
Review Article
J Cardiovasc Disord. 2024; 10(1): 1056.
Role of Curcumin in Modulating Autophagy for CVD Treatment
Hadiseh Mohammadi#; Sahar Ghoflchi1#; Mahla Palizkaran Yazdi; Mahdieh Aliyari; Reyhane Ghayour Vatanparast; Hossein Hosseini*
¹Department of Clinical Biochemistry, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. Email: hadisehmohammadi2001@gmail.com
*Corresponding author: Hossein Hosseini, PhD Department of Clinical Biochemistry, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. Tel: 99199-91766; +985138002355 Email: h_hosseini121@yahoo.com
#These authors have been equally contributed to this article.
Received: September 18, 2024 Accepted:October 08, 2024 Published: October 15, 2024
Abstract
This comprehensive review explores the intricate relationship between curcumin and autophagy in the context of Cardiovascular Disease (CVD). It provides a detailed overview of autophagy, its important role in maintaining cellular homeostasis, and its dysregulation in different types of cardiovascular disease, including atherosclerosis, coronary artery disease, and diabetic cardiomyopathy. This review investigates the mechanisms through which curcumin modulates autophagy, highlighting its potential as a therapeutic agent. By discussing the impact of curcumin on CVD, we offer valuable insights into its potential benefits in the prevention and treatment of CVD. Overall, this review highlights the significance of curcumin-mediated autophagy modulation as a promising avenue for addressing the global burden of cardiovascular disease.
Keywords: Curcumin, Cardiovascular disease, Autophagy, Coronary artery disease, Cardiomyopathy, Atherosclerosis
Abbreviation: CVD: Cardiovascular disease; AMPK: AMP-activated protein kinase; mTOR: Mammalian target of rapamycin; ULK1: Unc-51-like kinase 1; PAS: phagophore assembly site; PI3P: Phosphatidylinositol 3-phosphate; LC3: Light chain 3; STX17: Syntaxin 17; SNAP29: Synaptosome-associated protein 2; HOPS: homotypic fusion and vacuolar protein sorting; EGCG: Epigallocatechin gallate; NF-κB: Nuclear factor-kappa B; MAPKs : Mitogen-activated protein kinase; PI3K: Phosphatidylinositol 3-kinase; Akt: Protein kinase B; VSMC: Vascular smooth muscle cells; LDLs: Low-density lipoproteins; ox-LDL: Oxidized LDL; TFEB: Transcription factor EB; HUVECs: Umbilical vein endothelial cells; PPAR: Proliferator-activated receptor; PDT: Photodynamic therapy; a-SMA: Alpha-smooth muscle actin; SM22a: smooth muscle 22a; HAC: Hydroxyl acetylated curcumin; SDT: Sonodynamic treatment; CAD: Coronary artery disease; I/R: Ischemia-reperfusion; ROS: Reactive oxidative stress; DCM: Diabetic cardiomyopathy; HIIT: High-intensity interval training.
Introduction
Cardiovascular Disease (CVD) is a leading global health concern, with millions of people affected worldwide [1]. CVD is a broad term encompassing conditions that affect the heart and blood vessels. These conditions include heart failure, arrhythmias, cardiomyopathies, congenital heart defects, aortic aneurysms, valvular heart disease, and peripheral artery disease [2]. Autophagy is a cellular degradation and recycling process highly conserved in all eukaryotes [3]. Autophagy is a lysosome-dependent catabolic process that recycles cytoplasmic components such as damaged organelles, protein aggregates, and lipid droplets [4]. Accordingly, autophagy dysfunction is associated with various human diseases [5,6]. Natural compounds like curcumin offer a promising therapeutic approach to modulate autophagy and treatment of CVD. Curcumin exhibits significant cardioprotective effects in animal models while representing low toxicity and broad safety profiles [7]. The main goal of the current review is to discuss
Autophagy
Autophagy, a fundamental cellular process, is pivotal in maintaining cellular homeostasis. This lysosome-dependent degradation pathway is indispensable for the turnover of long-lived proteins and damaged organelles. Autophagy facilitates the recycling of cellular components and supports cellular adaptation to various stress conditions, such as nutrient deprivation, oxidative stress, and pathogen infection [8]. Autophagy plays a dual role in human disease, acting as a protective and a pathogenic mechanism. While autophagy can promote cell survival and protect against disease, excessive or defective autophagy can contribute to disease pathogenesis [5]. Numerous research has shown that autophagy is dysregulated in many diseases, such as cancer, neurodegenerative disorders, and cardiovascular disease.
Types of Autophagy
Autophagy is categorized into three primary types based on cargo sequestration mechanisms. The first type is chaperone-mediated autophagy, which selectively targets specific proteins for lysosomal degradation through chaperone-protein complexes. The second type (microautophagy) involves the direct engulfment of cytoplasmic material by the lysosomal membrane. The third type (macroautophagy), the most extensively studied type, is characterized by the formation of double-membrane autophagosomes that fuse with lysosomes for cargo degradation [4,9-13]. Beyond this classification, autophagy can also be differentiated as selective or non-selective. Selective autophagy targets specific cellular components like protein aggregates, damaged organelles, or pathogens for degradation, contributing to cellular quality control. In contrast, non-selective autophagy (bulk autophagy) indiscriminately degrades cytoplasmic content. Selective and non-selective autophagy dysregulation has been implicated in various human diseases [14]. Certain types of cellular components are recycled and removed by selective autophagy. For example, protein aggregates, lipid droplets, invading microorganisms, and damaged or unnecessary mitochondria are degraded through aggrephagy, lipophagy, xenophagy, and mitophagy, respectively [14].
Signaling of Autophagy
Autophagy is a tightly regulated cellular process influenced by a complex network of signaling pathways, especially those involving AMPK (AMP-activated protein kinase) and mTOR (mammalian target of rapamycin). Under nutrient-rich conditions, mTORC1, a downstream effector of mTOR, is activated and phosphorylates ULK1 (Unc-51-like kinase 1) at Ser757 and leads to inhibiting autophagy initiation. Conversely, during nutrient deprivation or cellular stress, AMPK is activated, leading to the phosphorylation of ULK1 at Ser317 and Ser777 and promoting autophagy initiation. Additionally, AMPK can phosphorylate mTORC1, further suppressing its activity [8,15,16].
