
Research Article
J Cardiovasc Disord. 2015;2(1): 1007.
Effect of Polyunsaturated Fatty Acids on the Evolution of Cognitive ability in Elderly Patients with Alzheimer’s Disease
Takashi Yamazaki*, Ken Nagata, Daiki Takano,Mayumi Saito, Tomomi Shinoda, Rena Muraoka,Yuchi Satoh, Yumi Fujimaki, and Tetsuya Maeda
Department of Neurology, Research Institute for Brain and Vessels, Japan
*Corresponding author: Takashi Yamazaki, Department of Neurology, Research Institute for Brain and Blood Vessels 6-10 Senshu-Kubota-Machi, Akita 010-0874, Japan
Received: October 29, 2014; Accepted: February 23, 2015; Published: February 24, 2015
Abstract
As the disease modifying therapy is not yet available for Alzheimer’s disease (AD), the management of modifiable Vascular Risk Factors (VRFs) including lipid metabolism is now considered to be the best strategy to minimize the impact of AD lesions, especially in elderly subjects.
Objective: To elucidate the effect of Polyunsaturated Fatty Acids (PUFAs) on the cognitive ability, we investigated the relationship between the plasma PUFA profile and neuropsychological performance in elderly AD patients.
Methods: Present study was based on133 elderly patients (51 men and 82 women) with probable AD, and their mean age was 78.6 years. Mini-mental State Exam (MMSE) and clock drawing test were used for neuropsychological evaluation. Blood samples were obtained for the measurement of PUFA profiles. Neuropsychological evaluation was repeated with one-year interval in 49 subjects, who were classified into two categories; stable group in which the MMSE score was c\unchanged or improved and deteriorating group in which the MMSE score worsened. A Receiver Operating Characteristic (ROC) curve was used to evaluate the relationship between the EPA/AA ratio and the evolution of cognitive ability.
Results: Total MMSE score correlated positively with the Eicosapentaenoic Acid (EPA)/ Arachidonic Acid (AA) ratio, and negatively with AA concentration. In the ROC curve analysis, the threshold EPA/AA ratio was estimated at 0.67 for the stable MMSE score with 66% sensitivity and 70% specificity [Odds Ratio (OR) = 4.43].
Conclusion: The EPA/AA ratio can be regarded as a predictive marker for the preservation of cognitive ability in elderly AD patients.
Keywords: Vascular Risk Factors; Alzheimer’s Disease; Eicosapentaenoic Acid (EPA); Arachidonic Acid (AA); Polyunsaturated Fatty Acid (PUFA); EPA/ AA Ratio
Introduction
According to the epidemiological studies, Vascular Risk Factors (VRFs) including hypertension, diabetes, dyslipidemia, heart failure and cerebrovascular lesions are associated with the onset and progression of Alzheimer’s Disease (AD) [1-2]. Such VRFs are also known to be the strong risk for vascular dementia and stroke, and they are regarded as common modifiable risk factors for AD and vascular dementia [3]. Since the disease modifying therapy is not yet available in the management of AD patients, treating modifiable VRFs is now considered to be the best strategy to minimize the impact of AD lesions, especially in elderly subjects [3].
The role of nutrition, a potentially modifiable vascular factor, and particularly that of dietary Polyunsaturated Fatty Acids (PUFA) has been drawing increasing attention for the prevention of cognitive decline and dementia. A number of epidemiological, clinical and experimental studies suggested the protective effects of the long chain omega-3 PUFAs such as Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) against coronary heart disease, arrhythmia atherosclerosis and hypertension [4-7].In addition to the vascular effects, the long chain omega-3 PUFAs are considered to be crucial to brain development and normal brain functioning [6]. Plasma omega-3 PUFAs were inversely related to the risk of dementia and depression [8]. Several epidemiological studies showed that fish consumption which is the primary dietary source of omega-3 PUFAs was associated with a reduced risk of cognitive decline and dementia [9-14]. Although the previous studies suggested that PUFAs were associated with the cognitive function in elderly subjects, the relationship between the PUFA profile and evolution of cognitive function in AD patients is still to be clarified. In the present study, we analyzed the PUFA profile in relation to the cognitive performance in Japanese elderly AD patients.
