
Research Article
J Cardiovasc Disord. 2015;2(1): 1011.
Usefulness of Stress Myocardial Perfusion Imaging and Baseline Clinical Factors to Predict Cardiovascular Events in Patients with Peripheral Artery Disease
Furuhashi T, Moroi M*, Minakawa M, Masai H, Kunimasa T, Fukuda H and Sugi K
Division of Cardiovascular Medicine, Toho University Ohashi Medical Center, Japan
*Corresponding author: Moroi M, Division of Cardiovascular Medicine, Toho University Ohashi Medical Center, 2-17-6 Ohashi, Meguro-ku, Tokyo 153- 8515, Japan
Received: March 19, 2015; Accepted: April 27, 2015; Published: April 28, 2015
Abstract
Introduction: Peripheral Artery Disease (PAD) is a well-established risk factor for poor cardiovascular prognosis. Stress Myocardial Perfusion Imaging (MPI) is a widely used diagnostic and prognostic tool for Coronary Artery Disease (CAD). Normal stress MPI generally correlates with excellent cardiovascular prognosis. We assessed the usefulness of stress MPI and baseline clinical factors as independent predictors of cardiovascular events in patients with PAD and suspected or known CAD.
Methods: Stress MPI was performed in 97 PAD patients. The mean followup period was 30 months. PAD was defined as an ankle-brachial index of <0.9 or a history of revascularisation for PAD. Advanced Chronic Kidney Disease (CKD) was defined as Stage IV to V CKD or CKD requiring haemodialysis. Cardiovascular events included cardiac death, nonfatal myocardial infarction and Braunwald class III unstable angina requiring hospitalisation.
Results: Cardiovascular events were observed in 28 patients (29%). Multivariate Cox regression analysis revealed that advanced CKD (hazard ratio = 4.03; P < 0.001); left ventricular ejection fraction (hazard ratio = 0.96; P = 0.008); and, summed stress scores on stress MPI (hazard ratio = 1.15; P = 0.013) were independent and significant predictors of cardiovascular events.
Conclusion: In PAD patients, advanced CKD, impaired left ventricular systolic function and abnormal summed stress scores on stress MPI can be significant and independent predictors of cardiovascular events. In patients who have these risk factors, aggressive management strategies (strengthened suboptimal therapies and careful observation) should be implemented as early as possible.
Keywords: Chronic Kidney Disease; Coronary Artery Disease; Myocardial Perfusion Defect; Prognosis
Abbreviations
ABI: Ankle-Brachial Index; CAD: Coronary Artery Disease; CKD: Chronic Kidney Disease; eGFR: Estimated Glomerular Filtration Rate; LVEF: Left Ventricular Ejection Fraction; MI: Myocardial Infarction; MPI: Myocardial Perfusion Imaging; PAD: Peripheral Artery Disease; QGS: Quantitative Gated SPECT; SSS: Summed Stress Score; SRS: Summed Rest Score; SDS: Summed Difference Score; SPECT: Single-Photon Emission Computed Tomography
Introduction
PAD is well established as a risk factor for cardiovascular disease, and previous angiographic studies have shown that 33–55% of patients with asymptomatic PAD have significant coronary stenosis [1-6]. CKD is also a major cardiovascular risk factor [7-10], and poor cardiovascular prognosis is characteristic of CKD patients treated with haemodialysis. Previous studies have established the usefulness of stress MPI to diagnose CAD and to assess cardiovascular prognosis [11-19]. Normal stress MPI results correlate with excellent cardiovascular prognosis, as evidenced by the annual cardiovascular event rate of <1% [20]. On the other hand, in CKD patients treated with haemodialysis, normal stress MPI results are not necessarily associated with good cardiovascular prognosis; of haemodialysis patients with normal stress MRI results, 4–9% per year experience cardiovascular events, with poor prognosis [21,22]. Here, we assess the usefulness of baseline clinical factors and stress MPI to predict cardiovascular events in patients with PAD and suspected or known CAD.
