
Research Article
J Cardiovasc Disord. 2014;1(1): 4.
Vascular Endothelial Growth Factor Gene Transfection in Endocardial Endothelial Cells Promote Capillary Growth in Vitro
Kuruvilla L1, Santhosh Kumar TR2, Panicker SR2, Kartha CC1*
1Division of Cellular & Molecular Cardiology, sree chitra tirunal institute for Medical Sciences and Technology, Trivandrum, India
2Cancer Biology, Rajiv Gandhi Center for Biotechnology, Trivandrum, India
*Corresponding author: Kartha CC, Disease Biology & Molecular Medicine, Rajiv Gandhi Center for Biotechnology, Trivandrum - 695014, Kerala, India
Received: August 16, 2014; Accepted: September 11, 2014; Published: September 15, 2014
Abstract
Background: Different types of cells have been employed both in in vitro and in vivo studies for revascularization of ischemic myocardium. Whether Endocardial Endothelial Cells (EEC) lying adjacent to the myocardium can be used in gene therapy for successful revascularization of ischemic myocardium remains unexplored. Here we present evidence for the first time that EECs can be made to take up Vascular Endothelial Growth Factor (VEGF) gene with consequent VEGF protein synthesis to promote angiogenesis in vitro.
Methods and Results: Immortalized EECs (iEEC) with stable h TERT tranfection were transfected with VEGF gene. The iEECs, Rat Aortic Endothelial Cells (RAECs) and VEGF transfected cells were seeded in various combinations onto matrigel and the number of tubes counted using tube formation assay. Cocultures of RAECs and VEGF transfected EECs gave the maximum number of tubes (24.6 ± 3.7) and also the maximum tube length (334 ± 45 μm) within the shortest period of time. iEECs alone did not show any tube formation whereas iEECs with VEGF gene formed a few incomplete tubes.
Conclusion: These results confirm that VEGF gene transfection in EECs can promote capillary growth in vitro.
Keywords: Endocardium; Endothelium; VEGF; Angiogenesis
Introduction
Significant number of patients with coronary artery disease failed to have complete revascularization even after Percutaneous Coronary Intervention (PCI) and Coronary Artery Bypass Grafting (CABG), resulting in refractory angina [1]. A combination of surgical laser Transmyocardial Revascularization (TMR) and therapeutic angiogenesis offers an attractive possibility for successful revascularization of ischemic myocardium. Following TMR, new capillary formation has been observed in the area of the channels which is a result of up-regulation of VEGF [2,3,4]. Different strategies for delivering these angiogenic factors has been attempted, such as direct intra coronary and intra venous delivery, percutaneous endocardial delivery catheters using fluoroscopic guidance or electromagnetic three-dimensional navigation system for therapeutic myocardial angiogenesis. EECs that line the cardiac cavities auto regulate cardiac performance by direct interaction with superfusing blood [5] and via various cardioactive mediators [6].We hypothesized that if the VEGF gene is directly delivered to the endocardial endothelial cells and is able to secrete VEGF protein in required levels, this may augment collateral blood vessel development constituting endogenous bypass conduits around the damaged areas in the myocardium. We tested this hypothesis in an in vitro system with EECs and aortic endothelial cells.
Materials and Methods
All fine chemicals including Medium199, FBS and all supplements were procured from Sigma-Aldrich, St. Louis, USA. MEM with D-valine was obtained from Pan Biotech, Germany. Matrigel basement membrane matrix (7.8 mg/ml) was purchased from BD biosciences, San Jose, CA, USA. Rabbit polyclonal anti-mouse VEGF and monoclonal anti-mouse Hsc70 antibodies were from Santa Cruz Biotechnology, Inc. Santa Cruz, CA, and USA. The pWZL Blast VEGF plasmid was procured from Addgene, plasmid 10909.
Cells and cell culture
Immortalized Endocardial Endothelial Cells (iEECs) and Rat Aortic Endothelial Cells (RAECs) were used for the experiments. The primary EECs and the iEECs with stable h TERT gene were obtained by the procedure described earlier [7]. RAECs were isolated from male Sprague-Dawley rats 2-3 months old according to the methods of McGuire PG and Orkin RW, 1987 [8]. Briefly, rat aortae were removed aseptically, cut into rings and placed in culture dishes containing Minimum Essential Medium with D-Valine and supplemented with 20% fetal bovine serum,75 μg/ml endothelial cell growth supplements. After 4-7 days, the aortic rings were removed; the cells harvested and used for experiments between passages 3 and 8. RAECs were characterized using immunocytochemical staining against factor VIII related antigen, by their cobblestone morphology and ability to grow in culture medium containing D-Valine.
