
Research Article
J Cardiovasc Disord. 2015; 2(3): 1019.
Is There a Relationship between Serum S100B Protein Level and Severity of Coronary Artery Disease in Patients with Acute Coronary Syndrome?
Ekici B1*, Sahin RO2, Altinsoy M1, Açikgoz S3, Ozdemir S4, Demirtas S4 and Korkmaz FS1
1Department of Cardiology, Ufuk UniversityFaculty of Medicine, Turkey
2Department of EmergencyMedicine, Ufuk UniversityFaculty of Medicine, Turkey
3Department of Cardiology, Kavaklidere Umut Hospital, Turkey
4Department of MedicalBiochemistry, Ufuk UniversityFaculty of Medicine, Turkey
*Corresponding author: Ekici B, Ufuk University Faculty of Medicine, Department of Cardiology, Ankara, Turkey, MevlanaBulvari (Konya Yolu) No:86-88, 06520 Balgat – Ankara, Turkey
Received: July 22, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
S100, a calgranulin family protein released from white blood cells, is involved in inflammatory cardiovascular disease. Elevated serum S100 protein levels have been reported to be associated with Coronary Artery Disease (CAD).
Objective: Our aim was to determine whether there is a relationship between serum S100B protein levels and severity and complexity of CAD in the Acute Coronary Syndromes (ACS).
Methods: A total of 81 patients (aged 61.12±13.57 years,49.4% men) who were admitted to the emergency room for the evaluation of the angina pectoris were enrolled. According to the clinical statusand cardiac enzymes levels coronary an giography was performed. The serum S100B protein (S100 A1B and S100BB) levels were measured 6 hour after admission. The extent and severity of the CAD were evaluated by the Gensini score.
Results: Mean serum S100B protein values were 0.11±0.12 μg/L in the control group, 0.20±0.48 μg/L in the group with non ST segment elevation my cordial infarction, and 0.29±0.79 μg/L in the group with ST segment elevation myo-cardial infarction (p=0.267). No correlation was found between serum S100B protein andGensiniscore (p=0.093, r=0.188). However, a statistically significant positive correlation was found between serum S100B protein and sixth our oftroponin-T levels (p=0.05 r=0.253).
Conclusion: We did not determine any correlation between serum S100B protein levels and severity of CAD. Also, there was no relationship between the type of ACS and serum S100B protein values. But, the results of the S100B levels tend to increase numerically in ACS groups when compared to control group. However, serum s100B protein is positively correlated with serum troponin-T levels in ourstudy. There fore, to clarify this issue, larges calestudies are needed.
Keywords: A Cute Coronary Syndrome: Severity of Coronary Artery Disease: S100B Protein
Abbreviations
CAD: Coronary Artery Disease; ACS: Acute Coronary Syndrome; STEMI: ST Segment Elevation Myocardial Infarction; NSTEMI: Non ST Segment Elevation Myocardial Infarction; CK-MB: Creatine Kinase-Myocardial Band; DM: Diabetes Mellitus; HT: Hypertension; HL: Hyperlipidemia; MPV: Mean Platelet Volume
Introduction
Subgroups of the S100 Ca2+-binding protein family are associated with inflammatory disorders, and their relationship to atherosclerotic process and its complications is emerging [1-3]. S100A12 constitutes ~2–5% of neutrophil cytosolic protein and is induced in monocytes by lipopolysaccharide and TNF-α, and in macrophages by IL-6. S100A12 is a monocyte chemoattractant and activates mast cells, resulting in neutrophil and monocyte recruitment in vivo. Thus, S100A12 may modulate processes that contribute to atherogenesis [4]. Recently, S100B protein expression was found to be induced after myocardial infarction [5], and it has been proposed as a biomarker of poor prognosis in patients undergoing cardiac surgery [6] Therefore, we investigated the relationship between serum S100B protein levels and severity and complexity of Coronary Artery Disease (CAD) in patients with Acute Coronary Syndrome (ACS).