Methods
Patients and study protocol
This was a retrospective study of patients who had undergone stress MPI. In total, 1015 consecutive patients with a suspected or confirmed history of CAD underwent thallium-201 stress MPI between 2008 and 2010. The following patients were excluded: 154 with no prognostic data following stress MPI; 760 with no evidence of PAD; and 4 with significant ischaemia (SDS of =2 on stress MPI) who achieved revascularization within 2 months of subsequent percutaneous coronary intervention [14]. In total, 97 patients with PAD were included in the study. PAD was defined on the basis of a previous medical history of angioplasty of peripheral arteries or an ABI of <0.9 [1-3].
The study protocol was approved by the Committee on Human Investigation of the Toho University Ohashi Medical Center (approval No. 12-62), and the study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki (as revised in Tokyo in 2004) and subsequent revisions. All included patients provided informed consent.
Information on the history of PAD was obtained from clinical records or patient interviews. In addition, data on age, sex, medication usage, past medical history and other coronary risk factors were routinely collected during stress MPI. The following were considered possible cardiovascular risk factors: cigarette smoking (current and past), history of hypertension, diabetes, hyperlipidaemia, or CKD; or a history of CAD in a first-degree relative (aged <55 years for men and <65 years for women). Hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg or current treatment with antihypertensive medications. Diabetes was defined as fasting blood glucose >126 mg/dL, glycosylated haemoglobin >6.5% (based on the National Glycohemoglobin Standardization Program definition) or current treatment with insulin or oral anti-diabetic medications. Hyperlipidaemia was defined as the presence of hypercholesterolaemia (total cholesterol >220 mg/dL), hypertriglyceridaemia (serum triglyceride >150 mg/dL) or current lipid-lowering therapy. CKD was defined according to the National Kidney Foundation criteria [23] as an eGFR of < 60 mL/min/1.73 m² or persistent proteinuria for at least 3 months. Advanced CKD was defined as confirmed (2 consecutive measurements =3 months apart) eGFR = 30 mL/min/1.73 m² (Stage IV to V of CKD) and haemodialysis for =1 month. The LVEF was measured by motionmode echocardiography within 1 month of stress MPI.
Stress MPI
Exercise or pharmacological stress tests were performed. Cardiac medications and caffeine ingestion were stopped for 1 day before the examination. All exercise tests were performed using a treadmill; no patient underwent ergometer exercise stress testing. The treadmill exercise test (Bruce protocol) was considered adequate if patients achieved 85% of the maximum predicted heart rate or developed chest pain. Patients with limited exercise capacity (who failed to achieve 85% of the predicted heart rate during the treadmill test) underwent a pharmacological stress test using intravenous adenosine infusion. Adenosine and thallium-201 were administered in different arms. An automated infusion pump was used to deliver intravenous adenosine (0.120 mg/kg/min) over 6 min. Thallium-201 (111 MBq; Fujifilm RI Pharma Co., Ltd., Tokyo, Japan) was injected into a peripheral vein either 1 min before cessation of the treadmill stress test or 3 min after initiation of adenosine infusion.
All myocardial perfusion SPECT data were acquired using a 3-headed gamma camera (MS-3; Siemens, Chicago, IL, USA) equipped with a low-energy cardiofocal collimator and a computer interface (ICON; Siemens, Chicago, IL, USA). Stress SPECT was performed 10 after stress testing, and resting SPECT was performed 4 after MPI. In total, 90 projections were obtained for 20 s each in 4/360° intervals and stored on 64 × 64 matrices. A 15% symmetrical energy window centred on the 70 keV peak was used. Tomographic reconstruction was performed by the standard filtered back-projection technique using a Butterworth filter with a cut-off frequency of 0.5 cycles/pixel, and an order of 5. No correction was made for attenuation or scatter.