Subjects and Methods
The present study was observational clinical study focusing on the role of PUFAs as a clinical marker for the evolution of cognitive changes in probable AD patients.Weenrolled133 patients (51 men and 82 women) who were diagnosed as having probable AD according to the NINCDS-ADRDA criteria. The irmean age was 78.6 years. Inclusion criteria were diagnosis of probable AD and general health status that would not interfere with the patient’s ability to visit our memory clinic in the Research Institute for Brain and Blood Vessels. Exclusion criteria were non-AD dementia, residence in a long-term care facility at the time of screening, and a history of stroke, cancer, liver disease, severe arrhythmia, major psychiatric disorders, or other major central nervous system diseases. This study was approved by the institution ethics committee, and all subjects provided informed written consent.
Fasting blood samples were collected from all subjects at baseline. Laboratory tests included measurements of Fasting Blood Sugar (FBS), insulin, low-density lipoprotein cholesterol (LDL-cholesterol), High-Density Lipoprotein cholesterol (HDL-cholesterol), plasma PUFA concentration including EPA, DHA and Arachidonic Acid (AA), Brain Natriuretic Peptide (BNP) as well as Apo Ee4. EPA/AA and ω-3/ω-6 ratios were calculated based on the plasma fatty acid composition that was determined by the capillary gas chromatography. Quartile analysis was added to the evaluation of the relationship between the MMSE score and EPA/AA ratio.
Neuropsychological evaluation included the Mini-Mental State Exam (MMSE) [15] and the 5-point Clock Drawing Test (CDT), which has a sensitivity of 86.7% and a specificity of 92.7% for detecting AD-associated cognitive decline [16]. In 49 subjects, the neuropsychological evaluation was carried out repeatedly with oneyear interval. All patients were on donepezil (5mg/day) during the observation period. According to the difference in the total MMSE score between the baseline and follow-up evaluations, those subjects were classified into the2 groups: stable group in whom the MMSEs core was unchanged or improved as compared with the baseline, and deterioration group in whom the MMSEs core was worsened.
Gender differences in the baseline demographic, vascular and neuropsychological parameters were evaluated with the Χ2 test for categorical variables and with the wilcoxon test. Spearman rank correlation coefficient was used in the evaluation of the relationship between cognitive performance and the demographic and laboratory data. Wilcoxon test was used in the evaluation of the effect of PUFA profile on the risk of cognitive decline between stable and deterioration groups. Independent measures analysis of variance was used to explore the relationship between the EPA/AA ratio and cognitive decline in the follow-up study. The cut-off value of the EPA/ AA ratio for distinguishing cognitive stability from deterioration was calculated using the Receiver Operating Characteristic (ROC) curve. All probability values of 5% or less (two-sided) were considered significant. These statistical analyses were performed using SAS statistical software (version 11.2; SAS Inc., Cary, NC).
Results
The mean baseline MMSE and CDT scores were 15.9 ± 5.3 and 2.7 ± 1.5, respectively. There was no significant difference in the mean age, blood pressure, presence of ApoE4, LDL-cholesterol, FBS, insulin HbA1c, and BNP between men and women. The EPA and DHA concentration did not differ between men and women, whereas the AA concentration was significantly higher in women than in men. The baseline MMSE score was significantly greater in men than in women (Table 1).
