
Case Report
Austin J Cardiovasc Dis Atherosclerosis. 2022; 9(1): 1051.
Case of Leukotriene Receptor Antagonist Related Bradycardia in Patient Taking Montelukast
Shankar A1*, Dang V1, Fadeyi O1, Shalom FM1,2 and Shams F1,2
1West Anaheim Medical Center, Anaheim, California
2HMC Anaheim Regional Medical Center, Anaheim, California
*Corresponding author: Abhirami Shankar, West Anaheim Medical Center, Anaheim, 604 S Beach Blvd, Apt 60, Anaheim, CA 92804, West Anaheim Medical Center, Anaheim, California
Received: September 21, 2022; Accepted: October 19, 2022; Published: October 26, 2022
Abstract
A 74-year-old male on Montelukast presented initially with tachycardia in septic shock masking his underlying bradycardia, which emerged with initiation of sepsis treatment. The patient’s heart rate responded to cessation of Montelukast showing a likely association between Montelukast and bradycardia. Further research is necessary to thoroughly investigate this relationship.
Keywords: Montelukast; Singulair; Bradycardia; Leukotriene; LTD4; LTRA; CysLT; Cysteinyl; Heart block; Long pause; Arrhythmia; Hypertension; Lipoxygenase
Abbreviations
CysLT: Cysteinyl-Leukotriene; LTD4: Leukotriene Receptor D4; LTRAs: Leukotriene Receptor Antagonists; SHR: Spontaneously Hypertensive Rats; WKY: Wistar-Kyoto
Case Report
74 y/o Caucasian male presented to the ED from a residential care facility due to altered mental status. Prior to admission, he was found to be lying on his side with drooling for an unknown period of time. Per facility staff, he had been more lethargic and drowsy since the last month. His mental status began to change after a Psychiatrist adjusted his medications for his aggressive behaviour. His baseline is AOx3.
Physical examination was remarkable for lethargy upon initial inspection, tachycardia on cardiovascular exam, decreased bilateral upper and lower extremity ROM on neurological exam and focal bullae noted on abdominal exam.
Physical examination was remarkable for lethargy upon initial inspection, tachycardia on cardiovascular exam, decreased bilateral upper and lower extremity ROM on neurological exam and focal bullae noted on abdominal exam.
Differential Diagnosis: Septic shock, UTI, Pneumonia, Toxic vs. metabolic encephalopathy, rhabdomyolysis.
Investigations
Vital signs: BP 66-116/33-74, T 101.7 F, HR 92-101, RR 30-42, SpO2 91-97% Labs were significant for BUN/Cr of 60/1.6, Mg 1.8, lactic acid 1.3, ammonia 53, total CK 1753, cortisol 16.49 and AST/ ALT of 146/131. UA was positive for UTI. UDS was initially positive for cocaine but ruled as lab error upon further investigation. ABG showed appropriate acid base balance and oxygenation. CBC, Troponin, BNP, TSH, A1c, serum toxicology and other labs were unremarkable.
Imaging
CXR: Left basilar subsegmental atelectasis.
CT Head: Unremarkable.
Previous Echo: EF~55%
Management
Patient was started on antibiotics, fluids and pressors. Patient then had an episode of bradycardia prior transportation from ER to ICU and Code 44 was called. The patient however had not lost his pulse. Atropine was given. EKG obtained during this incident showed sinus bradycardia without heart block, long pause or arrhythmia. Cardiology was consulted who initially increased his synthroid dose. As the patient continued to be bradycardic, Montelukast was discontinued. Subsequently, the patient’s heart rate improved and remained stable throughout his hospital stay.
Discussion/Literature Review
The patient’s cause of bradycardia can be attributed to his Risperidone, hypothyroidism, montelukast vs other etiologies. Risperidone commonly causes tachycardia although, at moderatehigh doses it has bradycardic effects as noted in an alcohol-withdrawal patient [1,2]. There have also been documented cases of Risperidone causing sinus bradycardia and 1st degree AV block [3,4]. However, our patient was on low dose Risperidone and his bradycardia resolved with discontinuing only Singulair while still on Risperidone.