The activated ULK1 complex, composed of ULK1, FIP200, ATG13, and ATG101, is recruited to the Phagophore Assembly Site (PAS). Following ULK1 activation, recruiting the Beclin 1-Vps34 complex to the PAS is essential for autophagosome nucleation. Vps34, a class III phosphatidylinositol 3-kinase, generates phosphatidylinositol 3-Phosphate (PI3P) at the phagophore membrane. PI3P serves as a platform for recruiting autophagy proteins, including WIPI2, further amplifying PI3P production. PI3P recruits other autophagy proteins, such as adaptor proteins of the ATG12-ATG5 complex and microtubule-associated protein 1 Light Chain 3 (LC3), to the phagophore membrane [4,17-20]. Two ubiquitin conjugation systems involving the ATG16L1 and ATG5-ATG12 complex promote the elongation of the phagophore membrane. LC3 is conjugated to Phosphatidylethanolamine (PE) on the phagophore membrane to form LC3-PE, essential for autophagosome closure. Syntaxin 17 (STX17) on the autophagosome interacts with synaptosome-associated protein 2 (SNAP29) Homotypic fusion and vacuolar Protein Sorting (HOPS) on the lysosome to mediate autophagosome-lysosome fusion. After fusion with the lysosome, the inner autophagosomal membrane and its enclosed cargo are degraded by lysosomal hydrolases. The breakdown products are then transported back into the cytoplasm to be reused for cellular processes [4,8,21,22] (Figure 1).
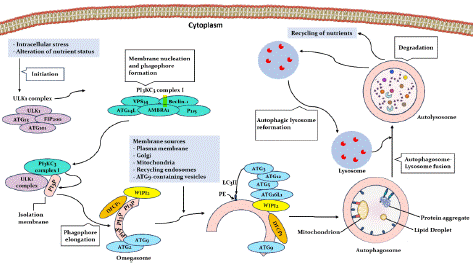
Figure 1: An overview of signaling of Autophagy.
Autophagy and Cardiovascular Diseases
According to the studies, autophagy acts as a double-edged sword in the cardiovascular system. While basal levels of autophagy are essential for maintaining cardiac function and promoting cellular survival, dysregulation of autophagy can contribute to the pathogenesis of various CVDs. Research has shown that impaired autophagy can result in the accumulation of damaged organelles and proteins, promoting cellular dysfunction and contributing to the development of atherosclerosis, myocardial infarction, and heart failure [23].
The complex interplay between autophagy and CVD is influenced by numerous factors, including genetic predisposition, environmental stressors, and aging [6,24]. Emerging evidence suggests that autophagy can be modulated by various therapeutic interventions, including pharmacological agents and lifestyle modifications [25]. Natural compounds with autophagy-modulating capacities have produced many possible candidates for treating disorders because of their low toxicity and wide safety margins. Recent studies have highlighted the therapeutic potential of natural products such as resveratrol, curcumin, berberine, trehalose, and epigallocatechin gallate (EGCG) in treating CVDs through autophagy regulation [7,26-28].
Curcumin
Curcumin, the principal curcuminoid of turmeric (Curcuma longa L.), chemically, is a diarylheptanoid consisting of two ferulic acid molecules joined by a seven-carbon chain. This unique curcumin structure allows interaction with various biological targets, including multiple kinases, transcription factors, and enzymes. The pleiotropic effects of curcumin are attributed to its ability to modulate multiple signaling pathways, such as the nuclear factor-kappa B (NF-κB), Mitogen-Activated Protein Kinase (MAPKs), and phosphatidylinositol 3-kinase (PI3K)/protein Kinase B (Akt) pathways [29-31). These effects make curcumin a promising therapeutic agent for various diseases (Figure 2).
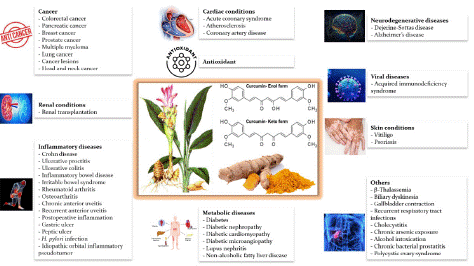
Figure 2: The therapeutic potential of curcumin against a wide range of human diseases.
A hallmark of the protective effects of curcumin is its ability to regulate autophagy. Curcumin stimulates autophagy by activating AMPK, a critical energy sensor. Concurrently, curcumin inhibits the mTOR, a central regulator of cell growth, thereby promoting the degradation of damaged cellular components. Moreover, the anti-inflammatory actions of curcumin, achieved through suppression of NF-κB, indirectly support autophagy and contribute to its overall cytoprotective effects [32,33]. Curcumin can also upregulate the expression of autophagy-related genes, such as LC3 and Beclin-1, and enhance the formation of autophagosomes [33]. The multifaceted effects of curcumin on autophagy make it an attractive therapeutic target for various diseases [34-36] (Table 1).
Authors (Ref)
Disease
Experimental model
Dose of Curcumin
Research findings
Li et al. (52)
Atherosclerosis
Human monocytic THP-1 cells
20 μmol/L
Cur enhances autophagy by regulating mTORC1 and promoting nuclear translocation of TFEB.
Cur enhances the binding of H3K4me1 and H3K27ac while reducing the binding of H3K9me3 and H3K27me3 at the ATG5 promoter.
Cur degrades BRD4 in FCs by activating autophagy.
Cur inhibits histone acetylation by binding to P300/CBP.
Cur reduces ROS.8 weeks old male C57BL/6 mice (Apoe–/–)
20 mg/kg body weight, daily
Gu et al. (58)
Atherosclerosis
THP-1 cells
1, 5, 10 μmol/L
NC increases the expression of LC3-II and decreases the level of p62.
NC enhances impaired autophagy flux via the PI3K/Akt/mTOR pathway.Zhao et al. (59)
Atherosclerosis
HUVECs cells
5 μmol/L
Cur regulates the autophagy-related AMPK/mTOR/p70S6K signaling pathway.
Cur increases the expression of PPARγ.Wang et al. (66)
Atherosclerosis
MOVAS cells
20 μmol/L
CUR-PDT increases the expression of Beclin-1 and LC3B- and the degradation of p62.