Total
(n = 133)
Women
(n = 82)
Men
(n = 51)
p
Age (years)
78.6 (7.2)
78.7 (7.2)
78.4 (7.3)
0.89
SBP (mmHg)
127.2 (11.6)
127.3 (11.2)
127.1 (12.5)
0.99
DBP (mmHg)
73.6 (6.1)
74.3 (5.4)
72.4 (7.0)
0.06
ApoE4 carrier
53.2%
55.2%
50.0%
0.59
EPA (Μg/ml)
56.7 (26.7)
56.3 (29.6)
57.4 (21.5)
0.31
DHA (μg/ml)
122.7 (32.7)
123.8 (34.0)
120.8 (30.6)
0.77
AA (μg/ml)
110.2 (29.2)
115.2 (29.0)
102.2 (27.8)
<0.01
LDL cholesterol (mg/dl)
117.9 (31.5)
118.1 (31.9)
117.6 (31.3)
0.92
HDL cholesterol (mg/dl)
64.9 (17.1)
68.3 (17.5)
57.1 (14.1)
<0.01
FBS (mg/dl)
119.3 (34.1)
115.5 (32.6)
124.6 (35.9)
0.06
Insulin (μU/ml)
19.6 (23.2)
16.3 (15.7)
24.2 (30.4)
0.18
HOMA-R
6.7 (9.8)
5.5 (8.1)
8.4 (11.7)
0.09
BNP (pg/ml)
87.5 (117.5)
95.9 (129.4)
74.3 (95.7)
0.19
MMSE
15.9 (5.3)
15.1 (5.3)
17.2 (5.2)
<0.05
CDT
2.7 (1.5)
2.8 (1.5)
2.7 (1.5)
0.82
Abbreviations: SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; EPA: Eicosapentaenoic Acid; DHA: Docosahexaenoic Acid; AA: Arachidonic Acid; EPA/AA Ratio: Eicosapentaenoic Acid/Arachidonic Acid Ratio; LDL Cholesterol: Low-Density Lipoprotein Cholesterol; HDL Cholesterol: High-Density Lipoprotein Cholesterol; FBS: Fasting Blood Sugar; BNP: Brain Natriuretic Peptide; MMSE: Mini-Mental State Examination; CDT: Clock Drawing Test, The Χ2 Test for Categorical Variables and the Wilcox on test or Statistics for Gender Differences in The Baseline Demographic, Vascular and Neuropsychological Parameters were used. HOMA-R Value Indicates A Marker of Insulin Resistance. HOMA-R= (Insulin Concentration) χ (FBS)/405
Table 1: Baseline Characteristics of cross-sectional Study Subjects.
The baseline MMSE score correlated positively with theω-3/ω-6 and the EPA/AA ratios, and negatively with AA concentration (p<0.05) (Table 2). There was no correlation between the 5-point CDT score and PUFA profiles (Table 2). In the quartile analysis of EPA/AA ratio, there was a tendency toward the total MMSE score to be preserved in the greater EPA/AA quartiles (Figure 1). There was no significant relationship between the MMSE score and cholesterol profile in our AD patients.
MMSE
p
CDT
p
Age (years)
-0.1540 [-0.3159 to 0.0167]
0.2053
-0.1040 [-0.2773 to 0.0759]
0.3252
Female n, (%)
-0.3523 [-2.1389 to 1.4343]
0.6971
0.1039 [-0.4003 to 0.6081]
0.6841
SBP (mmHg)
0.1258 [-0.0579 to 0.301]
0.3542
-0.0350 [-0.2252 to 0.1577]
0.8774
DBP (mmHg)
0.0247 [-0.1583 to 0.2061]
0.5413
-0.0654 [-0.2539 to 0.1279]
0.4390
ApoE4 carrier
-0.9358 [-2.6448 to 0.7731]
0.2807
-0.4781 [-0.9588 to 0.0026]
0.0512
EPA (μg/ml)
0.0132 [-0.0421 to 0.1830]
0.2239
0.0508 [-0.1288 to 0.2273]
0.3130
DHA (μg/ml)
-0.1275 [-0.2914 to 0.1830]
0.2149
-0.0217 [-0.1995 to 0.1574]
0.2744
AA (μg/ml)
-0.1641 [-0.3252 to 0.0063]
0.0273
-0.0527 [-0.2290 to 0.1270]
0.2246
ω-3/ω-6
0.1216 [-0.0497 to 0.2859]
0.0499
0.4665 [-0.2191 to 1.1521]
0.4691
EPA/AA
0.0991 [-0.0723 to 0.2649]
0.0302
0.4665 [-0.2191 to 1.1521]
0.0998
DHA/AA
0.0338 [-0.1373 to 0.2028]
0.2622
0.4665 [-0.2191 to 1.1521]
0.7496
LDL-cholesterol (mg/dl)
0.0023 [-0.1680 to 0.1725]
0.5896
0.0165 [-0.1625 to 0.1944]
0.3285
HDL-cholesterol (mg/dl)
-0.0142 [-2.1652 to 0.1158]
0.9486
-0.0193 [-0.1971 to 0.1597]
0.2155
Insulin (μU/ml)
1.1587 [-0.8171 to 3.1344]
0.3125
-0.1441[-0.3303 to 0.0528]
0.3462
FBS (mg/dl)
0.0689 [-0.1190 to 0.2520]
0.3757
-0.0009 [-0.1944 to 0.1926]
0.8452
BNP (pg/ml)
-0.0494 [-0.2190 to 0.1232]
0.3567
-0.0034 [-0.1833 to 0.1767]
0.7186
Abbreviations: SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; EPA: Eicosapentaenoic Acid; DHA: Docosahexaenoic Acid; AA: Arachidonic Acid; EPA/AA Ratio: Eicosapentaenoic Acid/Arachidonic Acid Ratio; LDL Cholesterol: Low-Density Lipoprotein Cholesterol; HDL Cholesterol: High-Density Lipoprotein Cholesterol; FBS: Fasting Blood Sugar; BNP: Brain Natriuretic Peptide; MMSE: Mini-Mental State Examination; CDT: Clock Drawing Test, The Spearman Rank Correlation Coefficient for Categorical Variables as used for the Influence of Vascular Risk Factors on the Cognitive Performance in AD Patients.
