
Review Article
J Cardiovasc Disord. 2015;2(1): 1009.
Brachial-Ankle Pulse Wave Velocity, Mortality, and Cardiovascular Events
Dai Ato¹* and Takeshi Takami²*
¹Science Representative, Sales Strategy Department,Japan
²Department of Internal Medicine, Clinic Jingumae,Japan
*Corresponding author: Ato D, Sales Strategy Department, OMRON COLIN Co., Ltd., 1-12-14 Koishikawa, Bunkyo-ku, Tokyo, 112-0002, Japan,
Takami T, Department of Internal Medicine, Clinic Jingumae, ConfortYagi 1F 5-4-41 Naizencho, Kashihara, Nara 634-0804, Japan
Received: December 18, 2014; Accepted: March 03, 2015; Published: March 06, 2015
Abstract
Pulse Wave Velocity (PWV) is used as an index of arterial stiffness worldwide. One standard PWV is the carotid-femoral PWV (cfPWV). Articles reporting the usefulness of brachial-ankle PWV (baPWV), which is measured at the brachium and ankle, are rapidly increasing.And the number of prognosis follow-up studies is approaching 40. Observing these reports, baPWV shows a prognostic predictability independent from classic atherosclerotic risk factors that are considered to improve upon the exclusion of patients with Peripheral Arterial Disease (PAD). On the other hand, it is difficult to exclude PAD only by the ordinal cut-off of ABI 0.9, especially in populations of patients with diabetes or those on hemodialysis. In this review, we summarize previously reported articles, identify the efficacy of baPWV in the various reports, consider why the predictability of baPWV is decreased in those populations, suggest possible solutions to avoid the use of inaccurate baPWV measurements, and present future perspectives.
Keywords: Brachial-Ankle Pulse Wave Velocity; Ankle-Brachial Index; Prognostic Predictability; Arterial Stiffness; Peripheral Arterial Disease; Cardiovascular Disease
Introduction
The prevention of atherosclerotic disease is an important issue in developed and developing countries. The prevention of this disease is based on controlling classical risk factors such as hypertension and Diabetes Mellitus (DM), but since it is impossible to perfectly predict future events by combining these factors, confirming arterial damage is important. CfPWV is an index vascular test with proven prognostic predictability, and many related studies have been performed in Europe [1,2].
An apparatus that simultaneously measures Ankle-Brachial Index (ABI) and PWV became available in December 1999 in Japan. This machine continuously measures PWV between the brachium and the ankle (baPWV) after the ABI. With its procedural convenience, this machine first became rapidly popular in Japan and East Asian countries. The clinical use of baPWV was first started in Japan, so Japan is virtually the origin of this biomarker. The product name of this machine is “form” and it means “let us watch the ‘form” of the pulse wave.” Abroad, the name of “VP-1000” or “VP-2000” (Vascular Profiler) has essentially the same intent. Fifteen years have passed since its debut, and to date, more than 1000 English-language articles have reported the use of this device, not only the ABI tested by this device indicated prognostic predictability like as many preceding studies worldwide, the number of the studies of the prognostic significance of baPWV has reached nearly 40. Moreover, in early 2014, the cut-off value of 18 m/s was announced in the Japanese Circulation Society (JCS) and Japanese Society of Hypertension (JSH) guidelines [3,4]. In this review, we summarize articles focusing on the current prognostic value of baPWV and suggest possible solutions to avoid the use of inaccurate baPWV measurements as well as future perspectives.
Researched Articles
Until the end of November 2014, 38 articles that mainly or additively researched the prognostic predictability of baPWV measured almost by VP in terms of all-cause mortality, cardiovascular mortality, and cardiovascular events were included. Cardiovascular events that were variously defined in different articles focusing on the events in these organs were considered in this review. The follow-up studies focusing on the progression to chronic kidney disease (>CKD3) or PAD were excluded. We summarized these articles by study population and checked the baPWV cut-off value and PAD exclusion criteria in each document because baPWV is underestimated in the limbs of patients with PAD [5]. We also included articles which provide the product name when the baPWV was measured by other apparatuses. The additive value to classical atherosclerotic risk factors is a crucial point in the consideration of the prognostic predictability of vascular function tests [6], so we classified the articles in which baPWV showed effectiveness as an independent prognostic biomarker after adjustment on Cox Multiple Regression Analysis (CMRA).
Measurement of baPWV and ABI
The method of measuring these indices by this apparatus was previously reported [7]. Briefly, after at least a 5-minute rest, four oscillometric cuffs are placed in both brachia and ankles, Electrocardiography (ECG) sensors are placed on both wrists, and a Phonocardiography (PCG) sensoris placed on the spine position. In the Hemodialysis (HD) population, the arm cuff is placed on the arm opposite the arteriovenous fistula. The original structure of the ankle cuff is composed of two parts, one that senses the pulse and another that occludes the artery. After automatically measuring the Blood Pressures (BP) of the four limbs, a plethysmogram is simultaneously measured for about 10 seconds. The pulse volume recording is performed on four limbs and the cuff pressure is kept at 55 mmHg. The Pulse-Transit Time (PTT) is calculated by the time difference between the up-stroke of the right brachium and both ankles as pulse transit Time brachial-ankle (Tba). The travel path of the pulse is defined as follows: Lba (cm) = 0.59 × height (cm) + 14.4 [8]. As such, baPWV is Lba/Tba. The baPWV value is reportedly significantly correlated with aortic PWV and the Pearson correlation coefficient is approximately 0.8 [7,9,10]. Its reproducibility is also reportedly good. The inter-observer coefficient is 0.98 [7] and the intra-observer coefficient is 0.89 [11].
In the evaluation of baPWV, the first step is to confirm whether there is decline of ABI to exclude baPWV underestimation [5] because PTT decreases when there is any significant stenosis in the pulse pathway. The ankle BP and ABI accuracy measured by this machine is also valid compared to the Doppler method [12-15], for example, the Pearson correlation coefficient of the ankle systolic BP is 0.95 [14]. Moreover, in this machine, Upstroke Time (UT), and percent mean arterial pressure (%MAP) are provided as quantitative indices of the pulse wave form. These parameters are supportively used to judge the existence of PAD in the clinical setting. Nevertheless, the studies included in this review did not add these supportive parameters to define PAD, so we only focused on whether they describe ABI/PAD exclusion criteria for baPWV analysis in this review. To easily image this point, we provided two examples of the printed results of this apparatus to demonstrate the change of the ankle pulse wave form from normal to PAD (Figures 1,2).
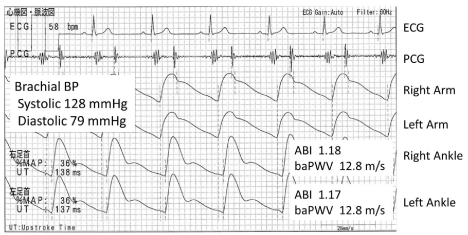
Figure 1: A typical normal case, 65 y.o. male. Both ABIs are normal and the
pulse wave forms of the both ankles are also normal. These ankle pulse wave
forms also show clear dicrotic wave in the early diastole. So these pulse wave
forms are ideal for the ankle and PAD is hardly suspected, therefore use of
baPWV is appropriate. 12.8 m/s is normal. So this male has an ideal result of
this vascular physiological examination for his age.
