
Short Communication
J Cardiovasc Disord. 2016; 3(1): 1022.
Suture Mediated Vascular Closure Devices: Technology in Review and on the Horizon
Angela Echeverria1*, Zvonimir Krajcer2
1Department of Vascular Surgery, Baylor College of Medicine, Houston, USA
2Department of Cardiology, Texas Heart Institute, Houston, USA
*Corresponding author: Angela Echeverria, Department of Vascular Surgery, Baylor College of Medicine, Houston, USA
Received: December 30, 2015; Accepted: January 15, 2016; Published: January 19, 2016
Short Communication
Vasoseal was the first Vascular Closure Device (VCD) introduced in 1994, (Data scope Corporation, Mahwah, NJ). Prostar (Abbott Vascular, Santa Clara, CA) and later Angioseal (St. Jude Medical, Minnetonka, MN) were introduced in 1996. Vascular closure devices in comparison to manual compression have decreased time to hemostasis and ambulation. Early in the development of VCDs there was a reported 10-20% failure rate [1].
Through further device modifications and ease of deployment the failure rates have significantly reduced. Currently, there are ten approved and marketed VCDs available in the United States. The devices currently in use a variety of methods for closure from a collagen plugs to suture to nitinol clip application. They can be deployed through as small as a five French (F) sheath with as few as 6 steps. Table 1 summarizes the currently approved VCDs in the United States as well as the method of closure, sheath size and steps of deployment [2].
Company
Product
Method of Closure
Sheath Size
Steps
St. Jude
Medical
Angio-Seal VIP, Evol
Collagen plug
and Anchor
6,8
11
Abbott
Prostar XL
Braided suture
8.5-10
<30
Vascular
ProGlide
Monofilament suture
5-Aug
12
Starclose SE
Nitinol clip
5,6
6
Access
Closure
Mynx Cadence
MynxGrip
Extravascular
PEG sealant
5-Jul
10
Arstasis
Arstasis One
Reentry closure
5-Jul
6
Cardiva
Medical
Catalist II& III
Kaolin, Chitosan &
5-Jul
6
Protamine
Vascade
Collagen
5-Jul
6
Cordis
Exsoseal
Extravascular PGA plug
5-Jul
6
Table 1: Available Suture Mediated and Vascular Closure Devices.
Currently there are two devices available for closure of large bore sheaths. Prostar XL (Abbott Vascular, Redwood City, CA) is a 10 F profile device with a braided suture. This device requires an operator tied knot (Figure 1). One Prostar XL device can be used to close

Figure 1: Prostar XL device (Abbott Vascular, Redwood City, CA).
arteriotomies up to 24 F sheaths. Prostar XL is approved in Europe in a pre-close fashion for Percutaneous Endovascular Aortic Repair (PEVAR). The second device, ProGlide (Abbott Vascular, Redwood City, CA) is a 6 F profile device with a monofilament suture within it and contains a pre-formed knot (Figure 2). Two ProGlides are requires to close an arteriotomy for up to a 21F sheath. ProGlide is the only device approved in the United States for pre-close technique in PEVAR.
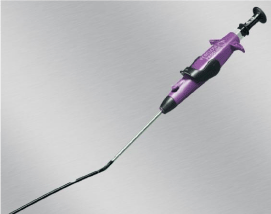
Figure 2: ProGlide device (Abbott Vascular, Redwood City, CA).
Until today a number of non-randomized single center PEVAR studies using Prostar XL and ProGlide (Abbott Vascular, Redwood City, CA) suture mediated closure devices (SMCD) have been published. More recently in 2011, Krajcer and colleagues reported a 96% and 97% technical success [3].
The review of literature revealed that the average technical success for the last five years using Prostar XL has been 96%. The review of these trials demonstrated that there is a considerable learning curve in the use of this device.
More recently ProGlide has been also reported in a variety of non-randomized single center PEVAR studies [4]. The average technical success with ProGlide for the last 5 years has been 96%. Several investigators have reported that there is considerably shorter learning curve with ProGlide than Prostar XL [5].