CUR-PDT increases the expression of a-SMA and SM22-a and decreases the expression of OPN.A7r5 cells
20 μmol/L
Zheng et al. (69)
Atherosclerosis
THP-1 cells
5 μg/mL
Translocation of BAX and cytochrome C, along with activation of the caspase cascade, was induced after HAC-SDT, leading to apoptosis.
HAC activates autophagy through the PI3K/AKT/mTOR pathway.
Accumulation of LC3 II, increased conversion of LC3 II/I, decrease in p62, and increase in beclin1 expression were observed during HAC-SDT.Yang et al. (77)
Coronary artery disease
Cardiomyocytes from ventricles of 1-day-old C57BL/6 mice
10 μmol/L
Cur inhibits mTOR signaling and activates autophagy.
PI3K/Akt and MAPK/Erk1/2 signaling influence autophagy by regulating the mTOR pathway.8 to 12-week-old male C57BL/6 mice
100 mg/kg body weight, daily
Deng et al. (32)
Coronary artery disease
Neonatal rat cardiomyocytes (NRCs)
5~200 μmol/L
Nap-Cur-NO reduces the levels of ROS and apoptosis (decreasing the expression of p-p38 MAPK and p-NF-κB and increasing Bcl-2).
Nap-Cur-NO inhibits autophagy by suppressing ROS-related signals (decreasing the expression levels of LC3B II/I and Beclin-1 and increasing the expression of p62).6–8 weeks, 20–25 g male C57BL/6 mice
-
Chen et al. (82)
Coronary artery disease
Rat cardiomyocyte H9c2 cells
0.5, 1.0 and 2.0 μmol/L
THC improves antioxidant activity by increasing SOD and CAT activities and decreasing MDA levels .
THC decreases the Bax/Bcl-2 ratio and caspase-3 level and inhibits apoptosis.
THC decreases Beclin1 expression and LC3 II/LC3 I ratio while increasing p62 expression. (reduction of autophagosome and autolysosome formation)
THC promotes the phosphorylation of the PI3K/AKT/mTOR pathway and induces the expression of HIF-1a.Sprague-Dawley rats, 180–200g
50 mg/kg body weight, daily
Sadeghi et al. (87)
Diabetic cardiomyopathy
8-week-old male Wistar rat, 270 ± 20g
100 mg/kg body weight, daily
Cur increases the expression of Beclin-1 and LAMP-2 genes.
Yao et al. (88)
Diabetic cardiomyopathy
H9c2 rat cardiac myoblast cells
10 μmol/L
Cur suppresses apoptosis by restoring autophagy.
Cur acts through AMPK and JNK1-mediated phosphorylation of Bcl-2 and Bim, which dissociates Beclin1-Bcl-2 (or Bim) complexes.2-months-old male C57BL/6 mice
200 mg/kg body weight, daily
Table 1: A summary of the effect of curcumin (Cur) in cardiovascular diseases through modulation of autophagy. Cur: curcumin, ROS: Reactive oxygen species, FCs: Foam cells, BRD4: Bromodomain-containing protein 4, mTOR: Mammalian target of rapamycin, HUVEC: Primary Human Umbilical Vein Endothelial Cells, PPARγ: Peroxisome proliferator-activated receptor gamma, PDT: Photodynamic therapy, Asma: Alpha Smooth Muscle Actin, LC3: light chain 3, HAC: Hydroxyl acetylated curcumin, TFEB: Transcription factor EB, NC: nicotinate-curcumin, PI3K: Phosphoinositide 3-kinases, Akt: Protein kinase B, THC: Tetrahydrocannabinol, SOD: Superoxide dismutase, CAT: Catalase, MDA: Malondialdehyde, HIF-1a: Hypoxia-inducible factor 1-alpha, AMPK: AMP-activated protein kinase, LAMP-2: Lysosome-associated membrane protein 2, JNK: c-Jun N-terminal kinases.
Although curcumin has been shown to have positive effects on health, its poor bioavailability has limited the practical applicability of curcumin. Low water solubility, inefficient absorption, rapid metabolism, and systemic clearance of curcumin limit its effectiveness when administered orally. To overcome these challenges, researchers have explored various strategies to enhance the bioavailability of curcumin, such as the use of adjuvants involving piperine and resveratrol or the development of curcumin delivery systems [37,38]. Moreover, the synthetic structure of curcumin analogs has been designed to improve its bioavailability and pharmacokinetic properties [39,40].
Curcumin and Autophagy in Cardiovascular Disease
Autophagy is essential for maintaining the cardiovascular health system. Its critical role in various cell types, especially those within the heart and blood vessels, underscores its potential as a therapeutic target for CVDs. The ability of curcumin to modulate autophagy makes it a promising candidate for the treatment of CVDs [35]. Atherosclerosis, coronary artery disease, and diabetic cardiomyopathy are three major types of cardiovascular diseases. In the following sections, we will delve into the role of curcumin in modulating autophagy within these specific cardiovascular diseases (Figure 3).
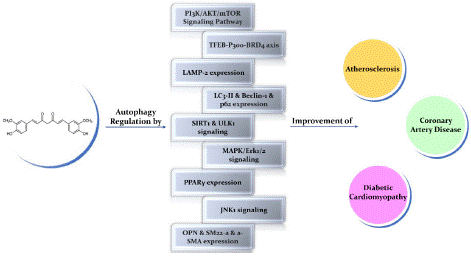
Figure 3: The protective impacts of curcumin in cardiovascular disease by regulating autophagy.
Curcumin and Autophagy in Atherosclerosis
Atherosclerosis is a chronic inflammatory disorder defined by the accumulation of plaques containing lipids in the artery walls due to several risk factors, such as high blood pressure, diabetes, smoking, or elevated cholesterol [41]. Several lines of evidence suggest that autophagy malfunctions play a critical role in the development of atherosclerotic plaques by affecting important cell types involved in the disease process, including macrophages, Vascular Smooth Muscle Cells (VSMC), and endothelial cells [42]. Although the deficiency of autophagy in each cell type plays a role in plaque progression, the underlying mechanisms are different. In this regard, abnormal autophagy in macrophages increases necrosis and apoptosis, and this, in turn, promotes plaque instability. Endothelial cells exhibit apoptosis and aging, while VSMC, which lack autophagy mechanisms, contribute to plaque formation by promoting senescence [41-45]. These cell-specific roles of autophagy in atherosclerosis highlight its potential as a strategic target for the development of novel therapeutic interventions to combat this disease.
