M. Association of Endothelial Glycocalyx and Tight and Adherens Junctions with Severity of Plasma Leakage in Dengue Infection. J Infect Dis. 2017; 215: 992-999.
- Uchimido R, Schmidt EP and Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019; 23: 16.
- Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, et al. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res. 2011; 165: 136-141.
- Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, et al. Urinary Glycosaminoglycans Predict Outcomes in Septic Shock and Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2016; 194: 439- 449.
- Marechal X, Favory R, Joulin O, Montaigne D, Hassoun S, Decoster B, et al. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock. 2008; 29: 572-576.
- Chelazzi C, Villa G, Mancinelli P, De Gaudio AR and Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015; 19: 26.
- Chignalia AZ, Yetimakman F, Christiaans SC, Unal S, Bayrakci B, Wagener BM, et al. The Glycocalyx and Trauma: A Review. Shock. 2016; 45: 338-348.
- Gonzalez Rodriguez E, Cardenas JC, Cox CS, Kitagawa RS, Stensballe J, Holcomb JB, et al. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand J Trauma Resusc Emerg Med. 2018; 26: 102.
- Rubio-Gayosso I, Platts SH and Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006; 290: H2247-2256.
- Miranda CH, de Carvalho Borges M, Schmidt A, Marin-Neto JA and Pazin- Filho A. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis. 2016; 247: 184-188.
- DellaValle B, Hasseldam H, Johansen FF, Iversen HK, Rungby J and Hempel C. Multiple Soluble Components of the Glycocalyx Are Increased in Patient Plasma After Ischemic Stroke. Stroke. 2019; 50: 2948-2951.
- Curry FE. Layer upon layer: the functional consequences of disrupting the glycocalyx-endothelial barrier in vivo and in vitro. Cardiovasc Res. 2017; 113: 559-561.
- Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms In Endocrinology: Vitamin D and COVID-19. Eur J Endocrinol. 2020; 183: R133-R147.
- Hastie CE, Pell JP and Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur J Nutr. 2021; 60: 545-548.
- Anastasi E, Ialongo C, Labriola R, Ferraguti G, Lucarelli M and Angeloni A. Vitamin K deficiency and covid-19. Scand J Clin Lab Invest. 2020; 80: 525- 527.
- Dofferhoff ASM, Piscaer I, Schurgers LJ, Visser MPJ, van den Ouweland JMW, de Jong PA, et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin Infect Dis. 2020.
- Wessels I, Rolles B and Rink L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front Immunol. 2020; 11: 1712.
- Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A and Aaseth J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti- Viral Resistance against Progressive COVID-19. Nutrients. 2020; 12: 2358.
- Pal A, Squitti R, Picozza M, Pawar A, Rongioletti M, Dutta AK, et al. Zinc and COVID-19: Basis of Current Clinical Trials. Biol Trace Elem Res. 2020: 1-11.
- Gamez-Gonzalez LB, Moribe-Quintero I, Cisneros-Castolo M, Varela-Ortiz J, Munoz-Ramirez M, Garrido-Garcia M, et al. Kawasaki disease shock syndrome: Unique and severe subtype of Kawasaki disease. Pediatr Int. 2018; 60: 781-790.
- Kanegaye JT, Wilder MS, Molkara D, Frazer JR, Pancheri J, Tremoulet AH, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009; 123: e783-789.
- Li Y, Zheng Q, Zou L, Wu J, Guo L, Teng L, et al. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFNgamma as biomarkers for early recognition. Pediatr Rheumatol Online J. 2019; 17: 1.
- Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021; 24: 145-157.
- Ohnishi Y, Yasudo H, Suzuki Y, Furuta T, Matsuguma C, Azuma Y, et al. Circulating endothelial glycocalyx components as a predictive marker of coronary artery lesions in Kawasaki disease. Int J Cardiol. 2019; 292: 236- 240.
- Weissgerber TL, Garcia-Valencia O, Milic NM, Codsi E, Cubro H, Nath MC, et al. Early Onset Preeclampsia Is Associated With Glycocalyx Degradation and Reduced Microvascular Perfusion. J Am Heart Assoc. 2019; 8: e010647.
- Johansson PI, Stensballe J and Ostrowski SR. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017; 21: 25.
- Okada H, Yoshida S, Hara A, Ogura S and Tomita H. Vascular endothelial injury exacerbates coronavirus disease 2019: The role of endothelial glycocalyx protection. Microcirculation. 2020: e12654.
- Luo L, Feng S, Wu Y, Su Y, Jing F and Yi Q. Serum Levels of Syndecan-1 in Patients with Kawasaki Disease. Pediatr Infect Dis J. 2019; 38: 89-94.
- Teuwen LA, Geldhof V, Pasut A and Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020; 20: 389-391.
- Pons S, Fodil S, Azoulay E and Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020; 24: 353.
- Buijsers B, Yanginlar C, de Nooijer A, Grondman I, Maciej-Hulme ML, Jonkman I, et al. Increased Plasma Heparanase Activity in COVID-19 Patients. Front Immunol. 2020; 11: 575047.
- Fraser DD, Patterson EK, Slessarev M, Gill SE, Martin C, Daley M, et al. Endothelial Injury and Glycocalyx Degradation in Critically Ill Coronavirus Disease 2019 Patients: Implications for Microvascular Platelet Aggregation. Crit Care Explor. 2020; 2: e0194.
- Stahl K, Gronski PA, Kiyan Y, Seeliger B, Bertram A, Pape T, et al. Injury to the Endothelial Glycocalyx in Critically Ill Patients with COVID-19. Am J Respir Crit Care Med. 2020; 202: 1178-1181.
- Hutchings SD, Watchorn J, Trovato F, Napoli S, Mujib SF, Hopkins P, et al. Microcirculatory, Endothelial and Inflammatory Responses in Critically Ill Patients with COVID-19 are Distinct from those Seen in Septic Shock: A Case Control Study. Shock. 2020.
- Ding M, Zhang Q, Li Q, Wu T and Huang YZ. Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir Med. 2020; 167: 105981.
- Yamaoka-Tojo M. Endothelial function for cardiovascular disease prevention and management. Int J Clinic Cardiol. 2017; 4: 103.
- Rorije NMG, Rademaker E, Schrooten EM, Wouda RD, Homan Van Der Heide JJ, Van Den Born BH, et al. High-salt intake affects sublingual microcirculation and is linked to body weight change in healthy volunteers: a randomized cross-over trial. J Hypertens. 2019; 37: 1254-1261.
- Vink H, Constantinescu AA and Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation. 2000; 101: 1500-1502.