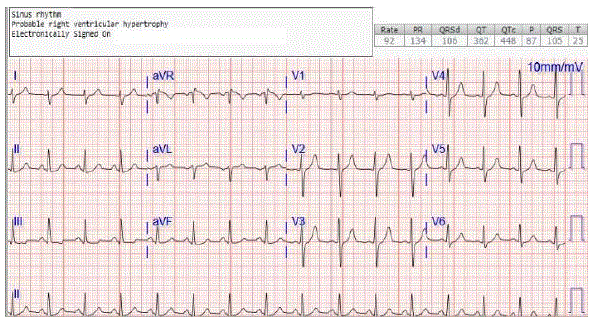
Figure 1: Initial EKG on admission: Sinus rhythm. HR 92 bpm. No evidence of heart block.
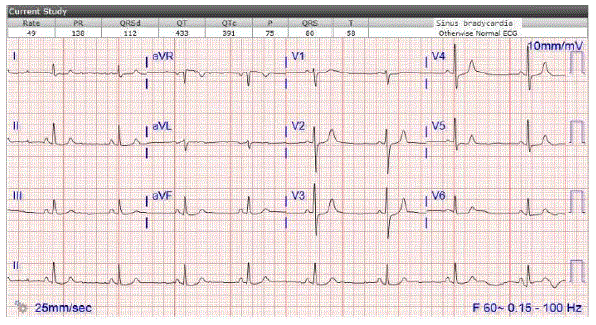
Figure 2: First episode of bradycardia in ED: Sinus bradycardia. HR 49 bpm. Patient was on Montelukast.
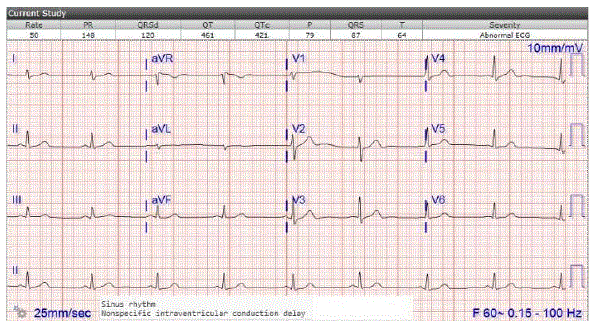
Figure 3: EKG the day Monteleukast was discontinued: Sinus rhythm. HR 50. Patient remains bradycardic despite increasing Levothyroxine dose.
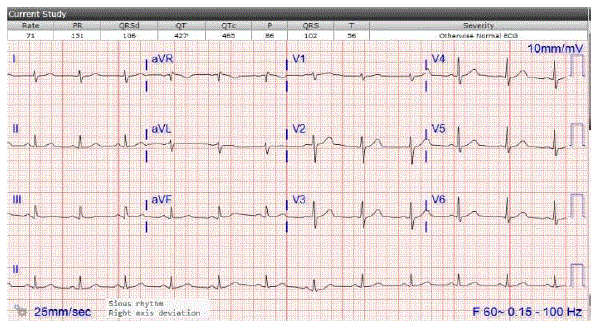
Figure 4: EKG 21 days after Montelukast was discontinued: Sinus rhythm. HR 70 bpm. Resolution of bradycardia following Montelukast discontinuation.
Hypothyoroid states raise after load due to elevated arterial stiffness from poor compliance and low nitric oxide production; hypothyroidism also decreases heart rate via impacted pacemakerrelevant gene transcription and effect on beta-adrenergic network in cardiac cells [5]. The patient’s synthroid dose was initially increased from 175 mcg to 200 mcg p.o. qD. But with no notable change in bradycardia.