Recent studies show that autophagy is critical in intracellularly processing Low-Density Lipoproteins (LDLs) within foam cells. LDLs are engulfed by autophagosomes and transported to lysosomes for degradation. Studies have shown that impaired autophagy flux contributes to lipid accumulation and foam cell formation, while activation of autophagy counteracts these processes. Therefore, enhancing autophagy function in foam cells presents a promising therapeutic strategy for improving atherosclerosis [46-51]. Studies have shown that curcumin could mitigate atherosclerosis by autophagy regulation, highlighting its potential as a therapeutic agent [23]. In this regard, Li et al. showed that oxidized LDL (ox-LDL) induces aberrant crosstalk between autophagy and inflammation in foam cells, mediated by the TFEB-P300-BRD4 axis, and this dysregulation contributes to atherogenesis. This group suggested that curcumin attenuates inflammation and promotes autophagy in foam cells by restoring the balance between these cellular processes, thereby mitigating the progression of atherosclerosis [52].
TFEB (Transcription factor EB), a master regulator of the autophagy-lysosome system, is typically phosphorylated by mTORC1 and inactivated [53]. Li et al. reported curcumin-induced autophagy through nuclear translocation of TFEB in foam cells. The nuclear translocation of TFEB was not significantly affected by the pharmacological suppression of autophagy, suggesting that TFEB was the upstream regulator of autophagy. Results of this study showed that the expression of p-mTOR was downregulated by curcumin and partially reversed by TFEB silencing in foam cells [54]. These findings suggested that curcumin promotes mTORC1-dependent TFEB nuclear translocation and enhances autophagy in foam cells. Additionally, curcumin influences chromatin accessibility by increasing H3K4me1 and H3K27ac binding to the ATG5 promoter while decreasing H3K9me3 and H3K27me3 binding to the ATG5 promoter. This chromatin remodeling likely contributes to TFEB-mediated autophagy activation. These results highlight the critical role of TFEB nuclear translocation and subsequent autophagy activation in curcumin-mediated lipid degradation and atherosclerosis reduction [52]. In another part of the study, Li et al. observed that BRD4 expression is significantly upregulated in foam cells; however, curcumin effectively inhibits this upregulation, likely by inducing autophagy-mediated degradation of BRD4. Given the role of BRD4 as a transcriptional autophagy regulator, its knockdown partially alleviates autophagy suppression in foam cells [52,55]. Li et al. observed that macrophage-specific TFEB knockout prevents curcumin-induced LC3 upregulation, H3 activation, and Reactive Oxidative Stress (ROS) reduction. These findings suggest that activated TFEB negatively regulates BRD4 in curcumin-treated foam cells [52]. These results demonstrate that curcumin effectively reduces BRD4 expression through autophagy activation, contributing to its anti-inflammatory and anti-atherosclerotic effects. Histone acetyltransferases, such as P300, are involved in histone acetylation. While the role of P300 in atherosclerosis is complex, studies suggest both pro- and anti-inflammatory effects [56]. Lu et al. reported that P300 promotes atherogenesis by stimulating proinflammatory genes in VSMC, while curcumin directly binds to P300/CBP and prevents histone acetylation [57]. As an additional mechanism, Li et al., in a different section of their study, proposed that curcumin reduces ROS, a known activator of P300, which led to decreased P300 activity and subsequent autophagy induction via TFEB activation [52]. Overall, Li et al. propose that by inhibiting mTORC1, curcumin facilitates TFEB nuclear translocation, restoring autophagy and autophagic flux in ox-LDL-injured foam cells. Simultaneously, curcumin suppresses ROS generation via autophagy activation, leading to decreased P300-mediated histone acetylation. This epigenetic modulation reduces BRD4 occupancy on inflammatory gene promoters, ultimately leading to inflammation downregulating. These findings underscore the multifaceted anti-inflammatory and autophagy-inducing properties of curcumin [52].
In another study, Gu et al. demonstrated impaired autophagy flux, as evidenced by reduced LC3-II and increased p62 levels in THP-1 cells exposed to ox-LDL. Their results showed that nicotinate-curcumin, a water-soluble curcumin derivative, restored autophagy flux, decreased foam cell formation, and lowered cholesterol levels in these cells [58]. In this regard, Zhao et al. investigated the protective effects of curcumin against ox-LDL-induced endothelial lipotoxicity in human umbilical vein endothelial cells (HUVECs) [59]. High levels of ox-LDL caused endothelial dysfunction, inflammation, and oxidative stress in HUVECs. Treatment with curcumin mitigated these effects by improving endothelial function, reducing tube formation and migration, and increasing peroxisome proliferator-activated receptor (PPARγ) expression. Mechanistically, curcumin regulated the autophagy-related AMPK/mTOR/p70S6K signaling pathway to protect endothelial cells [59]. Previous work by the same group showed that curcumin reduces ER stress and LOX1 expression in HUVECs exposed to palmitic acid [60]. These findings highlight the protective effects of curcumin on endothelial cells through autophagy induction and reduction of oxidative stress.
Studies have shown that activation of autophagy prevents atherosclerosis by inhibiting VSMC phenotypic switching from contractile to synthetic states and reducing their ability to proliferate and migrate these cells [61]. It has been reported that Photodynamic Therapy (PDT) can induce necrosis, apoptosis, and autophagy in target tissues. Given the unique impact of atherosclerotic plaques on the absorption and retention of curcumin, combining curcumin with PDT offers a promising approach to the treatment of atherosclerosis [62-65]. In this regard, Wang et al. investigated the effects of curcumin-PDT on ox-LDL-treated VSMCs. The combination therapy induced autophagy, as evidenced by increased LC3-II and Beclin-1 expression and decreased p62 levels. Furthermore, curcumin-PDT promoted the contractile VSMC phenotype through upregulated a-SMA (alpha-smooth muscle actin) and SM22a (smooth muscle 22a) and downregulated osteopontin as a synthetic marker [66]. These findings suggest that curcumin-PDT prevents VSMC phenotypic switching and foam cell formation through autophagy activation. Another study explored the combination of Hydroxyl Acetylated Curcumin (HAC) and Sonodynamic Treatment (SDT). HAC, a superior sonosensitizer to curcumin, effectively generates ROS upon ultrasound activation. While ROS typically induces apoptosis, in this context, it also stimulates autophagy [67,68]. The combination of HAC and SDT exhibited enhanced efficacy compared to individual treatments [69]. Zheng et al. demonstrated that HAC-SDT triggered both autophagy, mediated by the PI3K/AKT/mTOR pathway, and apoptosis, initiated by the mitochondrial-caspase cascade. HAC-SDT effectively inhibited foam cell formation in macrophages during atherosclerosis progression by inducing autophagy [69]. These findings underscore the pivotal role of ROS in this process and the potential of HAC-SDT as a therapeutic strategy.