Table 2: Vascular risk factors on cognitive performance in AD patients.
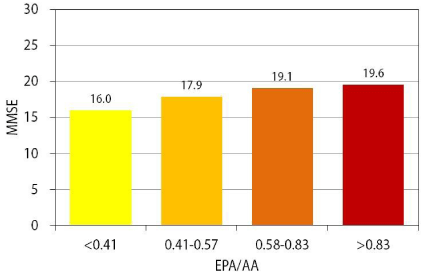
Figure 1: Comparison of mean MMSE score according to EPA/AA ratio
quartiles at baseline. There was a progressive increase in mean MMSE
score in the EPA/AA quartiles at baseline, suggesting that a high EPA/AA
ratio leads to preservation of cognitive function in elderly AD patients.
In the longitudinal follow-up study, 20 patients showed deterioration in MMSE score (deterioration group), whereas the MMSE score was unchanged or improved in 29 patients (stable group) (Table 3). The deterioration group was older than the stable group. There was no difference in the presence of ApoE4, baseline MMSE score, CDT score, blood pressure, FBS, insulin, HbA1c, LDLcholesterol, BNP or EPA/AA ratio between the deterioration and stable groups. The baseline MMSE score was significantly greater in the deterioration group than in the stable group (p <0.01), where as the baseline EPA/AA ratio was significantly greater in the stable group than in the deteriorating group (Table 3). In the ROC curve analysis, the threshold EPA/AA ratio was estimated at 0.67 for the stable MMSE score with 66% sensitivity and 70% specificity [Odds Ratio (OR) = 4.43] (Figure 2).
Total
(n = 49)
Stable
(n = 29)
Deterioration
(n = 20)
p
Age (years)
77.8 (7.0)
75.8 (6.7)
80.8 (6.6)
<0.01
Female gender
75.5%
69.0%
85.0%
0.21
MMSE
baseline
20.3 (4.3)
19.3 (3.8)
21.7 (4.7)
0.06
follow-up
20.3 (3.8)
20.7 (3.8)
19.4 (3.3)
0.28
CDT at baseline
3.4 (1.2)
3.6 (1.1)
3.1 (1.4)
0.18
SBP (mmHg) at baseline
126.8 (13.8)
125.7 (17.0)
128.5 (7.1)
0.48
DBP (mmHg) at baseline
67.4 (7.8)
67.7 (8.2)
67.0 (7.0)
0.78
ApoE4 carrier
47.9%
53.6%
40.0%
0.36
L/H ratio at baseline
2.1 (0.7)
2.1 (0.8)
2.0 (0.9)
0.58
HbA1c at baseline
5.5 (0.3)
5.5 (0.3)
5.4 (0.3)
0.51
BNP (pg/ml) at baseline
42.0 (34.7)
41.1 (36.1)
43.2 (33.5)
0.84
EPA/AA ratio
baseline
0.73 (0.15)
0.79 (0.37)
0.63 (0.31)
0.18
average
0.75 (0.34)
0.83 (0.37)
0.62 (0.26)
<0.05
follow-up
0.73 (0.35)
0.87 (0.42)
0.62 (0.26)
<0.05
Abbreviations: SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; EPA/AA Ratio: Eicosapentaenoic Acid/Arachidonic Acid Ratio; L/H Ratio: Low-Density Lipoprotein/High-Density Lipoprotein Ratio; BNP: Brain Natriuretic Peptide; MMSE: Mini-Mental State Examination; CDT: Clock Drawing Test
Table 3: Comparison between table group and deterioration group.