All experiments had the approval of the Institutional Animal Ethics Committee.
Transfection of iEECs with the VEGF gene
A commercially available mammalian expression vector, pWZL Blast VEGF plasmid containing the mouse VEGF-A (VEGF 120) gene insert and blasticidin resistance gene was used. Briefly, iEECs at about ~80% confluence were transiently transfected with 10μg of plasmid DNA using Lipofectamine 2000 reagent as per manufacturer's instructions, using serum free OptiMEM medium. Six hours after transfection, the transfection complex was replaced with complete medium. Forty-eight hours after transfection, the cells were incubated in complete medium containing 0.5 μg/ml of blasticidin for the selection of transfected population. The selected clones of cells were trypsinised and seeded on to matrigel for tube formation assay. For obtaining the Condition Medium (CM), VEGF transfected cells were incubated for 48 hours in complete medium and the cell culture supernatant taken after removing cellular debris by centrifugation.
Western blot analysis
The cell lysates from VEGF transfected cells were subjected to 15% PAGE and proteins transferred to a nitrocellulose membrane. Lysates from primary EECs and iEECs served as controls in the analysis. Hsc 70 protein expression was taken as internal control. VEGF expression was detected using rabbit polyclonal anti-mouse VEGF antibody at a dilution of 1:200. The bands were developed using the chromogen, diaminobenzidine.
Experimental design for matrigel tube formation assay
The spontaneous formation of capillary-like structures by endothelial cells on Matrigel basement membrane matrix was used to assess the angiogenic potential. Matrigel was coated on 35 mm cell culture dishes and incubated at 37°C for 1 h to promote gelling. Five different groups were seeded onto matrigel for the in vitro angiogenesis assay to calculate the number of tubes and length of the tube, namelyone set each of iEECs, VEGF transfected iEECs and RAECs alone and another set of RAECs with VEGF-iEECs as co-culture and the last group of RAEC supplemented with CM. The cells were seeded in 1 ml suspensions containing 5.25 x 104 cells. Cellular organization into tubular structures was monitored every three hours, starting from 2 hours using an inverted phase-contrast photomicroscope and photographs taken at 400x magnifications by scoring a three branch point event as one tube [9]. At 11 hours the photomicrographs were taken at 100X so as to capture a larger number of cells per field. Images of the tubes were captured using a digital camera Evolution TM MP and the length of the tubes measured using Image-Pro® Plus 5.1 software after 24 hours. Each assay was performed in duplicate and the results expressed as mean of 3 independent experiments.
Statistical analysis
All results were expressed as the mean ± SD. Significance of differences between groups was determined with ANOVA followed by Tukey test. p< 0.05 was considered statistically significant.
Results
VEGF gene is successfully transfected in iEECs
Western blot analysis was done to confirm whether iEECs transfected with the pWZL Blast VEGF plasmid had taken up the plasmid and were expressing VEGF protein. A band corresponding to the VEGF protein was observed at the 42 KDa position in VEGF transfected iEEC but not in primary EECs and iEECs which were used as controls, confirming successful VEGF transfection into iEECs with corresponding VEGF protein synthesis (Figure 1).
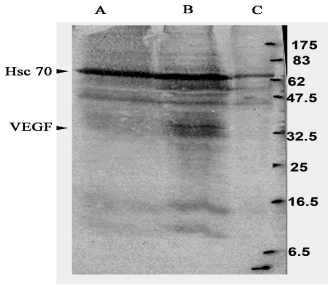
Figure 1: Western blot analysis to confirm whether the immortalized
endocardial endothelial cells transfected with VEGF are transiently
transfected with VEGF gene. Lane A: Immortalized endocardial endothelial
cells. Lane B: Immortalized endocardial endothelial cells transfected with
VEGF gene. Lane C: Primary endocardial endothelial cells. Hsc 70 was used
as a positive control for gene expression. A band corresponding to VEGF is
visible at 42 KDa.