Curcumin and Autophagy in Coronary Artery Disease
Atherosclerosis often leads to Coronary Artery Disease (CAD) and subsequent myocardial infarction. Both ischemic and reperfusion phases of myocardial injury stimulate cardiomyocyte autophagy. While these phases share similarities in inducing cellular stress, they differ in oxygen and nutrient availability and ROS production [23]. Depletion of ATP caused by ischemia stimulates AMPK activation and mTOR inhibition, leading to enhanced autophagy. This process is crucial for maintaining cellular energy and cardiomyocyte survival [70,71]. Studies have shown that improving autophagy with inducers can mitigate myocardial damage and improve cardiac outcomes following ischemic injury. Despite restored oxygen and nutrient supply during reperfusion, excessive ROS production occurs. This amplified oxidative stress activates autophagy, paradoxically leading to the exacerbation of reperfusion injury and cardiomyocyte death. While autophagy is beneficial during ischemia to maintain cell survival, its role during reperfusion becomes detrimental [23,70-75]. Curcumin has shown promise as a therapeutic for myocardial Ischemia-Reperfusion (I/R) injury [76]. Studies have demonstrated that curcumin promotes autophagy by inhibiting mTOR signaling; however, the role of autophagy in I/R injury is complex: beneficial during ischemia but detrimental during reperfusion [76]. Combination therapies involving curcumin and other agents have been explored to optimize therapeutic effects. For instance, combining curcumin with D942 enhances autophagy and reduces myocardial damage (77). Additionally, a hydrogel delivering curcumin and nitric oxide has shown promise in protecting cardiomyocytes from I/R injury by reducing oxidative stress and inhibiting autophagy [78-81]. Tetrahydrocurcumin, a derivative of curcumin, has also demonstrated cardioprotective effects. This compound reduces myocardial infarct size, improves cardiac function, and inhibits autophagy while promoting antioxidant defenses [82]. These findings highlight the complex interplay between autophagy, oxidative stress, and myocardial injury.
Curcumin and Autophagy in Diabetic Cardiomyopathy
Diabetic Cardiomyopathy (DCM) is a significant cause of diabetes-related mortality, affecting two-thirds of diabetic individuals [83]. DCM is characterized by left ventricular dysfunction, dilation, and cardiomyocyte death. Myocardial hypertrophy, fibrosis, and diastolic dysfunction are common cardiac abnormalities associated with DCM. Excessive fatty acid utilization in diabetic hearts leads to metabolic rigidity, increased oxidative stress, mitochondrial dysfunction, and, ultimately, heart failure [83]. The role of autophagy in diabetic cardiomyopathy is complex. While autophagy is initially activated, prolonged high-fat diet feeding suppresses cardiac autophagic flux. Evidence suggests that autophagy and mitophagy protect the heart through clearing damaged mitochondria and cellular waste. In line with this, inhibiting autophagy exacerbates cardiac dysfunction in diabetic models [23,84-86]. Notably, administering an autophagy inducer improved autophagy, mitophagy and cardiac function in diabetic mice. These findings highlight the potential of autophagy and mitophagy as therapeutic targets for preventing diabetic cardiomyopathy [84-86]. Curcumin has shown protective effects in diabetic cardiomyopathy by inducing autophagy-related cell death. A study combining High-Intensity Interval Training (HIIT) with curcumin showed increased expression of autophagy-related genes, suggesting a synergistic effect in improving cardiac function [87]. protective mechanisms of curcumin involve inhibiting cell death and restoring autophagy. In this regard, Yao et al. reported that curcumin by phosphorylating Bcl-2 and Bim, disrupts their interaction with Beclin-1, and promotes autophagy. Additionally, AMPK activation and subsequent mTOR inhibition contribute to this process [88].
Autophagy and apoptosis are intricately linked through shared regulatory proteins. Beclin-1, a key autophagy protein, interacts with pro- and anti-apoptotic proteins like Bcl-2 and Bim. This interaction inhibits autophagy by preventing the association of Beclin-1 with Vps34, a critical autophagy initiator. However, phosphorylation of Bcl-2 or Bim by JNK1 disrupts this complex, promoting autophagy [89-93]. The protective effects of curcumin in diabetic cardiomyopathy involve a complex interplay between autophagy and apoptosis. By phosphorylation of Bcl-2 and Bim, curcumin promotes autophagy and inhibits apoptosis. This process involves AMPK and JNK signaling, but the exact mechanisms underlying their interaction require further investigation [88]. Understanding the intricate relationship between autophagy and apoptosis is crucial for developing effective therapeutic strategies for diabetic cardiomyopathy.
Discussion and Conclusion
CVD is a leading global health concern, with millions of people affected worldwide. The intricate interplay between autophagy and cardiovascular health has opened new avenues for therapeutic interventions, mainly using natural compounds such as curcumin. Autophagy, a critical cellular process for maintaining homeostasis, plays a pivotal role in CVD [86]. Nutrient-sensing pathways, including AMPK, mTOR, and SIRT1, regulate this process, which responds to cellular stressors like oxidation and starvation [94,95]. Curcumin has emerged as a promising therapeutic agent due to its multifaceted properties. Antioxidant, anti-inflammatory, and autophagy-modulating capabilities offer significant potential for curcumin in combating several diseases [29,30]. Dysregulation of autophagy is implicated in the pathogenesis of several CVDs. Therefore, modulating autophagy through compounds like curcumin presents a promising therapeutic approach. Curcumin, by promoting autophagy, can help clear damaged cellular components, reduce oxidative stress, and mitigate inflammation, potentially improving cardiovascular health and preventing disease progression [28].
