
Review Article
J Cardiovasc Disord. 2017; 4(1): 1033.
An Overview of the Pleioptropic Effects of Statins and Their Impact on Postoperative Outcomes in Cardiovascular Surgery Patients
Hearps T and Du Toit EF*
Menzies Health Institute of Queensland, School of Medical Science, Griffith University, Gold Coast, QLD, Australia
*Corresponding author: EF du Toit, School of Medical Science, Griffith University, Gold Coast Campus, Parklands Drive, Southport, 9726, QLD, Australia
Received: May 02, 2017; Accepted:May 26, 2017; Published: June 02, 2017
Abstract
Obesity and its comorbidities (dyslipidaemia, hypertension and insulin resistance/diabetes) are all risk factors that significantly contribute to Cardiovascular Disease (CVD) morbidity and mortality. Although statins form the cornerstone of most CVD prevention strategies and are effective for the management of blood lipids, their pleiotropic cholesterol-independent effects have generated significant interest. There is compelling evidence to suggest that statins may influence perioperative outcomes after cardiovascular surgery.
The pleiotropic effects of statins include the attenuation of; 1) inflammation, 2) vascular dysfunction, 3) platelet and smooth muscle cell aggregation, and, 4) atherothrombogenic enzyme activity. These effects all potentially contribute to attenuation of atherosclerotic lesion formation, plaque development and rupture, and atherothrombosis. Clinical trials report a significantly diminished incidence of adverse cardiac outcomes when statin therapy is implemented during the perioperative period in cardiovascular surgery. Similarly, some studies have reported an increase in adverse postoperative cardiac outcomes after postoperative withdrawal of chronic statin therapy. The view that these beneficial effects of statins are due to their pleiotropic effects is further supported by data showing that normocholesterolaemic patients on statins also exhibit cardioprotective benefits in response to statin therapy. The data reviewed in this paper suggests that sustained perioperative statin therapy could potentially be adopted and incorporated into the perioperative treatment regime of patients undergoing high-risk cardiac and vascular surgical procedures to diminish the risk of postoperative adverse cardiac events.
Keywords: Statins; Pleiotropic Effects; Post-operative outcomes; Cardiac
Introduction
Adiposity is becoming increasingly prevalent, with overweight affecting 39 and obesity 13% of the global adult population [1]. It is well established that both obesity and the associated dyslipidaemia are risk factors for Cardiovascular Disease (CVD) with statin therapy used as a primary preventative measure for CVD in these at risk patients. Statins inhibit cholesterol biosynthesis and are widely prescribed to lower serum cholesterol and prevent CVD.
Although significant research focus has been on the lipid lowering effects of statins, less is known about the pleiotropic effects of statins and how they impact patient postoperative outcomes. The pleiotropic effects of statins are varied; with many of the beneficial effects being ascribed to not only directs cardiovascular effects, but also systemic effects of these compounds. There have been several studies investigating the role of these pleiotropic effects on clinical outcomes following ischaemic events, including myocardial infarctions [2,3], cardiac surgery, vascular surgery, and non- cardiovascular surgery [4-12]. Here we highlight the proposed pleiotropic mechanisms of statins and examine the clinical outcomes of studies using statin therapy.
Statins, their primary effects and their efficacy
Statins act to lower serum cholesterol levels by reducing hepatic and intestinal HMG-CoA reductase activity which is involved in the production of mevalonate [13] and the subsequent biosynthesis of cholesterol [14]. Statins vary in their action and the extent to which they lower plasma lipids. A dose- specific meta-analysis conducted by Edwards and Moore [15] found that statins lowered total cholesterol by 17-35%, LDL cholesterol by 24-49%, and increased HDL by roughly 5%. They also performed a study in which they determined that rosuvastatin and atorvastatin were the two most potent lipidlowering statins [16] which would suggest that hydrophilic statins have the greatest lipid lowering efficacy. Clinical outcomes after an Acute Myocardial Infarct (AMI) did however not differ significantly when comparing hydrophilic and lipophilic statins in a one year follow-up study [3].