Co-culture of RAECs with VEGF transfected iEECs induce higher rate of tube formation on matrigel
Cellular organization into tubular structures started occurring in the co-culture system by 2 hours whereas it was visible only by 5 hours in RAECs alone group. RAECs seeded in the co-culture dishes as well as those seeded alone displayed morphological changes such as elongation and alignment on matrigel to form two-dimensional tube-like structures. By 11 h, the tube length increased and there was a decrease in the number of tubes per field. The iEECs seeded alone on matrigel remained rounded without any visible tube formation. VEGF transfected iEECs did not exhibit any tube formation till 8 hours after which a few scattered and incomplete tubes had formed that were considered for the tube length assay (Figure 2). At 5 h, 8 h and 11 h respectively, the average number of tubes in ten different fields counted in co-cultured cells was 24.6 ± 3.7, 15.4 ± 2.2 and 8 ± 1.56. The number of tubes in RAECs alone during the same time intervals was 13 ± 2.5, 9.5 ± 2.3 and 4.3 ± 0.7 (Figure 3).
(A) Rat aortic endothelial cells on matrigel at 2, 5, 8 (400 X) and 11 h (100X)
(B) Co-culture of aortic-endothelial cells and VEGF transfected immortalized endocardial endothelial cells at 2, 5, 8 (400X) and 11 h (100X)
(C) Immortalized endocardial endothelial cells on matrigel at 11 h (100X)
(D) VEGF transfected immortalized endocardial endothelial cells on matrigel at 8 h (100X).
Arrows point to a few tubes.
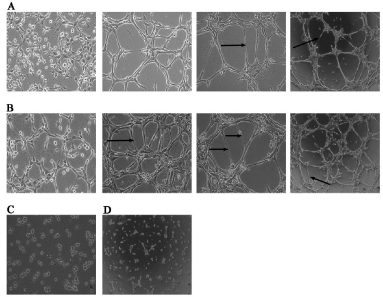
Figure 2: In vitro tube formation assay on matrigel basement membrane
matrix
(A) Rat aortic endothelial cells on matrigel at 2, 5, 8 (400 X) and 11 h (100X)
(B) Co-culture of aortic-endothelial cells and VEGF transfected immortalized
endocardial endothelial cells at 2, 5, 8 (400X) and 11 h (100X)
(C) Immortalized endocardial endothelial cells on matrigel at 11 h (100X)
(D) VEGF transfected immortalized endocardial endothelial cells on matrigel
at 8 h (100X).
Arrows point to a few tubes.
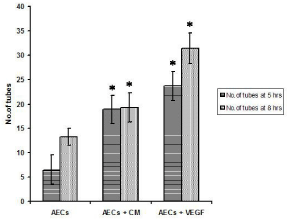
Figure 3: Number of tubes formed on matrigel at 5 hrs and 8 hrs. AECs -
Aortic endothelial cells seeded on matrigel, AECs + CM - aortic endothelial
cells on matrigel, grown in conditioned medium from VEGF transfected cells,
AECs + VEGF - co-culture of aortic endothelial cells and VEGF transfected
cells on matrigel.
AECs vs AECs + CM and AECs + VEGF, n= 10; p< 0.001 by ANOVA.
Maximum length of tubes on matrigel were observed with co-culture of RAECs and VEGF transfected iEECs
The average tube length formed on matrigel with VEGF transfected iEECs alone was 96 ± 11.7 μm where as that for RAECs alone was 137.9 ± 38 μm. The average tube length in RAECs supplemented with CM was 198.9 ± 47 m. The tube length in the RAECs and VEGF transfected iEECs co-culture was highest with 334 ± 45 μm (Figure 4)

Figure 4: Tube length measured at 24 hrs. VEGF TCs - Endocardial
endothelial cells transfected with VEGF gene and seeded on matrigel (n=7).
AECs - Aortic endothelial cells seeded on matrigel (n=29), AECs + CM -
aortic endothelial cells on matrigel grown in conditioned medium from VEGF
transfected cells (n=19), AECs + VEGF - co-culture of aortic endothelial cells
and VEGF transfected cells on matrigel (n= 34).
AECs vs AEC + CM= Non significant (NS); *AECs vs AECs + VEGF by
ANOVA, p < 0.001
Discussion
This communication presents evidence for the first time that EECs can be made to take up VEGF gene with consequent protein synthesis to augment angiogenic growth in vitro.