In conclusion, the multifaceted role of curcumin in modulating autophagy, reducing oxidative stress, and inhibiting inflammation makes it a compelling candidate for the treatment of CVDs. Future research should optimize its bioavailability and further elucidate the molecular mechanisms underlying its therapeutic effects. Such efforts will be crucial in translating the promising preclinical findings into effective clinical interventions for cardiovascular health.
Author Statements
Competing Interests
The authors declare no conflict of interest.
Authors' Contributions
All authors contributed to writing the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We gratefully acknowledge the contributions of the data collection team and the individuals who participated in this study. This project was implemented in collaboration with Mashhad University of Medical Sciences.
References
- Mendis S, Puska P, Norrving B, World Health O, World Heart F, World Stroke O. Global atlas on cardiovascular disease prevention and control / edited by: Shanthi Mendis. Geneva: World Health Organization. 2011.
- Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 385: 117-71.
- Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014; 20: 460-73.
- Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021; 17: 647-61.
- Wirawan E, Vanden Berghe T, Lippens S, Agostinis P, Vandenabeele P. Autophagy: for better or for worse. Cell Res. 2012; 22: 43-61.
- Rabinovich-Nikitin I, Kirshenbaum E, Kirshenbaum LA. Autophagy, Clock Genes, and Cardiovascular Disease. Can J Cardiol. 2023; 39: 1772-80.
- Wu X, Liu Z, Yu XY, Xu S, Luo J. Autophagy and cardiac diseases: Therapeutic potential of natural products. Med Res Rev. 2021; 41: 314-41.
- Liu S, Yao S, Yang H, Liu S, Wang Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023; 14: 648.
- Hurley JH, Young LN. Mechanisms of Autophagy Initiation. Annu Rev Biochem. 2017; 86: 225-44.
- Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018; 19: 349-64.
- Nakatogawa H, Ishii J, Asai E, Ohsumi Y. Atg4 recycles inappropriately lipidated Atg8 to promote autophagosome biogenesis. Autophagy. 2012; 8: 177-86.
- Melia TJ, Lystad AH, Simonsen A. Autophagosome biogenesis: From membrane growth to closure. J Cell Biol. 2020; 219: e20002085.
- Lawrence RE, Zoncu R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat Cell Biol. 2019; 21: 133-42.
- Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023; 24: 167-85.
- Jeon SM. Regulation and function of AMPK in physiology and diseases. Experimental & Molecular Medicine. 2016; 48: e245.
- Torii S, Yoshida T, Arakawa S, Honda S, Nakanishi A, Shimizu S. Identification of PPM1D as an essential Ulk1 phosphatase for genotoxic stress-induced autophagy. EMBO Rep. 2016; 17: 1552-64.
- Zhou Y, Manghwar H, Hu W, Liu F. Degradation Mechanism of Autophagy-Related Proteins and Research Progress. Int J Mol Sci. 2022; 23.
- Karabiyik C, Vicinanza M, Son SM, Rubinsztein DC. Glucose starvation induces autophagy via ULK1-mediated activation of PIKfyve in an AMPK-dependent manner. Developmental Cell. 2021; 56: 1961-75. e5.
- Brier LW, Ge L, Stjepanovic G, Thelen AM, Hurley JH, Schekman R. Regulation of LC3 lipidation by the autophagy-specific class III phosphatidylinositol-3 kinase complex. Molecular biology of the cell. 2019; 30: 1098-107.
- Fracchiolla D, Chang C, Hurley JH, Martens S. A PI3K-WIPI2 positive feedback loop allosterically activates LC3 lipidation in autophagy. J Cell Biol. 2020; 219.
- Shen Q, Shi Y, Liu J, Su H, Huang J, Zhang Y, et al. Acetylation of STX17 (syntaxin 17) controls autophagosome maturation. Autophagy. 2021; 17: 1157-69.
- Xu Y, Wan W. Acetylation in the regulation of autophagy. Autophagy. 2023; 19: 379-87.
- Abdellatif M, Ljubojevic-Holzer S, Madeo F, Sedej S. Autophagy in cardiovascular health and disease. Prog Mol Biol Transl Sci. 2020; 172: 87-106.
- Koutouroushis C, Sarkar O. Role of Autophagy in Cardiovascular Disease and Aging. Cureus. 2021; 13: e20042.
- Miyamoto S. Autophagy and cardiac aging. Cell Death Differ. 2019; 26: 653-64.
- Liu S, Ren J, Liu S, Zhao X, Liu H, Zhou T, et al. Resveratrol inhibits autophagy against myocardial ischemia-reperfusion injury through the DJ-1/MEKK1/JNK pathway. Eur J Pharmacol. 2023; 951: 175748.
- Zahedi N, Pourajam S, Zaker E, Kouhpayeh S, Mirbod SM, Tavangar M, et al. The potential therapeutic impacts of trehalose on cardiovascular diseases as the environmental-influenced disorders: An overview of contemporary findings. Environ Res. 2023; 226: 115674.
- Hashemzaei M, Entezari Heravi R, Rezaee R, Roohbakhsh A, Karimi G. Regulation of autophagy by some natural products as a potential therapeutic strategy for cardiovascular disorders. Eur J Pharmacol. 2017; 802: 44-51.
- Rózanski G, Kujawski S, Newton JL, Zalewski P, Slomko J. Curcumin and Biochemical Parameters in Metabolic-Associated Fatty Liver Disease (MAFLD)-A Review. Nutrients. 2021; 13(8).
- Geng-Ruei C, Hsieh WT, Chou LS, Lin CS, Wu CF, Lin JW, et al. Curcumin Improved Glucose Intolerance, Renal Injury, and Nonalcoholic Fatty Liver Disease and Decreased Chromium Loss through Urine in Obese Mice. Processes. 2021; 9: 1132.
- Chang GR, Hsieh WT, Chou LS, Lin CS, Wu CF, Lin JW, et al. Curcumin Improved Glucose Intolerance, Renal Injury, and Nonalcoholic Fatty Liver Disease and Decreased Chromium Loss through Urine in Obese Mice. Processes. 2021; 9: 1132.