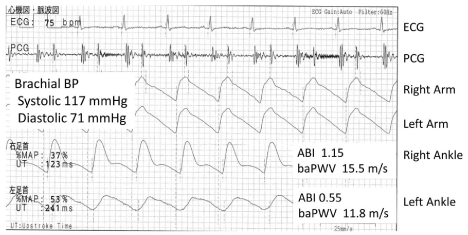
Figure 2: A typical PAD case, 49 y.o. male. The left ankle pulse wave form is
triangular and the height of this pulse wave is apparently lower than the right
ankle. The left UT and %MAP are also much higher. The left baPWV is much
lower than the right, but this is underestimated comparing to the right baPWV
because of the stenosis, and cannot be used. The right ankle wave form is
normal, but use of this baPWV 15.5 m/s is not appropriate as a prognostic
biomarker because these patients are already at high risk with PAD.
Whole Reports
The first study of the prognostic value of baPWV was published in 2005. To date, 38 articles including the reports by the other apparatuses have been published [16-53]. By the end of 2009, there were only five reports, and its number increased rapidly after 2012. Table 1 lists all of the articles, most of which came from Japan, several came from East-Asia and one each came from Greece and Russia. Of the 37 reports excluding the meta-analysis [28], 29 were effective and eight were ineffective. The ineffective studies were most common in the HD cohorts. The summaries and speculations in various cohorts are described below.
No.
Article
Population
Apparatus
Number of patients
Age
(average)Follow-up period
(years)End point
baPWV cut-off
Notice
30
Kuroiwa 2014 [45]
CDE
VP
450
76.6
3
All cause mortality
18.6 m/s
Multiple logistic regresion analysis
29
Seo 2014 [44]
CAD
VP
372
65
2.2
Cardiac death, Major adverse cardiac events
16.7 m/s
The lower baPWV is applied, include ABI < 0.9
28
Chang 2014 [43]
DM
VP
452
68
5.8
All cause mortality, Fatal and non-fatal cardiovascular events
?17 m/s with ABI < 0.9
Include ABI < 0.9
27
Sheng 2014 [42]
CDE
VP
3876
68.1
5.9
All cause mortality, non-cardiovascular mortality
23.3 m/s
Top decile versus whole population
26
Katakami 2014 [41]
DM
VP
1040
59
7.5 (median)
Fatal and non-fatal cardiovascular events
15.5 m/s
25
Ki 2014 [40]
CAD
VP
372
65
2.2
Cardiac death, Major adverse cardiac events
16.7 m/s
The lower baPWV is applied, include ABI < 0.9
24
Maeda 2014 [39]
DM
VP
3628
61
3.2
All cause mortality, Cerebrovascular events
24 m/s 14 m/s
23
Kim 2014 [38]
Stroke
VP
1765
65
3.3
All cause mortality, cardiovascular mortality
22.6 m/s
The higher baPWV is applied, include PAD
22
Kawai 2014 [37]
HT
VP
338
61
6.5
Fatal and non-fatal cardiovascular events
18.9 m/s
21
Sugamata 2014 [36]
CAD
VP
923
65
5.3
Major adverse cardiac events
1SD increase
(***)
20
Nagai 2013 [35]
Outpatients
VP
274
71
3.4
Fatal and non-fatal cardiovascular events
19.1 m/s
(***)
19
Takashima 2014 [34]
LR
VP
4164
58.9
6.5 (median)
Fatal and non-fatal cardiovascular events
18 m/s
18
Yoon 2013 [33]
CKD
VP
117
54
1.0 (median)
Fatal and non-fatal cardiovascular events
15.7 m/s
eGFR <90, the higher baPWV is applied (***)
17
Ishisone 2013 [32]
LR
VP
973
59
7.8
Fatal and non-fatal cardiovascular events
16.7 m/s (top quartile)
Cox multivariate analysis was performed for baPWV above 90th percentile
16
Ninomiya 2013 [31]
LR
VP
2916
60
7.1
Fatal and non-fatal cardiovascular events
17.6 m/s
Hisayama-Study
15
Kawai 2013 [30]
HT
Other
440
61
6.3
Fatal and non-fatal cardiovascular events
17.5 m/s
14
Han 2013 [29]
Outpatients
VP
185
62
1.7
Fatal and non-fatal cardiovascular events
17 m/s
Include ABI < 0.9
13
Vlachopoulos 2012 [28]
Meta-Analysis, combined
Almost VP
All cause mortality, cardiovascular mortality, Fatal and non-fatal cardiovascular events
lowest group vs highest group, 1 m/s increase
Meta-Analysis, including data of abstracts obtained from a few conference, several studies Include ABI < 0.9
12
Kato 2012 [27]
HD
Other
135
60
5.3
Cardiovascular mortality
16.6 m/s
11
Munakata 2012 [26]
HT
VP
662
60
3
Fatal and non-fatal cardiovascular events
17.5 m/s
J-TOPP Study
Table 1: Lists of articles researching prognostic predictability of baPWV.
10
Chen 2011 [25]
CKD
VP
145
69
1.2
Death or progression to commencement of dialysis
19.6 m/s
The higher baPWV is applied, baPWV is adjusted by mean BP for the analysis (***)
9
Inoue 2012 [24]
HD
VP
197
66
5.8
Fatal and non-fatal cardiovascular events
1cm/s increase
8
Orlova 2010 [23]
CAD
Other
161
57
3.5
Major adverse cardiac events
positive ?baPWV
Russia (***)
7
Nakamura 2010 [22]
CAD
VP
564
64
2.1 (median)
Fatal and non-fatal cardiac events
17.3 m/s
Effective in only DM patients
6
Turin 2010 [21]
LR
VP
2642
58.4
6.5
All cause mortality
17 m/s
5
Miyano 2010 [20]
CDE
VP
530
76
3
All cause mortality, cardiovascular mortality
19.6 m/s
4
Meguro 2009 [19]
HF
VP
72
68
1.2
Re-admittion because of HF exacerbation
17.5 m/s
3
Matsuoka 2005 [18]
CDE
VP
298
79.6
3.4
Cardiovascular mortality
25 m/s
2
Kitahara 2005 [17]
HD
VP
671
59
2.8
All cause mortality, Cardiovascular mortality
19.6 m/s 23 m/s
1
Tomiyama 2005 [16]
ACS
VP
215
63
2.2
Fatal and non-fatal cardiovascular events
17 m/s
Include ABI < 0.9
8
Park 2014 [53]
CAD
VP
203
57
4.2
Fatal and non-fatal cardiovascular events
Exclude ABI <0.9
7
Kuwahara 2014 [52]
HD
VP
300
61
7
All cause mortality
Include ABI < 0.9
6
Yoshida 2012 [51]
DM
VP
783
No average (30-75)
5.5
Fatal and non-fatal cardiovascular events
(***)
5
Tanaka 2011 [50]
HD
VP
445
63
3.6
All cause mortality, Cardiovascular mortality
Include ABI < 0.9
4
Amemiya 2011 [49]
HD
VP
186
61
4
All cause mortality
(***)
3
Chen 2010 [48]
HD
VP
212
59
2.4
All cause mortality, Cardiovascular mortality
Include ABI < 0.9
2
Kato 2010 [47]
HD
Other
194
64
3.3
All cause mortality, Fatal and non-fatal cardiovascular events
Include ABI < 0.9
1
Morimoto 2009 [46]
HD
VP
176
61
3.6
All cause mortality, Cardiovascular mortality
(****)
Table 1 of 1:
Local Residents
Four articles were found in the general examination cohorts [21,31,32,34] (Table 2). The difference of the baPWV cut-off of the each report is the smallest in this population. Approximately 1000 people were followed up more than 6.5 years. The mean age at enrollment was 60 years and the cut-off baPWV was very similar. Therefore, the baPWV cut-off could be summarized at 17–18 m/s in this population.