SPECT images were reoriented along the short horizontal and vertical long axes for analysis. SPECT data analysis was performed on the basis of agreement between at least two experienced nuclear medicine physicians (T.F. and M.M). Defects were classified as reversible (including partially reversible) or fixed (irreversible). SPECT images were assessed to determine the presence, location and severity of any perfusion defect. Observers assessed SPECT images visually and did not use software applications. The left ventricle was divided into 17 segments, each of which was assigned a score using a 5-point scoring system (0 = normal, 1 = mildly reduced, 2 = moderately reduced, 3 = severely reduced and 4 = uptake absent). The following scores were calculated: the Summed Score at Stress (SSS) which showed myocardial ischaemia and MI; the summed score at rest Summed Rest Score (SRS) which showed MI and prolonged myocardial ischaemia [24] and the difference between the Stress and rest Scores (SDS), which showed myocardial ischaemia. As demonstrated in previous studies, based on an excellent cardiovascular prognosis, an SSS of <4 was considered to be normal [12,25,26].
Endpoints and follow-up
Follow-up commenced after the assessment of clinical information and stress MPI. Cardiovascular events considered as endpoints were cardiac death, nonfatal MI and Braunwald class III unstable angina requiring hospitalisation. Cardiac deaths included sudden death, fatal MI and death due to heart failure or death due to arrhythmia. Sudden death was defined as witnessed cardiac arrest, death within 1 h of onset of acute symptoms or unexpected death in people who had been considered well for the previous 24 h. Braunwald class III unstable angina was defined as acute angina at rest within 48 h of onset. Patients were regularly followed up for a mean duration of 31 ± 21 months (range, 1–65 months).
Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as observed number of patients (percentage). To compare patient characteristics between groups, the Mann–Whitney U test was used for continuous variables, and Fisher’s exact test was used for categorical variables. Cox regression hazard analysis was used to assess the possible risk factors. Kaplan–Meier event-free curves were generated, and the risks of endpoints were compared between patient groups using logrank tests. Statistical analyses were performed using SPSS statistical software (SPSS Incorporated, Chicago, Illinois, USA). A P value <0.05 was considered statistically significant.
Results and Discussion
Patient characteristics
Table 1 summarizes patient characteristics and compares the results of patients who developed cardiovascular events with those of patients who did not. The frequency of advanced CKD, a familial history of CAD and a history of CAD were significantly higher in patients who developed cardiovascular events. Patients who developed cardiovascular events had lower LVEFs on echocardiography. Regarding stress MPI results, SSS and SRS were significantly higher in patients who developed cardiovascular events.
Overall
Events +
Events -
P
(n = 97)
(n = 28)
(n = 69)
Age, years
75 ± 11
74 ± 10
75 ± 11
0.49
Male
66 (68%)
22 (79%)
44 (64%)
0.23
Hypertension
85 (88%)
26 (93%)
59 (86%)
0.5
Diabetes
56 (58%)
17 (61%)
39 (57%)
0.82
Hyperlipidaemia
62 (64%)
17 (61%)
45 (65%)
0.82
CKD
71 (73%)
22 (79%)
49 (71%)
0.61
Advanced CKD
42 (43%)
17 (61%)
25 (36%)
0.04
Haemodialysis
28 (29%)
12 (43%)
16 (23%)
0.08
Smoking history
66 (68%)
21 (75%)
45 (65%)
0.47
Current smoking status
14 (14%)
6 (21%)
8 (12%)
0.22
Familial history of CAD
13 (13%)
7 (25%)
6 (9%)
0.05
Cerebral vascular disease
30 (31%)
9 (32%)
21 (30%)
>0.99
History of revascularisation for PAD
39 (40%)
11 (39%)
28 (41%)
>0.99
History of CAD