- Deng Y, Chen G, Ye M, He Y, Li Z, Wang X, et al. Bifunctional Supramolecular Hydrogel Alleviates Myocardial Ischemia/Reperfusion Injury by Inhibiting Autophagy and Apoptosis. J Biomed Nanotechnol. 2018; 14: 1458-70.
- Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L, et al. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012; 8: 812-25.
- Peng Y, Ao M, Dong B, Jiang Y, Yu L, Chen Z, et al. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des Devel Ther. 2021; 15: 4503-25.
- Pourbagher-Shahri AM, Farkhondeh T, Ashrafizadeh M, Talebi M, Samargahndian S. Curcumin and cardiovascular diseases: Focus on cellular targets and cascades. Biomed Pharmacother. 2021; 136: 111214.
- Li H, Sureda A, Devkota HP, Pittalą V, Barreca D, Silva AS, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. 2020; 38: 107343.
- Mehanny M, Hathout RM, Geneidi AS, Mansour S. Exploring the use of nanocarrier systems to deliver the magical molecule; Curcumin and its derivatives. J Control Release. 2016; 225: 1-30.
- Squillaro T, Cimini A, Peluso G, Giordano A, Melone MAB. Nano-delivery systems for encapsulation of dietary polyphenols: An experimental approach for neurodegenerative diseases and brain tumors. Biochem Pharmacol. 2018; 154: 303-17.
- Rajitha B, Belalcazar A, Nagaraju GP, Shaib WL, Snyder JP, Shoji M, et al. Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett. 2016; 373: 227-33.
- Rajitha B, Nagaraju GP, Shaib WL, Alese OB, Snyder JP, Shoji M, et al. Novel synthetic curcumin analogs as potent antiangiogenic agents in colorectal cancer. Mol Carcinog. 2017; 56: 288-99.
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019; 5: 56.
- Grootaert MOJ, Roth L, Schrijvers DM, De Meyer GRY, Martinet W. Defective Autophagy in Atherosclerosis: To Die or to Senesce? Oxid Med Cell Longev. 2018; 2018: 7687083.
- Ackermann K, Bonaterra GA, Kinscherf R, Schwarz A. Growth differentiation factor-15 regulates oxLDL-induced lipid homeostasis and autophagy in human macrophages. Atherosclerosis. 2019; 281: 128-36.
- Osonoi Y, Mita T, Azuma K, Nakajima K, Masuyama A, Goto H, et al. Defective autophagy in vascular smooth muscle cells enhances cell death and atherosclerosis. Autophagy. 2018; 14: 1991-2006.
- Kheloufi M, Vion AC, Hammoutene A, Poisson J, Lasselin J, Devue C, et al. Endothelial autophagic flux hampers atherosclerotic lesion development. Autophagy. 2018; 14: 173-5.
- Barascuk N, Skjųt-Arkil H, Register TC, Larsen L, Byrjalsen I, Christiansen C, Karsdal MA. Human macrophage foam cells degrade atherosclerotic plaques through cathepsin K mediated processes. BMC Cardiovasc Disord. 2010; 10: 19.
- Doonan RJ, Hafiane A, Lai C, Veinot JP, Genest J, Daskalopoulou SS. Cholesterol efflux capacity, carotid atherosclerosis, and cerebrovascular symptomatology. Arterioscler Thromb Vasc Biol. 2014; 34: 921-6.
- Weibel GL, Drazul-Schrader D, Shivers DK, Wade AN, Rothblat GH, Reilly MP, et al. Importance of evaluating cell cholesterol influx with efflux in determining the impact of human serum on cholesterol metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2014; 34: 17-25.
- Mei S, Gu H, Ward A, Yang X, Guo H, He K, et al. p38 mitogen-activated protein kinase (MAPK) promotes cholesterol ester accumulation in macrophages through inhibition of macroautophagy. J Biol Chem. 2012; 287: 11761-8.
- Li BH, Yin YW, Liu Y, Pi Y, Guo L, Cao XJ, et al. TRPV1 activation impedes foam cell formation by inducing autophagy in oxLDL-treated vascular smooth muscle cells. Cell Death Dis. 2014; 5: e1182.
- Kim HS, Montana V, Jang HJ, Parpura V, Kim JA. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: a potential role for reducing lipid accumulation. J Biol Chem. 2013; 288: 22693-705.
- Li X, Zhu R, Jiang H, Yin Q, Gu J, Chen J, et al. Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-P300-BRD4 axis. Acta Pharm Sin B. 2022; 12: 2280-99.
- Napolitano G, Esposito A, Choi H, Matarese M, Benedetti V, Di Malta C, et al. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat Commun. 2018; 9: 3312.
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015; 17: 288-99.
- Sakamaki JI, Wilkinson S, Hahn M, Tasdemir N, OPrey J, Clark W, et al. Bromodomain Protein BRD4 Is a Transcriptional Repressor of Autophagy and Lysosomal Function. Mol Cell. 2017; 66: 517-32.e9.
- Lu Y, Zhang L, Liao X, Sangwung P, Prosdocimo DA, Zhou G, et al. Kruppel-like factor 15 is critical for vascular inflammation. J Clin Invest. 2013; 123: 4232-41.
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004; 279: 51163-71.
- Gu HF, Li HZ, Tang YL, Tang XQ, Zheng XL, Liao DF. Nicotinate-Curcumin Impedes Foam Cell Formation from THP-1 Cells through Restoring Autophagy Flux. PLoS One. 2016; 11: e0154820.
- Zhao L, Luo R, Yu H, Li S, Yu Q, Wang W, et al. Curcumin protects human umbilical vein endothelial cells against high oxidized low density lipoprotein-induced lipotoxicity and modulates autophagy. Iran J Basic Med Sci. 2021; 24: 1734-42.
- Luo R, Zhao L, Li S, Chen P, Wang L, Yu H, et al. Curcumin Alleviates Palmitic Acid-Induced LOX-1 Upregulation by Suppressing Endoplasmic Reticulum Stress in HUVECs. Biomed Res Int. 2021; 2021: 9983725.
- Grootaert MO, da Costa Martins PA, Bitsch N, Pintelon I, De Meyer GR, Martinet W, et al. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy. 2015; 11: 2014-32.