Article
Apparatus
Number of patients
Age (average)
Follow-up period (years)
End point
baPWV
cut-offTakashima 2014 [34]
VP
4164
58.9
6.5 (median)
Fatal and non-fatal
cardiovascular events18 m/s
Ishisone 2013 [32]
VP
973
59
7.8
Fatal and non-fatal
cardiovascular events16.7 m/s
(top quartile)Ninomiya 2013 [31]
VP
2916
60
7.1
Fatal and non-fatal
cardiovascular events17.6 m/s
Turin 2010 [21]
VP
2642
58.4
6.5
All cause mortality
17 m/s
Abbreviations; VP: Vascular Profiler In Ishisone 2013, Cox multivariate analysis was performed for baPWV above 90th percentile.
Table 2: Lists of articles researching local resident.
Community-Dwelling Elderly
Four articles were found; with the exception of the Sheng study, the average age at enrollment was >75 years [18,20,42,45] (Table 3). The baPWV cut-off was in the range of 18.6–25 m/s. The Matsuoka study stated only that a value =25 m/s is an abnormally high value. If it was subjected to Receiver Operating Characteristic (ROC) curve analysis, it might be lower. Sheng reported a cut-off value of 23.3 m/s, but this analysis was done by comparison of the top decile to that of all patients. As such, similar to Matsuoka”s study, the value might be lower if analyzed by another method such as ROC. The population in these cohorts is about 10 years older than that of the local resident mentioned above, so it is reasonable that the cut-off would increase. Nevertheless, the further accumulation of reports on the baPWV cutoff in the elderly is eagerly awaited.
Article
Apparatus
Number of patients
Age (average)
Follow-up period (years)
End point
baPWV
cut-offKuroiwa 2014 [45]
VP
450
76.6
3
All cause mortality
18.6 m/s
Sheng 2014 [42]
VP
3876
68.1
5.9
All cause mortality, non- cardiovascular mortality
23.3 m/s
Miyano 2010 [20]
VP
530
76
3
All cause mortality,
cardiovascular mortality19.6 m/s
Matsuoka 2005 [18]
VP
298
79.6
3.4
Cardiovascular mortality
25 m/s
Abbreviations; VP: Vascular Profiler In Kuroiwa 2014, multiple logistic regression analysis was performed.
Table 3: Lists of articles researching community dwelling elderly.
Hypertensives, Outpatients, Chronic Kidney Disease
By summarizing these populations, we identified seven reports [25,26,29,30,33,35,37] (Table 4), all of which found baPWV effective. The cut-off values were in the range of 15.7–19.6 m/s. Yoon et al. reported that 15.7 m/s was the cut-off, but the younger mean patient age might be related. Chen et al. reported a 19.6 m/s cut-off, but the mean age at enrollment in that study was 69, which might affect this result. Moreover, these two reports did not mention the ABI/PAD exclusion criteria, so this might also be a factor. Nagai et al. reported a cut-off of 19.1 m/s, but the older mean age of 71 years is considered a reason. In the other four articles, mean age was near 60 and the cut-off was 17–18.9 m/s. As such, the baPWV cut-off could be summarized as 17–19 m/s in 60–70-year-old patients in these populations.
Article
Population
Apparatus
Number of patients
Age (average)
Follow-up period (years)
End point
baPWV
cut-offKawai 2014 [37]
HT
VP
338
61
6.5
Fatal and non-fatal
cardiovascular events18.9 m/s
Nagai 2013 [35]
Outpatients
VP
274
71
3.4
Fatal and non-fatal cardiovascular events
19.1 m/s
(***)Yoon 2013 [33]
CKD
VP
117
54
1
(median)Fatal and non-fatal
cardiovascular events15.7 m/s
(***)Kawai 2013 [30]
HT
Other
440
61
6.3
Fatal and non-fatal
cardiovascular events17.5 m/s
Han 2013 [29]
Outpatients
VP
185
62
1.7
Fatal and non-fatal
cardiovascular events17 m/s
Munakata 2012 [26]
HT
VP
662
60
3
Fatal and non-fatal
cardiovascular events17.5 m/s
Chen 2011 [25]
CKD
VP
145
69
1.2
Death or progression
to commencement of
dialysis19.6 m/s
(***)Abbreviations; VP: Vascular Profiler; HT: Hypertension; CKD: Chronic Kidney Disease; GFR: Glomerular Filtration Rate (***) Not mentioned about ABI exclusion criteria nor described about ABI value itself In Yoon 2013, effective only in the group of eGFR <90, the higher baPWV is applied. In Han 2013, patients with ABI < 0.9 are included. In Chen 2011, the higher baPWV is applied and baPWV is adjusted by mean BP for the analysis.
Table 4: Lists of articles researching hypertensives, outpatients, chronic kidney disease.
Coronary Artery Disease, Acute Coronary Syndrome, Heart Failure
There are eight articles in the heart disease cohort [16,19,22,23,36,40,44,53]; of them, seven found baPWV effective (Table 5). However, only three of the eight studies clearly described their ABI/PAD exclusion criteria. The baPWV effective studies that did not exclude PAD found a higher baPWV or did not use baPWV in the limbs with an ABI < 0.9, and these methods are considered the reason for the effective outcome. On the contrary, Park et al. reported that baPWV was not effectiveness even after excluding those with an ABI <0.9. Nakamura et al. reports that baPWV does not have predictability in groups of patients without DM, so it is not unnatural for similar results to be found among different study populations. Orlova et al. presented the only study from the Caucasian population. However, this result would have to be carefully applied to Asian populations, especially Japanese, since this study included only male patients, the average life expectancy of Russian men differs from that of Japanese men by >10 years, and the prevalence of disease differs between Caucasian and Japanese men. Review of these eight reports in the heart disease population revealed that 17–18 m/s could be set as a baPWV cut-off with some limitations depending on condition. Otsuka et al. reported results in a similar category [54]; as such, we did not add this paper to the list because it did not specify which device was used to measure baPWV.