Subjects and Methods
Study population
The sample was derived from a population of 132 consecutive patients who were admitted to the emergency department with chest pain. In total, 51 of them were excluded because they met the exclusion criteria (n: 35) and did not fulfill the inclusion criteria (n: 16). Finally, 81 patients were enrolled (age 61.12±13.57 (mean±SD)), including 40 men (49.4%) and 41 women subjects (50.6%). Our institutional review board approved the study, and we obtained informed consent from all individuals. All patients underwent coronary angiography. The inclusion criteria were age greater than 18 years, patients who were admitted to the hospital because of an acute myocardial infarction [ST Segment Elevation Myocardial Infarction (STEMI) or Non ST Segment Elevation Myocardial Infarction (NSTEMI)] or severe angina pectoris, a coronary angiogram clear enough to enable evaluation, and the patient’s consent. The exclusion criteria were current pregnancy, cardiomyopathy, any history of revascularization procedures (whether percutaneous transluminal coronary angioplasty or coronary artery bypass grafting), congenital heart disease, and any cerebral disorders. Noninvasive stress tests (treadmill exercise test, myocardial perfusion scintigraphy, and dobutamine stress echocardiography) were performed for the patients who were admitted to the emergency department with stable angina pectoris or had normal cardiac enzymes and coronary angiography was performed to the patients due to positive stress test results. The control group was identified as those with minimal CAD (Gensini score<20) or normal coronary arteries (Gensini score=0). NSTEMI patiens were identified as in group I and STEMI patients were identified as in group II.
Determining the severity of coronary artery disease
Selective coronary angiography was performed by the femoral approach using the Judkins technique and General Electric In nova 3100 angiographic system (Buc Cedex, France). Multiple views were obtained, with visualization of the left anterior descending and left circumflex coronary artery in at least 4 projections, and the right coronary artery in at least 2 projections. Coronary angiograms were recorded on compact discs in DICOM format. All angiograms were analyzed by two cardiologists blinded to the clinical data. The extent and severity of the CAD were evaluated according to the Gensini score. In this scoring system, a severity score is derived for each coronary stenosis based on the degree of luminal narrowing and its topographic importance. Reduction in the lumen diameter and the roentgenographic appearance of concentric lesions and eccentric plaques are evaluated [7].
Biochemical analyzes
A complete blood count and biochemical examination were performed in all patients at the administration using a vacuum tube. Troponin-T levels and creatine kinase-myocardial band (CKMB) levels were repeated after 6 hours. Serum S100B (S100 A1B and S100BB) testing was performed using the Roche Elecsys® 2010, S100 reagent kit 6 hour after admission (assay duration 18 minutes, measuring range 0.005–39 μg/L, cross reactivity against S100 < 1%). After centrifugation at 3000 rpm for 15 minute, plasma aliquots were stored at -80°C until analyses. Serum S100B protein, CK-MB, and troponin-T levels were assessed by the principles of electro-chemilum inescence immunoassay.
Statistical analysis
The data were analyzed with the IBM SPSS Statistics 21 for Windows. The normal distribution of variables was verified with the Kolmogorov-Smirnov test. We used to Kruskal-Wallis test to account for the differences among the groups (Table 1), but in order to analyze the specific sample pairs for significant differences, we used Conover- Inman test (Table 2). Spearman’s rho test was used in order to detect whether there was a correlation among the independent variables. A chi square (X2) test was used to investigate whether distributions of categorical variables differed within groups. Patients’ characteristics are summarized as mean±SD or as percentages. A p value less than 0.05 was considered statistically significant.
Control Group (n=24)
Group I (n=31)
Group II (n=26)
p value
Age (year)
53.66±14.58a,b
63.93±12.26a
64.65±11.70b
0.010
Gensini score
0.27±0.92 a,b
71.87±61.86 a
72.28±40.10 b
<0.001
Total-C (mg/dL)
184.41±45.10
188.71±58.51
194.94±44.84
0.780
HDL-C (mg/dL)
48.44±12.04a
35.06±13.47a,c
44.52±17.19c
0.003
LDL-C (mg/dL)
107.43±36.70
113.91±47.01
118.79±40.98
0.578
Triglyceride (mg/dL)
138.38±126.10 a,b
198.86±131.40 a
170.08±88.77 b
0.045
Uric acid (mg/dL)
5.43±2.16a
6.40±1.78a
6.64±2.10
0.067
CRP (mg/L)
6.94±14.62 a,b
39.06±44.52 a
53.46±10.41 b
0.001
Hemoglobin (g/dL)
12.55±1.96
13.37±1.87
13.2±1.85
0.391
Platelet (x103/µL)
253.45±72.95a
237.69±95.15a
254.44±89.52
0.072
MPV (fL)
8.55±1.14b
9.13±1.47c
10.02±1.37b,c
0.002
CK-MB( ng/mL) (6th h)
1.65±1.77 a,b
39.20±74.83a,c
59.64±65.30b,c
<0.001
Troponin-I (ng/mL) (6th h)
0.01±0.01 a,b
6.21±21.43a
10.03±18.55b
<0.001
S-100B protein (µg/L)
0.11±0.12
0.20±0.48
0.29±0.79
0.267
Abbreviations: Total-C: Total Cholesterol, HDL-C: High Density Lipoprotein Cholesterol, LDL-C: Low Density Lipoprotein Cholesterol, CRP: C -reactive protein, MPV: Mean Platelet Volume, CK-MB: Creatine Kinase-Myocardial Band. The Control Group was identified as those with Minimal CAD or Normal Coronary Arteries. Non ST Segment Elevation Myocardial Infarction Patients Were Identified as in Group I and ST Segment Elevation Myocardial InfarctionPatients were Identified as in Group II (Comparisons were made by Kruskal-Wallis Test). Significant Differences Were Found Between; A: Control vs Group I, B: Control vs Group II, C: Group I vs Group II (P<0.05).