A survey study conducted in Serbia inferred that myocardial revascularization is a good therapeutic option for CAD patients older than 65 years and improved their quality of life [10]. TMR using a carbon dioxide laser has been shown to relieve angina, increase vascular density, and improve myocardial contraction through sustained VEGF secretion [11]. Wu et al [12] demonstrated that ventricular endocardial cells generate endothelial cells of coronary arteries. Though endocardial cells have been previously thought to be terminally differentiated, Wu et al provide evidence that these cells are angiogenic and are activated to generate coronary plexuses. The initial results from our brief in vitro investigation suggest that VEGF synthesis by EECs may promote collateral vessel formation in areas around ischemic myocardium by stimulating new vessel formation from existing coronary micro/macro vasculature which can in fact supplement the effect of TMR. The formation of maximum number of tubes in co-culture system of VEGF transfected iEEC and RAEC supports this notion. We assume that the ability of VEGF-iEEC and RAEC co-culture system to produce the maximum number of tubes and the maximum tube length in a short period of 2 hours in comparison with RAECs, iEECs or the VEGF transfected iEECs alone, is probably due to the VEGF protein activating the VEGF receptors on RAECs. The formation of much higher number of tubes as well as longer tubes in the co-culture system of RAECs and VEGF transfected iEEC compared to RAEC in CM suggests that a continuous release of VEGF is required for better angiogenesis which may be applicable in in vivo settings both in animal models and patients. The iEECs seeded on matrigel basement membrane matrix not forming tubular structures may be because of immortalization with h TERT transfection. Further studies are to be done to confirm whether the EEC can be made to take up the VEGF gene, in an ex vivo model of the heart by targeted gene delivery, subsequent determination of efficacy of the gene transfer technique in vivo and sufficient VEGF protein secretion by EECs leading to revascularization of the ischemic myocardium. This could be taken up as a novel therapeutic modality that may supplement the beneficial effect of TMR for successful revascularization of ischemic myocardium. These findings offer new avenues for a novel therapeutic modality for conditions that are refractory to conservative measures and unresponsive to pharmacological therapy in the revascularization of ischemic myocardium.
Acknowledgements
We wish to acknowledge the following for their help in conducting this study. The Council of Scientific & Industrial Research for financial support, The Director, SCTIMST for facilities for the study, Dr. P. Sankara Sarma, Ac hutha Menon Center for Health Science Studies, SCTIMST for statistical analysis and Dr. PR. Umashankar, Vivarium, SCTIMST for providing the porcine hearts required for the study.
References
- SaririanM, EisenbergMJ. Myocardial laser revascularization for the treatment of end-stage coronary artery disease.J Am CollCardiol. 2003; 41: 173-183.
- Hardy RI, Bove KE, James FW, Kaplan S, Goldman L. A histologic study of laser-induced transmyocardial channels. Lasers Surg Med. 1987; 6: 563-573.
- Horvath KA, Chiu E, Maun DC, Lomasney JW, Greene R, Pearce W, et al. Up-regulation of vascular endothelial growth factor mRNA and angiogenesis after transmyocardial laser revascularization. Ann Thorac Surg. 1999; 68: 825-829.
- Pelletier MP, Giaid A, Sivaraman S, Dorfman J, Li CM, Philip A, et al. Angiogenesis and growth factor expression in a model of transmyocardial revascularization. Ann Thorac Surg. 1998; 66: 12-18.
- Brustaert DL, Andries LJ. The endocardial endothelium. Am J Physiol. 1992; 263: H985-1002.
- ShahAM, Grocott-MasonRM, PepperCB, MebazzaA, HendersonAH, Lewis MJ, et al. The cardiac endothelium:Cardioactive mediators. ProgCardiovasc Dis. 1996; 39: 263-284.
- Kuruvilla L, Santhoshkumar TR, Kartha CC. Immortalization and characterization of porcine ventricular endocardial endothelial cells. Endothelium. 2007; 14: 35-43.
- McGuire PG, Orkin RW. Isolation of rat aortic endothelial cells by primary explant techniques and their phenotypic modulation by defined substrata. Lab Invest. 1987; 57: 94-105.
- Oh SH, KimWY, KimJH, YounesMN, El-Naggar AK, Myers JN, et al. Identification of Insulin-Like Growth Factor Binding Protein-3 as a FarnesylTransferase Inhibitor SCH66336-Induced Negative Regulator of Angiogenesis in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2006; 12: 653-661.
- Redzek A, Susak S Zecevic D. Long term survival and quality of life after myocardial revascularization with respect to age and sex distribution. Med Pregl. 2007; 60: 317-321.
- Hamman BL, White CH, Cheung EH, Hebeler RF, Kourlis H Jr, Meyers TP, et al. Transmyocardial laser revascularization causes sustained VEGF secretion. Semin Thorac Cardiovasc Surg. 2006; 18:43-45.
- Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, et al. Endocardial cells from the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012; 151: 1083-1096.