Article
Population
Apparatus
Number of patients
Age (average)
Follow-up period (years)
End point
baPWV
cut-offSeo 2014 [44]
CAD
VP
372
65
2.2
Cardiac death,
Major adverse cardiac events16.7 m/s
Ki 2014 [40]
CAD
VP
372
65
2.2
Cardiac death,
Major adverse cardiac events16.7 m/s
Sugamata 2014 [36]
CAD
VP
923
65
5.3
Major adverse cardiac events
1 SD increase
(***)Orlova 2010 [23]
CAD
Other
161
57
3.5
Major adverse cardiac events
positive ?baPWV
(***)Nakamura 2010 [22]
CAD
VP
564
64
2.1
(median)Fatal and non-fatal
cardiac events17.3 m/s
Meguro 2009 [19]
HF
VP
72
68
1.2
Re-admission because
of HF exacerbation17.5 m/s
Tomiyama 2005 [16]
ACS
VP
215
63
2.2
Fatal and non-fatal
cardiovascular events17 m/s
Park 2014 [53]
CAD
VP
203
57
4.2
Fatal and non-fatal
cardiovascular eventsAbbreviations; VP: Vascular Profiler; CAD: Coronary Artery Disease; HF: Heart Failure; ACS: Acute Coronary Syndrome; SD: Standard Deviation (***) Not mentioned about ABI exclusion criteria nor described about ABI value itself. In Seo 2014 and Ki 2014, the lower baPWV is applied, include ABI <0.9. In Nakamura 2010, effective only in only DM patients. In Tomiyama 2005, ABI <0.9 is included. In Park 2014, ABI <0.9 is excluded.
Table 5: Lists of articles researching heart diseases.
Stroke
The subject of this cohort had acute ischemic stroke [38]. The measurement of baPWV is usually performed on the third admission day, so we must consider that this condition would differ from the general elective condition. The baPWV cut-off of 22.6 m/s is much higher for the cohort of this age than those in other studies, which must be considered in its application to other populations. Furthermore, sub-group analysis after excluding the patients with atrial fibrillation and a borderline ABI < 0.95 showed a higher Hazard Ratio (HR) of 0.5–0.6 with each 10 m/s increase. This stresses the importance of PAD exclusion criteria for baPWV evaluations.
Diabetes
Vlachopolous et al. already mentioned that the prognostic value of baPWV was lower among the DM population in a 2012 metaanalysis [28]. There are four studies and some difference of the results exists among these papers [39,41,43,51] (Table 6). Yoshida et al. reported no explanation about PAD exclusion criteria and that ABI was not an anthropometric parameter itself (it cannot be asserted that this subgroup is not excluded). In this study, the highest quartile of baPWV showed a significantly lower event-free survival rate on Kaplan-Meier analysis, so the predictability of baPWV was lost after CMRA adjustment including Framingham Risk Score as a covariate. Chang et al. included patients with an ABI < 0.9, but their stratification method combined values of baPWV = 17 m/s and ABI < 0.9. In other words, in the patients who have at least one limb with an ABI < 0.9, they reclassified patients by a baPWV > 17 m/s, which might be effective.
Article
Apparatus
Number of patients
Age (average)
Follow-up period (years)
End point
baPWV
cut-offChang 2014 [43]
VP
452
68
5.8
All cause mortality, Fatal and non-fatal cardiovascular events
>=17 m/s with ABI < 0.9
Katakami 2014 [41]
VP
1040
59
7.5 (median)
Fatal and non-fatal
cardiovascular events15.5 m/s
Maeda 2014 [39]
VP
3628
61
3.2
All cause cerebrovascular events
24 m/s
14 m/sYoshida 2012 [51]
VP
783
No average
(30-75)5.5
Fatal and non-fatal
cardiovascular events(***)
Abbreviations; VP: Vascular Profiler (***) Not mentioned about ABI exclusion criteria nor described about ABI value itself. In Chang 2014, ABI < 0.9 is included.
Table 6: Lists of articles researching diabetes mellitus.
Maeda et al. and Katakami et al. both reported baPWV efficacy. However, the cut-off baPWV differed extensively even though they excluded patients with ABI < 0.9 and the average patient age was very close. Furthermore, 24 m/s for total mortality and 14 m/s for cardiovascular events were reported by Maeda et al., so we speculate that this is the reason for the finding. Natsuaki et al. simultaneously reported on the prognostic predictability of ABI in the same study cohort [55]. In this report, patients with an abnormal (=0.9) or borderline (0.91–0.99) ABI were found to equally have a significant two-fold HR for total mortality and cardiovascular mortality compared to those with a normal (1.0–1.4) ABI. Furthermore, the risk of progression to the abnormal ABI was also significantly higher in patients with a borderline ABI than those with an ABI> 1.0. These results strongly imply that especially in patients with DM, a borderline ABI may indicate progression of systemic atherosclerosis, especially in other arteries. There is one case in which ABI is borderline or pseudo normal even when there is a significant stenosis in the arteries of the lower limbs that was considered to be caused by calcification, the so-called Moenckeberg”s arteriosclerosis, which is common in DM and HD [56,57]. As a result of these conditions, baPWV may have been already underestimated in patients with a borderline ABI, which then caused the discrepancy in baPWV cut-off values. On the other hand, Maeda et al. reported that the hazards of mortality and cardiovascular events increase linearly with baPWV but that the raw ratios of the mortality and coronary events differ by only about twofold between the lowest (<14.4 m/s) and highest (>19.8 m/s) quartiles. This ratio is much lower compared to those of general populations. Therefore, in the Maeda study, a number of high-risk patients with a borderline ABI whose risks were virtually the same as patients with an ABI = 0.9 were included in the lowest and the two middle quartiles of baPWV, which is considered the reason for the smaller difference in the occurrence of death and cardiovascular events between the lowest and the highest quartiles of baPWV. The same phenomenon likely exists in Katakami study, in which it could not be denied that there was an influence that decreased the baPWV values. Therefore, in the DM cohort, an ABI < 1.0 should be considered high-risk. And if the pulse wave form of the ankle is not normal, the use of baPWV as a prognostic biomarker should be pended.
Hemodialysis
Nine articles in our search results focused on the HD population [17,24,27,46-50,52]; of them, only three reported that baPWV was an effective prognostic marker (Table 7). Nevertheless, there are very important lessons of baPWV evaluation, especially in these studies. The four of six studies that did not show baPWV effectiveness did not exclude patients with PAD or an ABI < 0.9. Another paper did not mention ABI/PAD exclusion criteria. Therefore, only the Morimoto et al. study certainlyexcluded patients with an ABI < 0.9 among these six papers. On the contrary, the three studies that showed baPWV effectiveness all excluded those with an ABI < 0.9.
Article
Apparatus
Number of patients
Age (average)
Follow-up period (years)
End point
baPWV
cut-offKato 2012 [27]
Other
135
60
5.3
Cardiovascular mortality
16.6 m/s
Inoue 2012 [24]
VP
197
66
5.8
Fatal and non-fatal
cardiovascular eventslcm/s
increaseKitahara 2005 [17]
VP
671
59
2.8
All cause mortality,
Cardiovascular mortality19.6 m/s
23 m/sKuwahara 2014 [52]
VP
300
61
7
All cause mortality
Tanaka 2011 [50]
VP
445
63
3.6
All cause mortality,
Cardiovascular mortalityAmemiya 2011 [49]
VP
186
61
4
All cause mortality
(***)
Chen 2010 [48]
VP
212
59
2.4
All cause mortality,
Cardiovascular mortalityKato 2010 [47]
Other
194
64
3.3
All cause mortality, Fatal and non-fatal cardiovascular events
Morimoto 2009 [46]
VP
176
61
3.6
All cause mortality,
Cardiovascular mortality(****)
Abbreviations; VP: Vascular Profiler (***) Not mentioned about ABI exclusion criteria nor described about ABI value itself (****) Excluded ABI<0.9, and ABI is included in the cox regression analysis. In Kuwahara 2014, Tanaka 2011, Chen 2010, Kato 2010, ABI < 0.9 is included.