Table 1: Baseline characteristics and biochemical examinations of patients according to clinical status.
Control-Group I
p value
Control-Group II
p value
Group I-Group II
p value
Age (year)
0.008
0.006
0.826
Gensini score
<0.001
<0.001
0.378
Total-C
0.878
0.517
0.591
HDL-C
<0.001
0.116
0.040
LDL-C
0.736
0.318
0.469
Triglyceride
0.018
0.041
0.780
Uric acid
0.044
0.054
0.818
CRP
<0.001
0.001
0.705
Hemoglobin
0.191
0.315
0.758
Platelet
0.031
0.618
0.083
MPV
0.173
<0.001
0.012
CK-MB
<0.001
<0.001
0.010
Troponin-I
<0.001
<0.001
0.058
S-100B protein
0.234
0.137
0.711
Abbreviations: Total-C: Total Cholesterol, HDL-C: High Density Lipoprotein Cholesterol, LDL-C: Low Density Lipoprotein Cholesterol, CRP: C-Reactive Protein, MPV: Mean Platelet Volume, CK-MB: Creatine Kinase-Myocardial Band. A Conover–Inman test for all pairwise comparisons were used to compare groups of samples and a p value less than 0.05 was considered statistically significant. The control group was identified as those with minimal CAD or normal coronary arteries. Non ST segment elevation myocardial infarction patiens were identified as in group I and ST segment elevation myocardial infarction patients were identified as in group II.
Table 2: Statistical relationships between the groups in terms of baseline characteristics.
Results
Baseline characteristics of patients according to clinical status are shown in Table 1. Of the 81 patients, 23.5% had Diabetes Mellitus (DM), 61.7% had Hypertension (HT), 43.2% had Hyperlipidemia (HL), and 37.0% were current smokers. Twenty-four patients (29.6%) were assigned to control group (according to Gensini score, minimal CAD or normal coronary arteries). The remaining 57 patients constituted the study group, which was further divided into two groups. Thirtyone patients were assigned to group I (NSTEMI, 38.3%), and twentysix patients were assigned to group II (STEMI, 32.1%) (Anterior STEMI n:13; inferior STEMI n:10; high lateral STEMI n:3). Mean Gensini scores were 0.27±0.92, 71.87±61.86 and 72.28±40.10 in the control group, group I and group II, respectively (p<0.001). Higher Gensini scores were calculated in men than in women (54.5±58.6; 47.2±51.8, respectively). Serum levels of HDL-cholesterol were higher in the control group, whereas triglyceride, C-reactive protein, and Mean Platelet Volume (MPV) levels were higher in the study populations (p=0.003; p=0.045; p=0.001; p=0.002, respectively). Serum uric acid levels were numerically lower in the control group when compared to group I and II; however, there was no statistically significant relationship across the groups. No significant association was determined between serum S100B levels and DM, HT, HL, or smoking (p=0.911, p=0.137, p=0.841, and p=0.254, respectively). A significant inverse relationship was determined between Gensini score and HDL cholesterol (p=0.007, r=-0.315). Moreover, uric acid, CRP levels, and total cholesterol/HDL ratio showed a positive correlation with Gensini score (p=0.009, r=0.396; p=0.001, r=0.486, and p=0.024, r=0.267, respectively). Also there was a statistically significant positive correlation was found between serum S100B and C-reactive protein levels (p=0.024, r=0.332). In addition to, a significant relationship was determined between HT, smoking, and type of ACS (p=0.022, p=0.009, respectively). Conover-Inman test was performed for the binary comparisons among the groups and the p value less than 0.05 was considered statistically significant (Table 2). Serum S100B protein levels did not differ statistically significant between the control group and ACS groups. However, the results of the S100B levels tend to rise numerically in ACS groups when compared to control group (Table 1-2) (Figure 1) (p>0.05 for all comparisons, after Conover-Inman test). Also, no correlation was found between serum S100B protein and Gensini score (p=0.093, r=0.188). According to Spearman’s correlation analysis, a positive correlation was determined between serum S100B and troponin-T levels in men but not in women. (p=0.05 r=0.253). (p=0.869, r=0.033 in women; p=0.011, r=0.429 in men). No correlation was found between S100B and CK-MB levels (p=0.450; r=0.099)
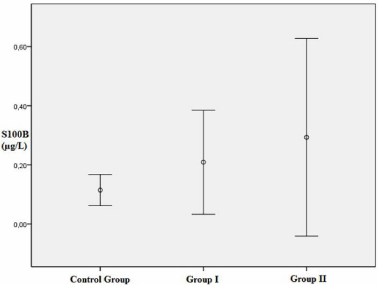
Figure 1: Serum S100B protein levels in the groups. The control group
was identified as those with minimal coronary artery disease or normal
coronary arteries. Non ST segment elevation myocardial infarction patients
were identified as in group I and ST segment elevation myocardial infarction
patients were identified as in group II.