45 (46%)
18 (64%)
27 (39%)
0.03
History of MI
19 (20%)
8 (29%)
11 (16%)
0.17
Laboratory data
Haemoglobin
11.7 ± 2.0
11.7 ± 1.8
11.6 ± 0.4
0.81
Albumin
3.9 ± 0.5
4.0 ± 0.5
3.9 ± 0.5
0.49
HbA1c
6.1 ± 1.0
6.2 ± 1.1
6.0 ± 1.0
0.55
LDL cholesterol
98 ± 28
95 ± 28
99 ± 29
0.68
HDL cholesterol
51 ± 16
49 ± 15
52 ± 16
0.32
LDL/HDL
2.1 ± 0.8
2.1 ± 0.8
2.1 ± 0.8
0.98
Echocardiography
LVEF
61 ± 14
54 ± 16
64 ± 13
0
Adenosine stress test
90 (93%)
25 (89%)
65 (94%)
0.41
MPI
SSS
2.5 ± 3.4
4.6 ± 4.8
1.6 ± 2.1
0.01
SRS
1.2 ± 2.6
2.6 ± 3.9
0.7 ± 1.5
0.02
SDS
1.2 ± 2.2
2.0 ± 3.1
0.9 ± 1.6
0.16
Medication
Antiplatelet
77 (79%)
22 (79%)
55 (80%)
>0.99
Aspirin
58 (60%)
18 (64%)
40 (58%)
0.65
Clopidogrel
22 (23%)
6 (21%)
16 (23%)
>0.99
Cilostazol
11 (11%)
5 (18%)
23 (27%)
0.29
ACE-I/ARB
68 (70%)
17 (61%)
51 (74%)
0.23
Beta-blocker
33 (34%)
10 (36%)
23 (33%)
0.82
Ca channel blocker
49 (51%)
14 (50%)
35 (51%)
>0.99
Diuretics
44 (45%)
15 (54%)
29 (42%)
0.37
Statin
45 (46%)
13 (46%)
32 (46%)
>0.99
CKD: Chronic Kidney Disease; CAD: Coronary Artery Disease; MI: Myocardial Infarction; LDL: Low-Density Lipoprotein; HDL: High-Density Lipoprotein; LVEF: Left Ventricular Ejection Fraction; MPI: Myocardial Perfusion Imaging; SSS: Summed Stress Score; SRS: Summed Rest Score; SDS: Summed Difference Score; ACE-I: Angiotensin-Converting Enzyme Inhibitor; ARB: Angiotensin II Receptor Blocker.
Table 1: Patients’ characteristics.
Outcomes
Cardiovascular events occurred in 28 patients with PAD (cardiac death in 12 patients, nonfatal MI in 11 patients and Braunwald III unstable angina in five patients). Univariate Cox regression hazard analysis (Table 2) revealed that advanced CKD, haemodialysis, reduced LVEF on echocardiography and abnormal parameters of stress MPI (SSS, SRS and SDS) were significant predictors of cardiovascular events. As previously reported, the minimum number of variables that should be entered into a multivariable model is a function of the number of events that occur during the study. In general, 1 variable for every 10 events is strongly recommended [27]. From the Cox univariate regression analysis, the relative risk level was calculated by multivariate Cox regression hazard analysis for cardiovascular events associated with advanced CKD, LVEF on echocardiography and SSS on stress MPI. Advanced CKD (hazard ratio = 4.03, P < 0.001), reduced LVEF on echocardiography (hazard ratio = 0.96, P = 0.008) and abnormal SSS on stress MPI (hazard ratio = 1.15, P = 0.013) were independent and significant risk factors for cardiovascular events (Table 3).
Hazard ratio
95% CI
P
Male
1.76
0.71–4.35
0.22
Age
1
0.97–1.06
0.99
Cerebral vascular disease
1.08
0.48–2.35
0.88
History of revascularisation for PAD
0.88
0.41–1.89
0.75
Hypertension
2.29
0.54–9.62
0.26
Diabetes
1.19
0.56–2.54
0.66
CKD
1.65
0.67–4.08
0.28
Advanced CKD
2.75
1.28–5.92
0.01
Haemodialysis
2.12
1.01–4.48
0.049
Hyperlipidaemia
1.41
0.66–3.03
0.37
Current smoking status
1.81
0.73–4.46
0.2
Smoking history
1.39
0.59–3.27
0.45
Familial history of CAD
2.55
0.99–6.02
0.05
History of CAD
2.37
0.99–5.24
0.05
History of MI
1.64
0.72–3.72
0.24
Laboratory data
Haemoglobin
0.97
0.80–1.17
0.73
Albumin
1.21
0.51–2.90
0.66
LDL
0.99
0.98–1.01
0.88
HDL
0.98
0.96–1.01
0.28
LDL/HDL
1.12
0.70–1.80
0.65
Echocardiography
LVEF
0.95
0.93–0.98
<0.001
Stress test
Adenosine stress test
0.6
0.18–2.00
0.41
Stress MPI
SSS
1.21
1.11–1.33
<0.001
SRS
1.18
1.07–1.31
0.001
SDS
1.19
1.03–1.37
0.017
CI: Confidence Interval; PAD: Peripheral Arterial Disease; CKD: Chronic Kidney Disease; CAD: Coronary Artery Disease; LDL: Low-Density Lipoprotein; HDL: High-Density Lipoprotein; LVEF: Left Ventricular Ejection Fraction; MPI: Myocardial Perfusion Imaging; SSS: Summed Stress Score; SRS: Summed Rest Score; SDS: Summed Difference Score.