Vascular closure devices are not indicated for every patient and procedure and are not without the potential for complication. The closure devices leave a retained foreign body and depending on the device utilized this is a suture, collagen plug or anchor or nitinol clip. In the vasculopath or patient requiring numerous percutaneous access procedures this can become problematic. The use of VCDs increases the risk of embolization, thrombosis and infection.
Technical success is also dependent on several factors. The use of VCD In the presence of severe peripheral vascular disease or circumferential arterial calcification is contraindicated. They are not approved to close brachial arteriotomies or antegrade access femoral artery access sites. They are also contraindicated for repair of femoral artery access sites above the inguinal ligament and below the common femoral artery. Operator experience, patient anatomy, procedure and device used all contribute to technical success of a VCD [6-9].
On the horizon are several large vessel closure devices. Table 2 summarizes these experimental devices. Some of the new generation devices allow for closure of large bore arteriotomy up to 24 F with a single device with as few as three steps for deployment. The Vivasure Device (Vivasure Medical, Galway, Ireland) is one of the experimental VCD with the placement of a synthetic absorbable low profile implant, sealing the arteriotomy from within. The Manta Vascular Closure System (Essential Medical, Malvern, PA) is another experimental
Category
Company
Device
Suture based
Sutura/Medtronic
Superstich
Suture based
SpiRx
SpiRx MSD
Patch or Plug
Vivasure
Vivasure VCD
Patch or Plug
Access Closure
Closure- GRIP
Scaffold&Cover
InSeal
Atum
Scaffold &Cover
ProMed
ProMed VCD
Patch or Plug
Essential Medical
Manta
Table 2: Investigational Suture Mediated and Vascular Closure Devices.
VCD, currently being investigated in Europe in a clinical trial for closure up to 18 F with a single device. This novel device achieves hemostasis by a sandwich technique of the arteriotomy between an intra-arterial patch and a bovine collagen plug while maintaining vascular access and delivering the implant over the wire.
References
- Schulz-Schüpke S, Helde S, Gewalt S, Ibrahim T, Linhardt M, Haas K, et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR-CLOSURE randomized clinical trial. JAMA. 2014; 312: 1981-1987.
- Caputo RP. Vascular Closure Techniques. Card Int Today. 6: 70.
- Krajcer Z, Strickman N, Mortazavi A, Dougherty K. Single-center experience of percutaneous abdominal aortic aneurysm repair with local anesthesia and conscious sedation: technique and results. J Cardiovasc Surg (Torino). 2012; 53: 695-706.
- Nelson PR, Krajcer Z, Kansal N, Rao V, Bianchi C, Hashemi H, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. 2014; 59: 1181-1193.
- Lee WA, Brown MP, Nelson PR, Huber TS, Seeger JM. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg. 2008; 47: 919-923.
- Balzer JO, Scheinert D, Diebold T, Michael Haufe, Thomas J Vogl, Giancarlo Biamino. Postinterventional transcutaneous suture of femoral artery access sites in patients with peripheral arterial occlusive disease: A study of 930 patients. CCI. 2001; 53: 174-181.
- Al-Khatib WK, Zayed MA, Harris EJ, Dalman RL, Lee JT. Selective use of percutaneous endovascular aneurysm repair in women leads to fewer groin complications. Ann Vasc Surg. 2012; 26: 476-482.
- Bechara CF, Barshes NR, Pisimisis G, Chen H, Pak T, Lin PH, et al. Predicting the learning curve and failures of total percutaneous endovascular aortic aneurysm repair. J Vasc Surg. 2013; 57: 72-76.
- Georgiadis GS, Antoniou GA, Papaioakim M, Georgakarakos E, Trellopoulos G, Papanas N, et al. A meta-analysis of outcome after percutaneous endovascular aortic aneurysm repair using different size sheaths or endograft delivery systems. J Endovasc Ther. 2011; 18: 445-459.