Pleiotropic effects of statins
A pleiotropic effect of statins refers to their effects that are independent of their lipid-lowering effects [17]. These effects are ascribed to a range of mechanisms elicited in the cardiovascular, immune and central nervous systems and include improving vascular endothelial function, modulating pro- coagulant and platelet activity, stabilising atherosclerotic plaques, inhibiting smooth muscle proliferation and attenuating inflammatory responses [4]. Many of the pleiotropic effects of statins are mediated through inhibition of isoprenylation, reducing the function of isoprenoids such as Rho and Rac, which impacts on inflammation and vascular function [5]. Statins also improve cardiovascular outcomes in patients with normal healthy cholesterol levels [4] which would suggest that the benefits are independent of their effects on circulating lipids.
Perioperative use of statins
A number of studies have investigated the impact of statins on cardiovascular outcomes when used in the perioperative period. The surgical procedures these studies assessed were generally limited to high risk invasive cardiac and vascular surgery. Data from these studies provide compelling evidence to suggest that statin therapy may reduce mortality, myocardial infarction and the duration of hospitalisation following both cardiac and non-cardiac surgery [4].
An early prospective randomised trial investigating the use of statins in the lead-up to cardiac surgery found that preoperative statin treatment significantly lowered the incidence of postoperative thrombocytosis and myocardial infarction following coronary artery bypass grafting in hypercholesterolemia patients [6]. The effect of preoperative statins on postoperative mortality (during the in-hospital stay) was investigated in a case controlled study [7]. Peri-operative statin use was significantly higher in those that survived in-hospital stay (25% vs 8%, p<0.001). The mortality odds ratio was 0.22 (0.10 to 0.47) when comparing statin users and nonusers [7]. Mortality rates were also 4.5 times greater in patients not on preoperative statin therapy [7]. A recent meta-analysis of preoperative statin use involving ~90,000 cardiac surgery patients, found that preoperative statin therapy resulted in a 31% odds reduction for all-cause mortality in the postoperative period (odds ratio 0.69; P < 0.0001) [8]. Statin therapy was also associated with a significant decrease in postoperative endpoints of atrial fibrillation (odds ratio = 0.71), stroke (odds ratio = 0.83) and duration of in-hospital treatment (weighted mean difference = -0.57) [8]. This study however reported that preoperative statin treatment did not reduce postoperative myocardial infarction or renal failure [8].
A study conducted by Kennedy and co-workers [9] found that patients who were on statin therapy prior to carotid endarterectomies had reduced in-hospital mortality and ischaemic stroke. A metaanalysis assessing the clinical outcomes of 2292 patients undergoing cardiac or non-cardiac surgery found that perioperative statin treatment decreased: 1) the risk of atrial fibrillation in cardiac surgery patients and, 2) Myocardial infarction risk in both cardiac and noncardiac patients. Statins however had no effect on mortality rates in either cardiac or non-cardiac patients [2]. These investigators proposed that the use of statin therapy to ameliorate adverse cardiac outcomes in patients undergoing high-risk surgical interventions may be warranted.
Impact of discontinuation of statins in the perioperative period
Discontinuation of statin therapy during the postoperative period appears to have significant adverse effects on the cardiovascular outcomes following high-risk interventional procedures [4]. An increasing number of studies report improved postoperative cardiac outcomes due to attenuated inflammation associated with statin therapy [10], while statin withdrawal possibly causes an inflammatory upsurge and adverse cardiac outcomes [11].
A prospective study compared the postoperative cardiovascular outcomes of patients who either continued or discontinued statin therapy after surgery [12]. The odds risk ratio as a predictor of myonecrosis in the continuation and discontinuation groups was 0.38 and 2.1 respectively, which translates to a relative risk reduction of 5.4 for those on continued statin therapy. Withdrawal of statins following vascular surgery served as an independent predictor of postoperative myonecrosis (odds ratio = 2.9). The outcomes of this and another study [18] strongly suggest that postoperative discontinuation of statin therapy contributes to adverse cardiovascular outcomes in these patients.
Mechanisms underpinning the pleiotropic effects of statins
The pleiotropic effects of statins potentially play an important role in the reduction of ischaemic events in the perioperative period. The most significant pleiotropic effects that potentially reduce the risk of adverse cardiovascular events include improving endothelial function, stabilising atherosclerotic plaques, inhibiting the thrombogenic response, decreasing oxidative stress, attenuating inflammation, modulating platelet function and inhibiting smooth muscle aggregation [17]. The mechanisms underlying each of these pleiotropic effects and how they decrease adverse cardiovascular events will be explored.