Table 2: Univariate Cox Regression Analysis.
Hazard ratio
95% CI
P
Advanced CKD
4.03
1.77–9.17
<0.001
LVEF
0.96
0.93–0.99
0.008
SSS
1.15
1.03–1.28
0.013
CI: Confidence Interval; CKD: Chronic Kidney Disease; LVEF: Left Ventricular Ejection Fraction; SSS: Summed Stress score.
Table 3: Multivariate Cox regression analysis.
The Kaplan–Meier survival curves shown in Figure 1 reveal a superior prognosis (concerning cardiovascular events) in patients with normal stress MPI (SSS =3) compared with patients with abnormal stress MPI (SSS >3). However, as shown in Figure 1, even patients with normal stress MPI did not have a very good prognosis for cardiovascular events (3 events; 4% in the first year), although their cardiovascular prognosis was better than that of patients with abnormal stress MPI. Although 74 of 97 (76%) patients had normal stress MPI results, we also assessed the prognosis of patients with abnormal stress MPI (SSS >3 and <7 versus SSS =7). The difference was not significant in our relatively small sample, but the prognosis tended to be better in patients with SSS >3 and <7 than in patients with SSS =7 [3 events in 9 patients (33%) versus 10 events in 14 patients (71%), P = 0.08].
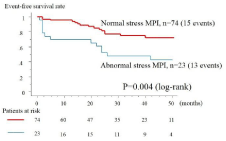
Figure 1: Kaplan–Meier survival curves for the absence of cardiovascular
events in patients with normal stress Myocardial Perfusion Imaging (MPI) or
Summed Stress Score (SSS) =3 on stress MPI versus those with abnormal
stress MPI (SSS >3). The prognosis was significantly superior in patients with
normal stress MPI compared with patients with abnormal stress MPI.
This study also examined the predictive value of the number of risk factors (abnormal stress MPI, advanced CKD and LVEF <50%) that indicated poor cardiovascular prognosis. The number of risk factors identified by multivariate Cox regression analysis was then determined as follows: 0 risk factor, n = 38, 3 events (8%); 1 risk factor, n = 40, 14 events (35%); 2 risk factors, n = 15, 8 events (53%); and 3 risk factors, n = 4, 3 events (75%). In univariate Cox regression analysis, the number of risk factors was identified as a predictor of cardiovascular events (hazard ratio = 2.32, P < 0.001). Figure 2 compares the Kaplan–Meier survival curves for patients with different numbers of risk factors (0, 1 and 2–3 factors). The prognosis of cardiovascular events was significantly different with different numbers of co-existing risk factors.
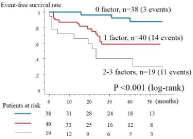
Figure 2: Kaplan–Meier survival curves for the absence of cardiovascular
events in patients with co-existing risk factors [advanced Chronic Kidney
Disease (CKD), Left Ventricular Ejection Fraction (LVEF) < 50% and abnormal
stress Myocardial Perfusion Imaging (MPI)]. The prognosis for cardiovascular
events differed significantly with different numbers of co-existing risk factors.