Discussion
The first members of the S100 protein family were discovered more than 40 years ago and were purified from bovine brain. The protein complex was termed “S100” owing to its solubility in 100% ammonium sulfate solution [8]. So far, more than 20 members of S100 protein family have been defined [9]. The role of S100 proteins in atherosclerosis has been studied in recent years. Enhanced expression of S100 proteins has been identified in vascular inflammation of diabetic apolipoprotein E-/- mice [10,11]. S100 proteins may activate nuclear factor- B thereby inducing expression of proinflammatory cytokines and adhesion molecules important steps in the formation of atherosclerosis. There is emerging evidence that S100A8/S100A9 dimer, a secretion product of neutrophils is an early and sensitive marker of plaque instability in the atherogenic process. It has been reported that, S100A8/S100A9 protein and mRNA could be found in macrophages and foam cells of human atherosclerotic plaque [12]. Increased levels of serum S100A12 concentrations in type 2 diabetes have been identified and could contribute to vascular inflammatory responses via endothelial activation and atherosclerotic changes [13].
Due to the low molecular weight and specific tissue distribution of S100A1, it may leak from damaged myocardial cells into the blood circulation of patients with ACS. Therefore, S100A1 could be used as a “negative marker” to detect ischemic-damaged cardiomyocytes from normal cells, which could be helpful for postmortem diagnosis of acute myocardial infarction. It has been reported that serum S100A1 concentrations are significantly elevated after acute myocardial infarction and have higher sensitivity and specificity than CK-MB and cardiac troponin-I within the early hours (0–6 h) of the onset of ACS. The prompt release of S100A1 into the bloodstream most likely reflects irreversible changes in cardiomyocytes due to hypoxia. However, little is known regarding the expression pattern of S100A1 in ischemic cardiomyocyte lesions. In patients with acute myocardial ischemia, a rise in the serum concentration of S100 protein may be associated with the role of S100 protein as a cardioprotective factor with antiapoptotic function [14-16]. In accordance with the literature, a statistically significant positive correlation between serum S100B protein and troponin-T levels was found in our study. In contrast to the literature, we found this correlation only in man. It can be explained by the frequency and severity of CAD is higher in men than in women in our study. It was previously reported that serum levels of S100B, S100A6 and S100P were higher in ACS group than instable angina and control groups [17]. In contrast to these findings, we did not determine a statistically significant relationship between serum S100B protein levels and type of the ACS. However, S100B protein levels tend to increase numerically in the ACS groups when compared to the control group. This situation can be explained by the small scale of our study. No correlation between Gensini score and serum S100 A12 was reported in the literature [4]. Likewise, no correlation was found between serum S100B protein and the Gensini score in our study. To clarify this issue further large-scale studies are needed.
It was previously reported that elevated levels of S100A12 is associated with CAD than healthy controls and levels correlated positively with C-reactive protein [4]. Similarly, a statistically significant positive correlation was found between serum S100B and C-reactive protein levels in our study. In patients with CAD and in population studies, C-reactive protein is an independent predictor of adverse cardiovascular outcomes [18,19]. Together with the positive associations of raised circulating C-reactive protein levels with poor prognosis, and adverse sequelae in patients with CAD [20].