Table 7: Lists of articles researching hemodialysis patients.
Kitahara et al. reported that CMRA analysis of all 785 patients with an ABI < 0.9 revealed that baPWV lost its prognostic value and that ABI was the most powerful prognostic predictor (p < 0.0001). Next, they re-analyzed 671 patients after excluding those with an ABI < 0.9 by CMRA, baPWV was independently effective from other confounding factors even including ABI as an adjustment. It should be considered that an ABI that is borderline (0.90–0.99) or >1.3 was also independent prognostic factor in this 671-patient analysis.
Two reports of Kato et al. are also interesting. In their 2010 article [47], they analyzed baPWV and PAD and found that baPWV lost its effectiveness after CMRA. They also reported that an ABI < 1.0 is an independent prognostic predictor. On the other hand, in their 2012 report [27], upon reanalyzing the population after excluding those with an ABI < 0.9 and adding a 2-year follow-up extension, they found that baPWV was effective as an independent predictor of cardiovascular death. In addition, ABI was not included in the CMRA model in this report since it did not differ significantly between the deceased and surviving patients. More interestingly, in this article, BP-adjusted PWV showed a statistical tendency for cardiovascular death on Kaplan-Meier analysis, but this trend disappeared after adjustment for age, sex, and DM by CMRA. This implies that BPadjusted PWV cannot always necessarily provide better results as a prognostic biomarker. In this study, the average baPWV was 16.0 m/s, median baPWV was 15.2 m/s, and highest tertile was =16.6 m/s, all of which were lower than those of other HD studies, and the differences in device use is thought to have greater influence than excluding patients = 75 years.
Next, the reports of Amemiya et al. [49] and Inoue et al. [24] were from the same institution, and it is important that Inoue et al. demonstrate baPWV effectiveness by certainlyexcluding ABI < 0.9 even though the cohorts differed between these two studies. In addition to these reports that indicate baPWV effectiveness excluding apparent PAD, Morimoto et al. did not demonstrate this [46], so we speculate that this is the reason. As we have shown, Kitahara et al. proved that not only higher baPWV also borderline ABI are independent prognostic predictors even after the exclusion of ABI < 0.9. In this study, even an ABI of 1.0–1.09 also shows a tendency or close value (p = 0.113 for total mortality, p = 0.086 for cardiovascular mortality after CMRA). On the other hand, Ono et al., one of the coauthors of the Kitahara article, previously reported on ABI prognostic values in 1010 HD patients [12] but did not measure or describe baPWV. In this 2003 report, needless to say, in addition to patients with an ABI < 0.9 or 0.90–0.99, those with an ABI of 1.0–1.09 showed worse prognosis than those with a normal ABI of 1.1–1.29 (HR, 1.92 and 95% Confidence Interval [CI], 1.02–3.59 for total mortality; HR, 2.82 and 95% CI, 1.22–6.54 for cardiovascular mortality after CMRA). Actually, Ono mentions that an ABI < 1.1 should already be noted from the view of prognosis. Furthermore, one study described that the best cut-off ABI was 1.1 for predicting total morality in patients with HD by ROC analysis, on which the Area Under the Curve (AUC) was 0.79, sensitivity was 0.9, and specificity was 0.62 [58]. This report comes from the same institute of Amemiya and Inoue. Therefore, in the HD population, it is implied that even an ABI < 1.1 can indicate a worse prognosis depending on the cohort.
As described above, Kato et al. showed that an ABI < 1.0 indicated worse prognosis [47] and Natsuaki et al. proved worse prognosis in patients with DM and a borderline ABI [55]. Considering this knowledge, in the patients at higher risk of lower limb artery calcification such as those with DM and HD, a borderline ABI indicates the progression of systemic atherosclerosis in the arteries body wide. This also implies that even in patients with an ABI > 1.0, a pseudo-normal ABI and prognostic deterioration should be considered depending on the cohort. Actually, the best cutoff ABI for patients with PAD > 50% square stenosis by computed tomography angiography was 0.99 in the ordinal PAD cohort [59]. In addition, Okamoto et al. reported that the sensitivity of an ABI < 0.9 for PAD in the HD cohort was only 29.9% [60]. Furthermore, other reports have shown that even a difference in ABI or ankle BP can predict future prognosis independently from ABI itself [61,62]. The Morimoto study excluded patients with an ABI < 0.9, but CMRA was performed including ABI as an adjustment, so some patients whose ABI was 0.91–1 or slightly higher might have worse prognosis, meaning baPWV underestimation but higher risk at the same time. As a result, the prognostic significance of baPWV is considered lost. In the HD studies, the prognostic value of baPWV was hardly proven, at least without the exclusion of patients with an ABI < 0.9.
Overestimation of ABI on Upper-Limb PAD
Above we discussed the overestimation of ABI that resulted from arterial calcification in the patients on HD, and there is a considerable pitfall of measuring ABI and baPWV in the HD population. As we know, ABI is calculated by dividing the ankle systolic BP by the higher brachial BP [56,57]. Measuring the brachial BP of the Hemodialysis Access (HA) side is generally contraindicated, so the brachial BP is mostly measured on the non-HA side. If PAD exists in the non-HA side (i.e. subclavian artery), its ABI is inevitably overestimated because the denominator used to calculate ABI is lower than when there is no upper-limb PAD (Figure 3). Furthermore, in this condition, baPWV is also likely overestimated. The PTT from the heart to the brachium possibly prolongs because of the stenosis, so the time difference of the brachium and the ankle shortens and the denominator for the baPWV calculation decreases. As a result, baPWV is overestimated (Figure 3).
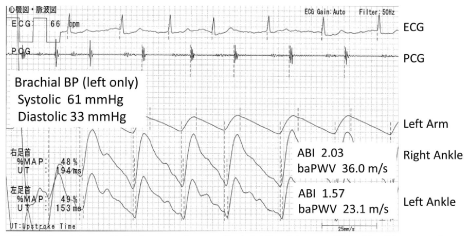
Figure 3: A hemodialysis case of upper limb PAD of the non-HA side, 72 y.o.
male. The brachial BP is able to be measured only on the left arm because
the right arm has a HA. This brachial BP is very low and the pulse wave form
of the left arm is triangular and blunt. So this apparently shows the existence
of PAD. As a result, the ABIs are abnormally high. And these pulse wave
forms are also apparently triangle on the both side. So PADs of both legs
are also strongly suspected. Therefore, both ABI and baPWV are not reliable
at all.
Of the articles we reviewed, no report defined exclusion criteria that considered this phenomenon, at least in the methodology explanation. Actually, much data such as ABI > 1.5 and baPWV > 40 m/s were seen in the Kitahara report [17]. An abnormally high ABI is also caused by arterial calcification and aortic valve regurgitation, and it is unclear whether an abnormally high ABI is a product after exclusion of the upper-limb PAD pitfall. Of course, a patient with a pseudo-high baPWV will definitely be at high risk. The BP difference of the upper limb mostly caused by upper-limb PAD is reportedly a prognostic predictor, at least in non-HD cohorts [63,64]. We must interpret the results in HD patients in whom ABI and baPWV are tested in only one brachium much more carefully than those in other populations. For these reasons, when using baPWV as a clinical biomarker in HD, a concept may require discussion. That is, it should only be indicated when at least upper-limb PAD is definitely denied and ABI is normal (i.e. =1.0), ideally when the pulse wave form of the ankle is normal on both sides. Clinicians should consider stratifying the risk of HD patients by ABI only unless a patient is in the condition mentioned above. This concept should be also considered in the DM population, especially in terms of the ankle pulse wave form, although ABI overestimation by upper-limb PAD is deniable in most cases.