Discussion
Atherosclerosis is a systemic degenerative inflammatory vascular disease and is the primary underlying cause of CAD. About half of all patients who die from CAD have no previous diagnosis or symptoms of cardiac disease [28]. Previous studies have shown that PAD is a risk factor for cardiovascular disease [1-3]. In fact, previous angiographic studies have shown that 33–55% of asymptomatic patients with PAD have significant coronary stenosis [4-6]. The present study demonstrated that advanced CKD, reduced LVEF and the grade of defects in the stress phase assessed by stress MPI results were significant and independent predictors of cardiovascular events in patients with PAD. In addition, previous studies at our institution have shown that PAD is a key factor among patients with normal stress MPI [29], and abnormal stress MPI and PAD are independent and significant predictors of cardiovascular events in haemodialysis patients [30]. Even in patients who have normal stress MPI, PAD is a significant predictor of poor cardiovascular outcomes.
The relationship between CKD and adverse cardiovascular prognosis has been established by large community-based studies [7-10], and in patients who undergo stress MPI, lower eGFR is an independent and significant predictor of cardiovascular events [31- 33]. In univariate Cox regression analysis, the present study showed that advanced CKD (including haemodialysis in 28 patients) was a stronger predictor of adverse outcomes than CKD and haemodialysis. However, CKD itself is not a useful predictor of cardiovascular events. Interestingly, 71 patients (73%) in our study had CKD, but cardiovascular events were not frequent in these CKD patients. Seven of 26 (27%) non-CKD patients had abnormal stress MPI. However, no cardiovascular events occurred in this group during the first year, while 2 of 19 (11%) non-CKD patients with normal stress MPI experienced cardiovascular events. The cardiovascular prognosis was not particularly good in non-CKD patients with normal stress MPI.
In this analysis, 74 of 97 patients presented with normal stress MPI. We could not adequately assess the prognosis of the 23 patients with abnormal stress MPI. We conclude that patients with a SSS >3 and <7 showed a tendency toward better outcomes than patients with a SSS =7, but these observations await statistical confirmation in larger studies.
LVEF has been widely used to assess prognosis in cardiovascular disease [34-37], and the values of LVEF measured by echocardiography, electrocardiogram-gated SPECT and left ventriculography are closely correlated [38]. The present study showed that reduced LVEF, assessed by echocardiography, could be a predictor of cardiovascular events.
Aggressive management strategies (careful observation, strengthening of so-called suboptimal therapies and coronary revascularisation when myocardial ischaemia is observed) are needed as early as possible to prevent adverse cardiac events in patients with PAD, particularly in those with co-existing advanced CKD, reduced left ventricular systolic function and high SSS on stress MPI, regardless of the presence or absence of typical angina symptoms.
Study limitations
An important limitation of this study is that, because it was a singlecentre retrospective trial, selection bias could be included. Baseline patient characteristics such as clinical data related to symptoms or history may not be completely accurate. Electrocardiogramgated SPECT was only performed in 28 patients, in 9 of whom cardiovascular events were observed. During the period when stress MPI was assessed (2008–2010), the technique was not performed regularly at our institution because of the limited informationprocessing ability of the available instruments. Moreover, we believed that decreased myocardial thallium washout was suitable to detect myocardial ischaemia, particularly in patients with multiplevessel disease and balanced ischaemia. Compared with technetium, thallium was not suitable for QGS and increased radiation exposure. We could not assess the predictive value of cardiac function measured by QGS, including the LV end-diastolic and end-systolic volumes, LVEF and peak filling rate because we did not use QGS in all patients. The periods between stress MPI and gathering of laboratory data (i.e. examination of ABI or echocardiography) varied, but all these data were obtained within a 3-month period before and after stress MPI. Finally, we cannot exclude the possibility that cardiovascular events were missed because of insufficient follow-up.
Conclusion
In conclusion, although the present study has several limitations, we believe our findings provide valuable insights into the usefulness of both background risk factors and stress MPI as predictors of cardiovascular risk in patients with PAD. In patients with PAD, baseline clinical factors (such as advanced CKD), cardiac functions and stress MPI can be powerful predictors of cardiovascular events.