It has been reported that elevated MPV is associated with ACS, mortality following ACS, and congestive heart failure. MPV is a useful means of identifying larger platelets, which are hemostatically more active and a risk factor for developing coronary atherosclerosis, leading to ACS [21,22]. Likewise, we found a statistically significant association between MPV and type of ACS in our study. Accordingly, the MPV levels in the ACS groups were significantly higher than in the controls.
Study Limitations
Our study had some limitations. First, the study population was relatively small. A larger study population would provide a higher statistical power. Also, we did not examine the different subgroups of S100 protein.
Conclusion
This study indicates that higher serum levels of S100B protein are associated with elevated cardiac troponin-T and C-reactive protein levels. However, we did not find any association between ACS type and serum S100B protein. Likewise, no correlation was found between severity of CAD and S100B protein. Therefore, we cannot generalize the S100B is a new marker of CAD due to the small sample size of the study and the lack of strong correlations between S100B and cardiac enzymes. As a result, S100B protein isn’t a sufficiently sensitive biomarker to determine acute coronary syndromes. Further large scale studies are necessary to evaluate the role of the serum S100B protein levels in prediction of the ACS.
References
- Mori Y, Kosaki A, Kishimoto N, Kimura T, Iida K, Fukui M, et al. Increased plasma S100A12 (EN-RAGE) levels in hemodialysis patients with atherosclerosis. Am J Nephrol. 2009; 29: 18-24.
- Altwegg LA, Neidhart M, Hersberger M, Müller S, Eberli FR, Corti R, et al. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007; 28: 941-948.
- Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008; 155: 49-55.
- Goyette J, Yan WX, Yamen E, Chung YM, Lim SY, Hsu K, et al. Pleiotropic roles of S100A12 in coronary atherosclerotic plaque formation and rupture. J Immunol. 2009; 183: 593-603.
- Ambrozic J, Bunc M, Osredkar J, Brvar M. S100B protein in benzodiazepine overdose. Emerg Med J. 2008; 25: 90-92.
- Mussack T, Biberthaler P, Kanz KG, Wiedemann E, Gippner-Steppert C, Jochum M. S-100B, SE-selectin and P-selectin for avaluation of hypoxic brain damage in patients after cardiopulmonary resuscitation: pilot study. World J Surg. 2001; 25: 539-543.
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983; 51: 606.
- Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965; 19: 739-744.
- Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun. 2004; 322: 1111-1122.
- Kislinger T, Tanji N, Wendt T, Qu W, Lu Y, Ferran LJ Jr, et al. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2001; 21: 905-910.
- Eue I, Pietz B, Storck J, Klempt M, Sorg C. Transendothelial migration of 27E10+ human monocytes. Int Immunol. 2000; 12: 1593-1604.
- McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, et al. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005; 280: 41521-41529.
- Kosaki A, Hasegawa T, Kimura T, Iida K, Hitomi J, Matsubara H, et al. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2004; 89: 5423-5428.
- Usui A, Kato K, Sasa H, Minaguchi K, Abe T, Murase M, et al. S-100ao protein in serum during acute myocardial infarction. Clin Chem. 1990; 36: 639-641.
- Kiewitz R, Acklin C, Minder E, Huber PR, Schäfer BW, Heizmann CW. S100A1, a new marker for acute myocardial ischemia. Biochem Biophys Res Commun. 2000; 274: 865-871.
- Bi H, Yang Y, Huang J, Li Y, Ma C, Cong B. Immunohistochemical detection of S100A1 in the postmortem diagnosis of acute myocardial infarction. Diagn Pathol. 2013; 8: 84.
- Cai XY, Lu L, Wang YN, Jin C, Zhang RY, Zhang Q, et al. Association of increased S100B, S100A6 and S100P in serum levels with acute coronary syndrome and also with the severity of myocardial infarction in cardiac tissue of rat models with ischemia-reperfusion injury. Atherosclerosis. 2011; 217: 536-542.
- Yeh ET, Palusinski RP. C-reactive protein: the pawn has been promoted to queen. Curr Atheroscler Rep. 2003; 5: 101-105.
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002; 347: 1557-1565.
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999; 106: 506-512.
- Cameron HA, Phillips R, Ibbotson RM, Carson PH. Platelet size in myocardial infarction. Br Med J (Clin Res Ed). 1983; 287: 449-451.
- Niu X, Yang C, Zhang Y, Zhang H, Yao Y. Mean platelet volume on admission improves risk prediction in patients with acute coronary syndromes. Angiology. 2015; 66: 456-463.