There was however, an observed dose-dependent and temporal change in heart rate concordant with Montelukast administration, especially given his age and mild hepatic impairment [7]. He’d had no bradycardic events prior to taking Montelukast, thus Singulair was discontinued and he was monitored on the telemetry floor as it would take a few days for Singulair to clear the system. Over the next few days, the patient’s bradycardia resolved and his heart rate remained stable. Montelukast is a Leukotriene Receptor Antagonist (LTRA) selective for the CysLT (Cysteinyl-leukotriene) type 1 receptor [7]. Cysteinyl LTs (LTC4, LTD4, LTE4) are arachidonic acid based inflammatory mediators causing potent bronchoconstriction [7]. They have also been implicated in other conditions such as coronary artery disease, cardiovascular disease and stroke [7]. Specifically, LTD4 has a direct effect on up/down regulation of vascular smooth muscle and its spectrum of sympathetic responses [7]. Studies isolating epicardial coronary arteries challenged with LTC4 and LTD4 concentrations showed that coronary arterial myocytes produced CysLTs leading to responsive vasoconstriction [7]. A study found that 1-20 mcg/kg of Leukotriene D4 given to conscious spontaneously hypertensive rats (SHR/genetically hypertensive rats) and Wistar-Kyoto (WKY) rats (i.e. bred normotensive controls for the SHR) triggered acute blood pressure increase in both, although in SHR it preceded by a prolonged hypotensive phase [8]. SHR rats were tachycardic while hypertensive and bradycardic while hypotensive and these effects were more prominent and protracted than in WKY rats [8]. The study insinuated a direct effect of LTD4 on the heart and vascular smooth muscle [8]. Normotensive (WKY) rats and some SHR rats were noted to have bradycardic effect of LTD4 resulting from sinus bradycardia rather than from impaired conduction such as transient Type II AV block to complete heart block8. The study suggested foundational differences in susceptibility of SHR and WKY rats to lipoxygenase products (LTD4) [8]. Given these results, it is not improbable that LTD4 antagonists such as singulair can cause the opposite effects of LTD4 agonists and induce bradycardia in hypertensive subjects. This corroborates with the bradycardic response seen in our patient with baseline hypertension taking Singulair, especially evident after treatment of his initial septic shock. A phase IV clinical investigation using FDA data based on frequently updated reports of subjects who had side effects while taking Singulair (Montelukast sodium) found that among those taking Singulair, bradycardia was observed especially in women, age >=60 and those taking the drug for <1 month [9]. But this doesn’t definitively exclude similar effects in other populations. As documented on May 23, 2022, of the 103,210 patients reportedly having side effects while taking Singulair, 239 persons (0.23%) had Bradycardia [9].
Follow Up
Patient was followed for 48 days after discharge and was not on Montelukast during this period. His heart rate was monitored and it was normal throughout with no bradycardic episodes.
Conclusion/Limitations
This case shows a likely temporal and dose dependent association between heart rate and montelukast ingestion. The patient’s bradycardia didn’t respond to increasing Synthroid, but resolved solely after discontinuing montelukast while still on Risperidonewhich would’ve been the next drug to be discontinued if he’d remained bradycardic off montelukast. Animal model studies on SHR/WKY rats demonstrate direct effects of LTD4 receptors on heart rate and coronary vasculature. There is an ongoing phase IV clinical study by EHealthMe, although this is wholly based on FDA data and consumer/patient personal reports with no public body oversight. Thus, there is a need for more regulated and newer research to elucidate this association and to help guide medical therapy in the future. From this report and future studies, we hope that patients and clinicians managing conditions such as asthma, COPD, allergic rhinitis, urticaria, etc. on LTRAs can benefit from further understanding of the adverse effects of LTRAs, including its negative chronotropic effects on the heart.
References
- Goyal RS, Goyal SB. Symptomatic bradyarrhythmia secondary to risperidone. The American journal of psychiatry. 2003; 160: 2243-2243.
- Jegede O, Taher K. Risperidone-Associated Atrioventricular Block. American Journal of Therapeutics. 2018; 26: e610-e612.
- Arun P, Gupta N. Symptomatic Bradycardia: an Adverse Event Following Risperidone Initiation in an Elderly Male. East Asian Archives of Psychiatry; Hong Kong J Psychiatry. 2001; 11: 21-22.
- Jegede O, Taher K. Risperidone-Associated Atrioventricular Block. American Journal of Therapeutics. 2018; 26: e610-e612.
- Merck & Co., Inc. . (n.d.). Singulair (Montelukast Sodium).
- Tran KT, Golden P, Lark T, Jacob NJ. Bradycardia at low doses of risperidone. The American journal of psychiatry. 2004; 161: 2325-a-2326.
- Zukowska-Grojec Z, Bayorh MA, Kopin IJ, Feuerstein G. Leukotriene D4: cardiovascular and sympathetic effects in spontaneously hypertensive rats (SHR) and Wistar-Kyoto (WKY) rats. J Pharmacol Exp Ther. 1982; 223: 183- 189.
- HealthLatLLC/Johnson Chen. (n.d.). Singulair and bradycardia - A phase IV clinical study of FDA Data. eHealthMe. 2022.