Acknowledgments
We thank Chihiro Hamazaki and Takeharu Ando for their technical assistance in the use of stress MPI.
Disclosure of Financial Interest
Kaoru Sugi has received scholarship funds from Sanofi Aventis, Mochida Pharmaceutical, Daiichi-Sankyo and Dai Nippon Sumitomo Pharmaceutical and lecture fees from Bayer Healthcare and Boehringer Ingelheim.
References
- Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999; 19: 538-545.
- Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004; 109: 733-739.
- Aboyans V, Lacroix P, Postil A, Guilloux J, Rolie F, Comu E, et al. Subclinical peripheral arterial disease and incompressible ankle arteries are both long-term prognostic factors in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2005; 46: 815-820.
- Kioka Y, Tanabe A, Kotani Y, Yamada N, Nakahama M, Ueda T, et al. Review of coronary artery disease in patients with infrarenal abdominal aortic aneurysm. Circ J. 2002; 66: 1110-1112.
- Her K, Choi C, Park Y, Shin H, Won Y. Concomitant peripheral artery disease and asymptomatic coronary artery disease: a management strategy. Ann Vasc Surg. 2008; 22: 649-656.
- Marsico F, Ruggiero D, Parente A, Pirozzi E, Musella F, Lo Iudice F, et al. Prevalence and severity of asymptomatic coronary and carotid artery disease in patients with lower limbs arterial disease. Atherosclerosis. 2013; 228: 386-389.
- Meisinger C, Doring A, Lowel H, KORA Study Group. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006; 27: 1245-1250.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351: 1296-1305.
- Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003; 41: 47-55.
- Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004; 15: 1307-1315.
- Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996; 93: 905-914.
- Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998; 97: 535-543.
- Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Stress myocardial perfusion single-photon emission computed tomography is clinically effective and cost effective in risk stratification of patients with a high likelihood of coronary artery disease (CAD) but no known CAD. J Am Coll Cardiol. 2004; 43: 200-208.
- Sharir T, Germano G, Kavanagh PB, Lai S, Cohen I, Lewin HC, et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999; 100: 1035-1042.
- Nishimura T, Nakajima K, Kusuoka H, Yamashina A, Nishimura S. Prognostic study of risk stratification among Japanese patients with ischemic heart disease using gated myocardial perfusion SPECT: J-ACCESS study. Eur J Nucl Med Mol Imaging. 2008; 35: 319-328.
- Berman D, Hachamovitch R, Lewin H, Friedman J, Shaw L, Germano G. Risk stratification in coronary artery disease: implications for stabilization and prevention. Am J Cardiol. 1997; 79: 10-16.
- Berman DS, Kiat H, Friedman JD, Wang FP, van Train K, Matzer L, et al. Separate acquisition rest thallium-201/stress technetium-99 m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography: a clinical validation study. J Am Coll Cardiol. 1993; 22: 1455-1464.
- Berman DS, Hachamovitch R, Kiat H, Cohen I, Cabico JA, Wang FP, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99 m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 1995; 26: 639-647.
- Galassi AR, Azzarelli S, Tomaselli A, Giosofatto R, Ragusa A, Musumeci S, et al. Incremental prognostic value of technetium-99m-tetrofosmin exercise myocardial perfusion imaging for predicting outcomes in patients with suspected or known coronary artery disease. Am J Cardiol. 2001; 88: 101-106.
- Shaw LJ, Hendel R, Borges-Neto S, Lauer MS, Alazraki N, Burnette J, et al. Prognostic value of normal exercise and adenosine (99m)Tc-tetrofosmin SPECT imaging: results from the multicenter registry of 4,728 patients. J Nucl Med. 2003; 44: 134-139.