- Huang L, Chen Q, Yu L, Bai D. Pyropheophorbide-a methyl ester-mediated photodynamic therapy induces apoptosis and inhibits LPS-induced inflammation in RAW264.7 macrophages. Photodiagnosis Photodyn Ther. 2019; 25: 148-56.
- Rkein AM, Ozog DM. Photodynamic therapy. Dermatol Clin. 2014; 32: 415-25, x.
- Li KT, Chen Q, Wang DW, Duan QQ, Tian S, He JW, et al. Mitochondrial pathway and endoplasmic reticulum stress participate in the photosensitizing effectiveness of AE-PDT in MG63 cells. Cancer Med. 2016; 5: 3186-93.
- Jain M, Zellweger M, Wagnières G, van den Bergh H, Cook S, Giraud MN. Photodynamic therapy for the treatment of atherosclerotic plaque: Lost in translation? Cardiovasc Ther. 2017; 35.
- Wang G, Zhu Y, Li K, Liao B, Wang F, Shao L, et al. Curcumin-mediated Photodynamic Therapy Inhibits the Phenotypic Transformation, Migration, and Foaming of Oxidized Low-density Lipoprotein-treated Vascular Smooth Muscle Cells by Promoting Autophagy. J Cardiovasc Pharmacol. 2021; 78: 308-18.
- Su X, Wang P, Yang S, Zhang K, Liu Q, Wang X. Sonodynamic therapy induces the interplay between apoptosis and autophagy in K562 cells through ROS. Int J Biochem Cell Biol. 2015; 60: 82-92.
- Wang X, Wang P, Zhang K, Su X, Hou J, Liu Q. Initiation of autophagy and apoptosis by sonodynamic therapy in murine leukemia L1210 cells. Toxicol In Vitro. 2013; 27: 1247-59.
- Zheng L, Li Y, Li X, Kou J, Zhong Z, Jiang Y, et al. Combination of Hydroxyl Acetylated Curcumin and Ultrasound Induces Macrophage Autophagy with Anti-Apoptotic and Anti-Lipid Aggregation Effects. Cell Physiol Biochem. 2016; 39: 1746-60.
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007; 100: 914-22.
- Yan J, Yan JY, Wang YX, Ling YN, Song XD, Wang SY, et al. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br J Pharmacol. 2019; 176: 3126-42.
- Sciarretta S, Yee D, Nagarajan N, Bianchi F, Saito T, Valenti V, et al. Trehalose-Induced Activation of Autophagy Improves Cardiac Remodeling After Myocardial Infarction. J Am Coll Cardiol. 2018; 71: 1999-2010.
- Kanamori H, Takemura G, Goto K, Maruyama R, Ono K, Nagao K, et al. Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol. 2011; 300: H2261-71.
- Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, et al. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013; 288: 915-26.
- Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012; 125: 3170-81.
- Mokhtari-Zaer A, Marefati N, Atkin SL, Butler AE, Sahebkar A. The protective role of curcumin in myocardial ischemia-reperfusion injury. J Cell Physiol. 2018; 234: 214-22.
- Yang K, Xu C, Li X, Jiang H. Combination of D942 with curcumin protects cardiomyocytes from ischemic damage through promoting autophagy. J Cardiovasc Pharmacol Ther. 2013; 18: 570-81.
- Gao J, Zheng W, Zhang J, Guan D, Yang Z, Kong D, et al. Enzyme-controllable delivery of nitric oxide from a molecular hydrogel. Chem Commun (Camb). 2013; 49: 9173-5.
- Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013; 65: 667-79.
- Yang C, Wang Z, Ou C, Chen M, Wang L, Yang Z. A supramolecular hydrogelator of curcumin. Chem Commun (Camb). 2014; 50: 9413-5.
- Chen G, Li J, Song M, Wu Z, Zhang W, Wang Z, et al. A Mixed Component Supramolecular Hydrogel to Improve Mice Cardiac Function and Alleviate Ventricular Remodeling after Acute Myocardial Infarction. Advanced Functional Materials. 2017; 27: 1701798.
- Chen X, Xie Q, Zhu Y, Xu J, Lin G, Liu S, et al. Cardio-protective effect of tetrahydrocurcumin, the primary hydrogenated metabolite of curcumin in vivo and in vitro: Induction of apoptosis and autophagy via PI3K/AKT/mTOR pathways. Eur J Pharmacol. 2021; 911: 174495.
- Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018; 122: 624-38.
- Hsu HC, Chen CY, Lee BC, Chen MF. High-fat diet induces cardiomyocyte apoptosis via the inhibition of autophagy. Eur J Nutr. 2016; 55: 2245-54.
- Che Y, Wang ZP, Yuan Y, Zhang N, Jin YG, Wan CX, et. al. Role of autophagy in a model of obesity: A long-term high fat diet induces cardiac dysfunction. Mol Med Rep. 2018; 18: 3251-61.
- Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, et al. Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy. Circ Res. 2019; 124: 1360-71.
- Sadeghi S, Delphan M, Shams M, Esmaeili F, Shanaki-Bavarsad M, Shanaki M. The high-intensity interval training (HIIT) and curcumin supplementation can positively regulate the autophagy pathway in myocardial cells of STZ-induced diabetic rats. BMC Res Notes. 2023; 16: 21.
- Yao Q, Ke ZQ, Guo S, Yang XS, Zhang FX, Liu XF, et al. Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J Mol Cell Cardiol. 2018; 124: 26-34.
- Zhu H, He L. Beclin 1 biology and its role in heart disease. Curr Cardiol Rev. 2015; 11: 229-37.
- Ewings KE, Wiggins CM, Cook SJ. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle. 2007; 6: 2236-40.
- Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis. 2014; 19: 555-66.
- Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014; 114: 549-64.
- Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf). 2009; 196: 65-80.
- Sanches-Silva A, Testai L, Nabavi SF, Battino M, Pandima Devi KP, Tejada S, et al. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol Res. 2020; 152: 104626.
- Ding X, Zhu C, Wang W, Li M, Ma C, Gao B. SIRT1 is a regulator of autophagy: Implications for the progression and treatment of myocardial ischemia-reperfusion. Pharmacol Res. 2024; 199: 106957.