Perspective
Here we provided an outline of 38 articles that evaluated the prognostic predictability of baPWV. Except for a meta-analysis of 2012 [28], 29 found it effective and eight found it ineffective. In the 2012 meta-analysis article, publication bias was evaluated by funnel plot analysis. The authors concluded that it is unlikely that there would be number of the articles estimated necessary to lose baPWV effectiveness using the fail-safeNtest. This meta-analysis was published online in early summer 2012, and they utilized the data of 16 previously published studies (of the summarized studies, 13 were effective and three were not, and these numbers were derive from the efficacy in the original article, not about the data used in this meta-analysis, and we excluded the data of the abstracts of various conferences). Approximately 2.5-fold more studies are currently available, and the balance of effective and ineffective results did not change. The data including PAD was included in this meta-analysis. Therefore, at present, even if we consider publication bias, baPWV as a whole has additive value as a prognostic predictor independent from classical risk factors. As such, we consider why baPWV has prognostic predictability. Aortic PWV has proven prognostic predictability independent from classical risk factors [1,2], while baPWV has shown a strong correlation with aortic PWV [7,9,10]. This is the same by evaluating the artery lengths, which is based on magnetic resonance imaging measurements [10]. At the same time, among various PWV, aortic PWV is the strongest factor that regulates baPWV on multiple regression analysis [10]. Therefore, it is reasonable that baPWV shows similar characteristics like cfPWV regarding prognostic predictability.
It is frequently noted that baPWV has the characteristic of being measured much higher than the real speed of the pulse wave. This is mainly caused by the condition in which heart-brachial PWV is smaller than heart-ankle PWV (haPWV) [10]. This means that the ratio of PTT heart-brachium for its distance is larger than the ratio of PTT heart-ankle for its distance, which makes Tba smaller for the defined length Lba (mentioned in the measurement paragraph) compared to Tha for its distance. Consequently, baPWV is higher than haPWV, and it increases almost parallel to aortic PWV with age [10]; in reality, this phenomenon does not cause serious issues in the clinical setting.
There were some contradicting studies among the articles reviewed, and some future subjects were proposed. It is known that baPWV tends to be effective in populations in which PAD can be effectively excluded by an ABI < 0.9; if we do this certainly, the effectiveness of baPWV is considered remarkably high. As such, it would be reasonable and easily stratify the risks for future mortality and cardiovascular events. First, a patient with an ABI = 0.9 is considered at very high risk. Second, a patient with an ABI > 0.9 and a baPWV = 18 m/s is considered at high risk. Third, a patient with an ABI > 0.9 and a baPWV 14–18 m/s is considered at middle risk. Finally, a patient with an ABI > 0.9 and a baPWV < 14 m/s is considered at lower risk (Figure 4). These baPWV cut-off values could be defined by age. This stratification method is applicable easily in the clinical setting and was recently named the Steno-Stiffness Index (SSI), which was recently introduced at the Artery Society 2014 [65]. On the other hand, in populations in which PAD is not efficiently excluded only by an ABI < 0.9, especially those with DM and HD, baPWV effectiveness is reduced. Thus, PAD exclusion criteria would have to be stricter in these patients, even though it will lose some degree of its simplicity. This precise SSI considers borderline ABI in combination with UT and %MAP to confirm the pulse wave form if necessary (Figure 5) [66]. To ensure a more precise SSI stratification, the best exclusion criteria of PAD for baPWV use as a prognostic biomarker is expected to be validated in the various cohorts as well as non-Asian populations. The use of a precise SSI would be more appropriate in western countries than in the Japanese general population. Because the prevalence of PAD morbidity in Japan is approximately 3% in the general elderly population approximately 70 years of age [67,68,69,70], it is expected to be much higher in the western countries [71,72]. The arterial path length is thought to differ between Asian and Caucasianpopulations, which might lead to the different baPWV cut-off values even upon PAD exclusion.
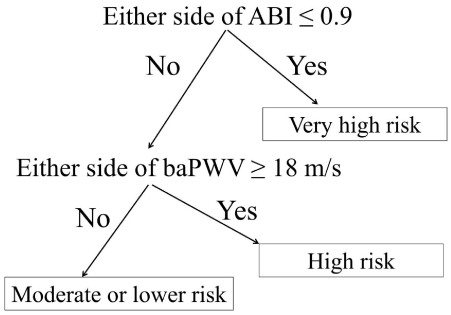
Figure 4: Risk stratification flow chart by the combination of ABI and baPWV.
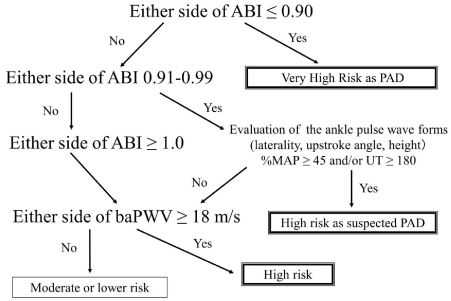
Figure 5: Precise risk stratification by ABI, baPWV (modified for this review
article).
There are still a number of studies in which baPWV was evaluated without PAD exclusion, and some avoided the risk of poorer baPWV effectiveness, but this might have occurred by chance. Even if baPWV is accurate in the absence of PAD and ABI is <0.9 in the other side limb, we must recognize that this low ABI would be a stronger prognostic predictor depending on the population. Kitahara et al. reported that even the highest quartile of baPWV (=23.8 m/s) loses its effectiveness as a predictor after CMRA adjustment to include patients with an ABI < 0.9, and ABI was the strongest predictor in this analysis [17]. Inter-study discrepancies are expected to persist if we continue to use baPWV without appropriate PAD exclusion. Therefore, in studies going forward, it is expected that baPWV should be standardized and not used as a prognostic predictor when PAD exists even only in one lower limb. Moreover, the higher, lower, right only, left only, or average baPWV can be used. According to the SSI concept, use of the higher baPWV is recommended.
Here we describe the expectation for knowledge accumulation of baPWV prognostic predictability. A larger meta-analysis conducted by the raw data in which patients with PAD patients are appropriately excluded according to the SSI concept is awaited in the future and should be repeated periodically. Improvements in the Net- Reclassification Index (NRI) as well as aortic PWV are also expected [2]. NRI improvement was demonstrated in the general population of the Hisayama study [31]. Moreover, to establish baPWV as a surrogate biomarker for the treatment of atherosclerotic diseases, a long-term intervention study targeting baPWV is desired.
Conclusion
Here we summarized available studies of baPWV prognostic predictability, which is independent from classical atherosclerotic risk factors. This efficacy is considered to improve when patients with PAD are appropriately excluded in the examination of various study populations.