- Hase H, Joki N, Ishikawa H, Fukuda H, Imamura Y, Saijyo T, et al. Prognostic value of stress myocardial perfusion imaging using adenosine triphosphate at the beginning of haemodialysis treatment in patients with end-stage renal disease. Nephrol Dial Transplant. 2004; 19: 1161-1167.https://www.ncbi.nlm.nih.gov/pubmed/16340725
- Caglar M, Mahmoudian B, Aytemir K, Kahraman S, Arici M, Kabakci G, et al. Value of 99 mTc-methoxyisobutylisonitrile (99 mTc-MIBI) gated SPECT for the detection of silent myocardial ischemia in hemodialysis patients: Clinical variables associated with abnormal test results. Nucl Med Commun. 2006; 27: 61-69.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39: S1-266.
- Lee DS, Yeo JS, Chung JK, Lee MM, Lee MC. Transient prolonged stunning induced by dipyridamole and shown on 1- and 24-hour poststress 99mTc-MIBI gated SPECT. J Nucl Med. 2000; 41: 27-35.
- Ladenheim ML, Pollock BH, Rozanski A, Berman DS, Staniloff HM, Forrester JS, et al. Extent and severity of myocardial hypoperfusion as predictors of prognosis in patients with suspected coronary artery disease. J Am Coll Cardiol. 1986; 7: 464-471.
- Berman DS, Abidov A, Kang X, Hayes SW, Friedman JD, Sciammarella MG, et al. Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion SPECT interpretation. J Nucl Cardiol. 2004; 11: 414-423.
- Hachamovitch R. Assessing the prognostic value of cardiovascular imaging: a statistical exercise or a guide to clinical value and application? Circulation. 2009; 120: 1342-1344.
- Ni H, Coady S, Rosamond W, Folsom AR, Chambless L, Russell SD, et al. Trends from 1987 to 2004 in sudden death due to coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 157: 46-52.
- Furuhashi T, Moroi M, Masai H, Kunimasa T, Nakazato R, Fukuda H, et al. Correlation of chronic kidney disease, diabetes and peripheral artery disease with cardiovascular events in patients using stress myocardial perfusion imaging. Ann Nucl Med. 2011; 25: 634-642.
- Furuhashi T, Moroi M, Joki N, Hase H, Minakawa M, Masai H, et al. Predictors of cardiovascular events in hemodialysis patients after stress myocardial perfusion imaging. Hemodial Int. 2013; 17: 568-575.
- Hakeem A, Bhatti S, Dillie KS, Cook JR, Samad Z, Roth-Cline MD, et al. Predictive value of myocardial perfusion single-photon emission computed tomography and the impact of renal function on cardiac death. Circulation. 2008; 118: 2540-2549.
- Yoda S, Nakanishi K, Tano A, Kasamaki Y, Kunimoto S, Matsumoto N, et al. Risk stratification of cardiovascular events in patients at all stages of chronic kidney disease using myocardial perfusion SPECT. J Cardiol. 2012; 60: 377-382.
- Furuhashi T, Moroi M, Joki N, Hase H, Minakawa M, Masai H, et al. Prediction of cardiovascular events in pre-dialysis chronic kidney disease patients with normal SPECT myocardial perfusion imaging. J Cardiol. 2014; 63: 154-158.
- Cohn PF, Gorlin R, Cohn LH, Collins JJ Jr. Left ventricular ejection fraction as a prognostic guide in surgical treatment of coronary and valvular heart disease. Am J Cardiol. 1974; 34: 136-141.
- Battler A, Slutsky R, Karliner J, Froelicher V, Ashburn W, Ross J Jr. Left ventricular ejection fraction and first third ejection fraction early after acute myocardial infarction: value for predicting mortality and morbidity. Am J Cardiol. 1980; 45: 197-202.
- Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003; 42: 736-742.
- Bosch X, Théroux P. Left ventricular ejection fraction to predict early mortality in patients with non-ST-segment elevation acute coronary syndromes. Am Heart J. 2005; 150: 215-220.
- Gimelli A, Landi P, Marraccini P, Sicari R, Frumento P, L’Abbate A, et al. Left ventricular ejection fraction measurements: accuracy and prognostic implications in a large population of patients with known or suspected ischemic heart disease. Int J Cardiovasc Imaging. 2008; 24: 793-801.