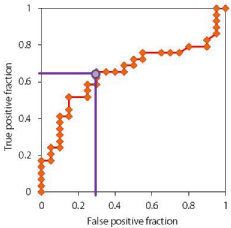
Figure 2: ROC curve of EPA/AA ratio for the stability of cognitive performance
in the one year follow-up study. ROC curve analysis demonstrated that the
EPA/AA ratio predicted stable MMSE score with 66% sensitivity and 70%
specificity [Odds Ratio (OR) = 4.43] at a cut-off value of average EPA/AA
ratio 0.67, further underscoring the relationship between the EPA/AA ratio
and cognitive stability in AD patients.
Discussion
The present results illustrated that the long-chain omega-3 PUFA was closely associated with the cognitive function, and the EPA/AA ratio can be regarded as a predictive marker for the preservation of cognitive ability in elderly AD patients. This may also endorse the results of previous studies.
Since the long chain omega-3 PUFAs such as EPA and DHA are the essential fatty acids most abundant in the brain tissue, they are thought to be crucial for normal cognitive function [17]. DHA plays important roles in the neuronal membrane plasticity, synaptogenesis and neurogenesis, all of which are involved in the synaptic transmission and the well-being of normal brain functions. Because of the neurobiological property of DHA, Hashimoto and coworkers [18] emphasized the significant preventive effect of DHA against dementia, although the DHA/AA ratio was not associated with the evolution of cognitive function in our results.
Previous study showed that the greater proportion of EPA was associated with a lower incidence of dementia [13]. Recent brain imaging study demonstrated that EPA had protective effects against the accumulation of amyloid-β (Aβ) in AD patients [12].There was limitation in the present observation, because we use the plasma concentration of PUFA instead of that on the red cell membrane. Plasma PUFA concentrations reflects a shorter-term lipid intake as compared with the PUFA from Red Blood Cell (RBC) membranes [19], because the PUFA turnover is more rapid in the plasma than in the RBC membrane. The direct measurement of PUFA in plasma may better reflect their bioavailability as precursors of other active molecules, such as eicosanoids, particularly for EPA [20].
The beneficial effects of the long-chain omega-3 PUFAs against dementia are explained by their major structural and functional roles in neuronal membranes, vascular and anti-inflammatory properties, as well as the potential ability to modulate neuroinflammation and the expression of neuronal pasticity-related gene expression [8]. Many theories have been proposed to account for the beneficial effects of EPA on cognitive function. Different ratios of omega-3 to omega-6 PUFAs may suppress neuroinflammation and oxidative stress in the central nervous system [21]. A previous biochemical study suggested that EPA suppresses inflammation by competitive antagonism of AA conversion by Cyclooxygenase-2 (COX-2) [22]. In the laboratory settings, the stimulation of COX-2 activity led to increased Prostaglandin E2 (PGE2) production, which in turn stimulated proteolysis of amyloid precursor protein to form amyloid beta, likely by enhancing gamma-secretase activity [23-24]. Therefore, the inhibition of PGE2 formation by omega-3 PUFAs may be an effective method to reduce the production and accumulation of Aâ. COX-2 also promoted amyloid plaque deposition and accelerated Aâ-mediated apoptotic brain damage in a transgenic AD model [25]. Moreover, the elevated PGE2 levels and COX-2 over expression have been observed in the brains of AD patients [26-27], and COX- 2 expression correlates with Aâ deposition and AD progression [28]. Inflammation and oxidative stress may contribute to the early development of amyloid plaques and neurofibrillary tangles [29-32], while PGE2-dependent amplification of COX-2 may act to sustain and exacerbate chronic inflammation. EPA and DHA also compete with AA for incorporation into cell membrane phospholipids. Thus, the EPA/AA ratio may reflect low neuroinflammation and oxidative stress as well as higher neuronal repair capacity in AD patients with greater cognitive stability.
Conclusion
Long-chain omega-3 PUFA is closely associated with the cognitive function, and the EPA/AA ratio can be regarded as a predictive marker for the preservation of cognitive ability in elderly AD patients.