References
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010; 55: 1318-1327.
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014; 63: 636-646.
- Yamashina A, Kario K, Kohara K, Sada M, Sugawara J, Suzuki H, et al. [Guidelines for noninvasive vascular function test]. (JCS2013). Japanese.
- Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014; 37: 253-390.
- Motobe K, Tomiyama H, Koji Y, Yambe M, Gulinisa Z, Arai T, et al. Cut-off value of the ankle-brachial pressure index at which the accuracy of brachial-ankle pulse wave velocity measurement is diminished. Circ J. 2005; 69: 55-60.
- Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006; 355: 2631-2639.
- Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002; 25: 359-364.
- Suzuki E, Kashiwagi A, Nishio Y, Egawa K, Shimizu S, Maegawa H, et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care. 2001; 24: 2107-2114.
- Munakata M, Ito N, Nunokawa T, Yoshinaga K. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am J Hypertens. 2003; 16: 653-657.
- Sugawara J, Hayashi K, Tanaka H. Arterial path length estimation on brachial-ankle pulse wave velocity: validity of height-based formulas. J Hypertens. 2014; 32: 881-889.
- Matsui Y, Kario K, Ishikawa J, Eguchi K, Hoshide S, Shimada K. Reproducibility of arterial stiffness indices (pulse wave velocity and augmentation index) simultaneously assessed by automated pulse wave analysis and their associated risk factors in essential hypertensive patients. Hypertens Res. 2004; 27: 851-857.
- Ono K, Tsuchida A, Kawai H, Matsuo H, Wakamatsu R, Maezawa A, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol. 2003; 14: 1591-1598.
- Koji Y, Tomiyama H, Ichihashi H, Nagae T, Tanaka N, Takazawa K, et al. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of the presence of coronary artery disease in subjects with a high risk of atherosclerotic cardiovascular disease. Am J Cardiol. 2004; 94: 868-872.
- Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol. 2003; 91: 1519-1522.
- Richart T, Kuznetsova T, Wizner B, Struijker-Boudier HA, Staessen JA. Validation of automated oscillometric versus manual measurement of the ankle-brachial index. Hypertens Res. 2009; 32: 884-888.
- Tomiyama H, Koji Y, Yambe M, Shiina K, Motobe K, Yamada J, et al. Brachial -- ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. 2005; 69: 815-822.
- Kitahara T, Ono K, Tsuchida A, Kawai H, Shinohara M, Ishii Y, et al. Impact of brachial-ankle pulse wave velocity and ankle-brachial blood pressure index on mortality in hemodialysis patients. Am J Kidney Dis. 2005; 46: 688-696.
- Matsuoka O, Otsuka K, Murakami S, Hotta N, Yamanaka G, Kubo Y, et al. Arterial stiffness independently predicts cardiovascular events in an elderly community -- Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother. 2005; 59: 40-44.
- Meguro T, Nagatomo Y, Nagae A, Seki C, Kondou N, Shibata M, et al. Elevated arterial stiffness evaluated by brachial-ankle pulse wave velocity is deleterious for the prognosis of patients with heart failure. Circ J. 2009; 73: 673-680.
- Miyano I, Nishinaga M, Takata J, Shimizu Y, Okumiya K, Matsubayashi K, et al. Association between brachial-ankle pulse wave velocity and 3-year mortality in community-dwelling older adults. Hypertens Res. 2010; 33: 678-682.
- Turin TC, Kita Y, Rumana N, Takashima N, Kadota A, Matsui K, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: findings from the Takashima study, Japan. Hypertens Res. 2010; 33: 922-925.
- Nakamura M, Yamashita T, Yajima J, Oikawa Y, Sagara K, Koike A, et al. Brachial-ankle pulse wave velocity as a risk stratification index for the short-term prognosis of type 2 diabetic patients with coronary artery disease. Hypertens Res. 2010; 33: 1018-1024.
- Orlova IA, Nuraliev EY, Yarovaya EB, Ageev FT. Prognostic value of changes in arterial stiffness in men with coronary artery disease. Vasc Health Risk Manag. 2010; 6: 1015-1021.
- Inoue T, Ogawa T, Ishida H, Ando Y, Nitta K. Aortic arch calcification evaluated on chest X-ray is a strong independent predictor of cardiovascular events in chronic hemodialysis patients. Heart Vessels. 2012; 27: 135-142.
- Chen SC, Chang JM, Liu WC, Tsai YC, Tsai JC, Hsu PC, et al. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6: 724-732.
- Munakata M, Konno S, Miura Y, Yoshinaga K. J-TOPP Study Group. Prognostic significance of the brachial-ankle pulse wave velocity in patients with essential hypertension: final results of the J-TOPP study. Hypertens Res. 2012; 35: 839-842.
- Kato A, Takita T, Furuhashi M, Maruyama Y, Miyajima H, Kumagai H. Brachial-ankle pulse wave velocity and the cardio-ankle vascular index as a predictor of cardiovascular outcomes in patients on regular hemodialysis. Ther Apher Dial. 2012; 16: 232-241.
- Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. 2012; 60: 556-562.
- Han JY, Choi DH, Choi SW, Kim BB, Ki YJ, Chung JW, et al. Predictive value of brachial-ankle pulse wave velocity for cardiovascular events. Am J Med Sci. 2013; 346: 92-97.
- Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Maekawa Y, et al. Cut-off value of brachial-ankle pulse wave velocity to predict cardiovascular disease in hypertensive patients: a cohort study. J Atheroscler Thromb. 2013; 20: 391-400.
- Ninomiya T, Kojima I, Doi Y, Fukuhara M, Hirakawa Y, Hata J, et al. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: the Hisayama Study. J Hypertens. 2013; 31: 477-483.
- Ishisone T, Koeda Y, Tanaka F, Sato K, Nagano M, Nakamura M. Comparison of utility of arterial stiffness parameters for predicting cardiovascular events in the general population. Int Heart J. 2013; 54: 160-165.
- Yoon HE, Shin DI, Kim SJ, Koh ES, Hwang HS, Chung S, et al. Brachial-ankle pulse wave velocity predicts decline in renal function and cardiovascular events in early stages of chronic kidney disease. Int J Med Sci. 2013; 10: 1430-1436.
- Takashima N, Turin TC, Matsui K, Rumana N, Nakamura Y, Kadota A, et al. The relationship of brachial-ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. J Hum Hypertens. 2014; 28: 323-327.
- Nagai K, Shibata S, Akishita M, Sudoh N, Obara T, Toba K, et al. Efficacy of combined use of three non-invasive atherosclerosis tests to predict vascular events in the elderly; carotid intima-media thickness, flow-mediated dilation of brachial artery and pulse wave velocity. Atherosclerosis. 2013; 231: 365-370.
- Sugamata W, Nakamura T, Uematsu M, Kitta Y, Fujioka D, Saito Y, et al. The combined assessment of flow-mediated dilation of the brachial artery and brachial-ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease. J Cardiol. 2014; 64: 179-184.
- Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Oguro R, et al. Prognostic impact of regional arterial stiffness in hypertensive patients. Heart Vessels. 2014.
- Kim J, Song TJ, Song D, Lee KJ, Kim EH, Lee HS, et al. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension. 2014; 64: 240-246.