References
- Van Der Putt R, Dineen C, Janes D, Series H, McShane R. Effectiveness of acetylcholinesterase inhibitors: diagnosis and severity as predictors of response in routine practice. Int J Geriatr Psychiatry. 2006; 21: 755-760.
- Fratiglioni L, Qiu C. Prevention of cognitive decline in ageing: dementia as the target, delayed onset as the goal. Lancet Neurol. 2011; 10: 778-779.
- Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci. 2012; 322: 141-147.
- Mariani J, Doval HC, Nul D, Varini S, Grancelli H, Ferrante D, et al. N-3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2013; 19: 2: 005033.
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, et al. Early protection against sudden death by omega3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infaro Miocardico (GISSI)-Prevenzione. Circulation. 2002; 105: 1897-1903.
- Neuringer M, Anderson GJ, Connor WE. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annu Rev Nutr. 1988; 8: 517-541.
- Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004; 39: 212-220.
- Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Lannglier B, et al. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev. 2004; 44: 509 –538.
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014; 14: 643.
- Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997; 42: 776-782.
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003; 60: 940-946.
- Mosconi L, Murray J, Davies M, Williams S, Pirraglia E, Spector N, et al. Nutrient intake and brain biomarkers of Alzheimer's disease in at-risk cognitively normal individuals: a cross-sectional neuroimaging pilot study. BMJ Open. 2014; 4: e004850.
- Samieri C, Feart C, Letenneur L, Dartigues JF, Peres K, Auriacombe S, et al. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008; 88: 714-721.
- Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol. 2009; 5: 140-152.
- Folstein M, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for clinician. J Psychiatr Res. 1975; 12: 189-198.
- Esteban-Santillan C, Praditsuwan R, Ueda H, Geldmacher DS. Clock drawing test in very mild Alzheimer's disease. J Am Geriatr Soc. 1998; 46: 1266-1269.
- Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000; 71: 171-175.
- Hashimoto M, Maekawa M, Katakura M, Hamazaki K, Matsuoka Y. Possibility of polyunsaturated fatty acids for the prevention and treatment of neuropsychiatric illnesses. J Pharmacol Sci. 2014; 124: 294-300.
- Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003; 133: 925-932.
- Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008; 47: 147-155.
- Jicha GA, Markesbery WR. Omega-3 fatty acids: potential role in the management of early Alzheimer's disease. Clin Interv Aging. 2010; 5: 45-61.
- Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007; 282: 22254-22266.
- Xiang Z, Ho L, Yemul S, Zhao Z, Qing W, Pompl P, et al. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer's disease neuropathology. Gene Expr. 2002; 10: 271-278.
- Qin W, Ho L, Pompl PN, Peng Y, Zhao Z, Xiang Z, et al. Cyclooxygenase (COX)-2 and COX-1 potentiate beta-amyloid peptide generation through mechanisms that involve gamma-secretase activity. J Biol Chem. 2003; 278: 50970-50977.
- Strokin M, Sergeeva M, Reiser G. Prostaglandin synthesis in rat brain astrocytes is under the control of the n-3 docosahexaenoic acid, released by group VIB calcium-independent phospholipase A2. J Neurochem. 2007; 102: 1771-1782.
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999; 830: 226-236.
- Kitamura Y, Shimohama S, Koike H, Kakimura Ji, Matsuoka Y, Nomura Y, et al. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer's disease brains. Biochem Biophys Res Commun. 1999; 254: 582-586.
- Ho L, Purohit D, Haroutunian V, Luterman JD, Willis F, Naslund J, et al. Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch Neurol. 2001; 58: 487-492.
- Cunnane SC, Plourde M, Pifferi F, Begin M, Feart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer's disease. Prog Lipid Res. 2009; 48: 239-256.
- Boudrault C, Bazinet RP, Ma DW. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer's disease. J Nutr Biochem. 2009; 20: 1-10.
- Hooijmans CR, Kiliaan AJ. Fatty acids, lipid metabolism and Alzheimer pathology. Eur J Pharmacol. 2008; 585: 176-196.
- 32. Montine TJ, Morrow JD. Fatty acid oxidation in the pathogenesis of Alzheimer's disease. Am J Pathol. 2005; 166: 1283-1289.