- Maeda Y, Inoguchi T, Etoh E, Kodama Y, Sasaki S, Sonoda N, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality and cardiovascular events in patients with diabetes: the Kyushu Prevention Study of Atherosclerosis. Diabetes Care. 2014; 37: 2383-2390.
- Ki YJ, Choi DH, Lee YM, Lim L, Song H, Koh YY. Predictive value of brachial-ankle pulse wave velocity for long-term clinical outcomes after percutaneous coronary intervention in a Korean cohort. Int J Cardiol. 2014; 175: 554-559.
- Katakami N, Osonoi T, Takahara M, Saitou M, Matsuoka TA, Yamasaki Y, et al. Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc Diabetol. 2014; 13: 128.
- Sheng CS, Li Y, Li LH, Huang QF, Zeng WF, Kang YY, et al. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014; 64: 1124-1130.
- Chang LH, Lin HD, Kwok CF, Won JG, Chen HS, Chu CH, et al. The combination of the ankle brachial index and brachial ankle pulse wave velocity exhibits a superior association with outcomes in diabetic patients. Intern Med. 2014; 53: 2425-2431.
- Seo HJ, Ki YJ, Han MA, Choi DH, Ryu SW. Brachial-ankle pulse wave velocity and mean platelet volume as predictive values after percutaneous coronary intervention for long-term clinical outcomes in Korea: A comparable and additive study. Platelets. 2014: 1-7.
- Kuroiwa Y, Miyano I, Nishinaga M, Takata J, Shimizu Y, Okumiya K, et al. Association between level of brachial-ankle pulse wave velocity and onset of activities of daily living impairment in community-dwelling older individuals. Geriatr Gerontol Int. 2014.
- Morimoto S, Yurugi T, Aota Y, Sakuma T, Jo F, Nishikawa M, et al. Prognostic significance of ankle-brachial index, brachial-ankle pulse wave velocity, flow-mediated dilation, and nitroglycerin-mediated dilation in end-stage renal disease. Am J Nephrol. 2009; 30: 55-63.
- Kato A, Takita T, Furuhashi M, Kumagai H, Hishida A. A small reduction in the ankle-brachial index is associated with increased mortality in patients on chronic hemodialysis. Nephron Clin Pract. 2010; 114: 29-37.
- Chen SC, Chang JM, Tsai JC, Hsu PC, Lin TH, Su HM, et al. A new systolic parameter defined as the ratio of brachial pre-ejection period to brachial ejection time predicts overall and cardiovascular mortality in hemodialysis patients. Hypertens Res. 2010; 33: 492-498.
- Amemiya N, Ogawa T, Otsuka K, Ando Y, Nitta K. Comparison of serum albumin, serum C-reactive protein, and pulse wave velocity as predictors of the 4-year mortality of chronic hemodialysis patients. J Atheroscler Thromb. 2011; 18: 1071-1079.
- Tanaka M, Ishii H, Aoyama T, Takahashi H, Toriyama T, Kasuga H, et al. Ankle brachial pressure index but not brachial-ankle pulse wave velocity is a strong predictor of systemic atherosclerotic morbidity and mortality in patients on maintenance hemodialysis. Atherosclerosis. 2011; 219: 643-647.
- Yoshida M, Mita T, Yamamoto R, Shimizu T, Ikeda F, Ohmura C, et al. Combination of the Framingham risk score and carotid intima-media thickness improves the prediction of cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2012; 35: 178-180.
- Kuwahara M, Hasumi S, Mandai S, Tanaka T, Shikuma S, Akita W, et al. Rate of ankle-brachial index decline predicts cardiovascular mortality in hemodialysis patients. Ther Apher Dial. 2014; 18: 9-18.
- Park KH, Han SJ, Kim HS, Kim MK, Jo SH, Kim SA, et al. Impact of framingham risk score, flow-mediated dilation, pulse wave velocity, and biomarkers for cardiovascular events in stable angina. J Korean Med Sci. 2014; 29: 1391-1397.
- Otsuka K, Fukuda S, Shimada K, Suzuki K, Nakanishi K, Yoshiyama M, et al. Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res. 2014; 37: 1014-1020.
- Natsuaki C, Inoguchi T, Maeda Y, Yamada T, Sasaki S, Sonoda N, et al. Association of borderline ankle-brachial index with mortality and the incidence of peripheral artery disease in diabetic patients. Atherosclerosis. 2014; 234: 360-365.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007; 33: 1-75.
- Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011; 58: 2020-2045.
- Otani Y, Otsubo S, Kimata N, Takano M, Abe T, Okajima T, et al. Effects of the ankle-brachial blood pressure index and skin perfusion pressure on mortality in hemodialysis patients. Intern Med. 2013; 52: 2417-2421.
- Ichihashi S, Hashimoto T, Iwakoshi S, Kichikawa K. Validation study of automated oscillometric measurement of the ankle-brachial index for lower arterial occlusive disease by comparison with computed tomography angiography. Hypertens Res. 2014; 37: 591-594.
- Okamoto K, Oka M, Maesato K, Ikee R, Mano T, Moriya H, et al. Peripheral arterial occlusive disease is more prevalent in patients with hemodialysis: comparison with the findings of multidetector-row computed tomography. Am J Kidney Dis. 2006; 48: 269-276.
- Chen SC, Chang JM, Tsai YC, Tsai JC, Su HM, Hwang SJ, et al. Association of interleg BP difference with overall and cardiovascular mortality in hemodialysis. Clin J Am Soc Nephrol. 2012; 7: 1646-1653.
- Lin CY, Leu JG, Fang YW, Tsai MH. Association of interleg difference of ankle brachial index with overall and cardiovascular mortality in chronic hemodialysis patients. Ren Fail. 2015; 37: 88-95.
- Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012; 379: 905-914.
- Sheng CS, Liu M, Zeng WF, Huang QF, Li Y, Wang JG. Four-limb blood pressure as predictors of mortality in elderly Chinese. Hypertension. 2013; 61: 1155-1160.
- Vlachopoulos C. AT the Artery Conference 2014.
- Yamashina A. [Proceedings of the 14th Conference of Clinical Research on Blood Pressure and Pulse Wave, Tokyo, Japan].
- Fujiwara T, Saitoh S, Takagi S, Ohnishi H, Ohata J, Takeuchi H, et al. Prevalence of asymptomatic arteriosclerosis obliterans and its relationship with risk factors in inhabitants of rural communities in Japan: Tanno-Sobetsu study. Atherosclerosis. 2004; 177: 83-88.
- Ishida A, Miyagi M, Kinjo K, Ohya Y. Age- and sex-related effects on ankle-brachial index in a screened cohort of Japanese: the Okinawa Peripheral Arterial Disease Study (OPADS). Eur J Prev Cardiol. 2012; 21: 712-718.
- Shigematsu H. [Epidemiology of peripheral arterial disease in Japan. IGAKU-NO-AYUMI 2013; 245].
- Ohnishi H, Sawayama Y, Furusyo N, Maeda S, Tokunaga S, Hayashi J. Risk factors for and the prevalence of peripheral arterial disease and its relationship to carotid atherosclerosis: the Kyushu and Okinawa Population Study (KOPS). J Atheroscler Thromb. 2010; 17: 751-758.
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013; 382: 1329-1340.
- Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009; 120: 2053-2061.