Statins and endothelial function
While statins protect against atherosclerosis by lowering cholesterol, endothelial function in patients on statins is improved before overt changes in cholesterol levels are detected. These observations have led to the proposal that statins directly enhance endothelial function [19]. Statins are known to preserve tissue Nitric Oxide (NO) signalling, protecting against vascular endothelial injury and myocardial dysfunction [20]. NO is a key mediator of vascular dilatation and protection and is derived from endothelial Nitric Oxide Synthase (eNOS) which is a critical enzyme for the maintenance of normal vascular function [21].
Statins upregulate eNOS activity, augment NO production and enhance endothelial function while also protecting the myocardium [22]. Statin treatment also blocks mevalonate synthesis in cultured endothelial cells [23]. However, under conditions where ROS generation may be exacerbated by risk factors such as smoking, hypertension and hypercholesterolaemia, Reactive Oxygen Species (ROS) can react with NO to form peroxynitrite which uncouples eNOS. Endothelial NOS then produces more ROS, particularly peroxynitrite, in place of NO, thus exacerbating oxidative stress, as a consequence of the imbalance between ROS production and elimination [24]. Increased vascular ROS production impairs endothelial function and contributes to vascular dysfunction [25] and myocardial injury [26]. Wagner and co-workers [27] found that statin treatment of healthy rats attenuated ROS formation and improved aortic endothelial function.
The loss of these effects of statins on the endothelium potentially underpins the increase in adverse postoperative outcomes associated with discontinuation of statin therapy. When statin therapy is withdrawn a rebound effect occurs in which isoprenylation of Rho is disinhibited, down-regulating eNOS activity to below pre-statin therapy levels, thus lowering NO bioavailability [5] (Figure 1).
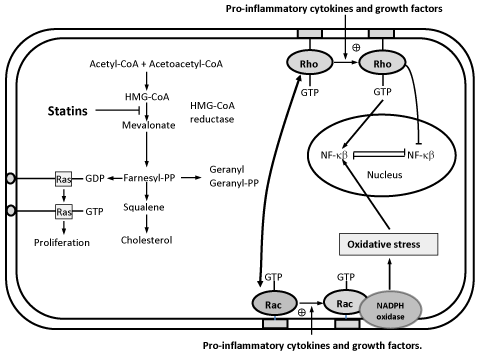
Figure 1: Illustration of the proposed effects of statin withdrawal and the
subsequent ‘statin rebound’ on isoprenylation of Rho and NO bioavailability.
Reproduced and modified from Le Manach et al. [5].
Statins potentially decrease oxidative stress and upregulate eNOS function to confer cardioprotective effects, both through direct protection against atherosclerosis and vascular dysfunction and indirectly by reducing myocardial oxidative stress and ROS induced injury.
Statins and attenuation of inflammation
In addition to enhancing endothelial function, statins also diminish inflammatory responses by reducing ROS production and modulation of macrophage and cytokine activity [5].
Since atherosclerosis involves a complex inflammatory process characterised by the infiltration of monocytes or macrophages into the vascular endothelium [28], statin induced reduction in ROS formation and oxidative stress serves to reduce the oxidation of LDL cholesterol which is a trigger of vascular inflammation [29]. Human monocyte-derived macrophages treated with simvastatin produced less ROS and subsequently produced lower levels of oxidised LDL cholesterol (ox-LDL) [30]. A key event that initiates the atherosclerotic process is the sub-endothelial deposition of ox- LDL which triggers the pro-inflammatory complement cascade and plaque formation [31]. The sub-endothelial deposition of ox-LDL stimulates monocytes to adhere to the endothelium, turning them into macrophages which absorb the ox-LDL, become foam cells, and form fatty streaks and plaques [29].
In addition to reducing LDL oxidation, statins also exert their anti-inflammatory effects by upregulating eNOS expression and NO release to attenuate monocyte adhesion to the endothelium. This NO induced attenuation of atherogenesis is absent in eNOS knockout mice treated with statins suggesting that NO is central to the antiinflammatory effect of statins [32].
The concept that these anti-inflammatory effects of statins are due to its pleiotropic effects is supported by a study demonstrating that statins elicit a reduction in the levels of circulating inflammatory markers prior to a reduction in cholesterol levels [33]. Ridker and coworkers [33] found that statin therapy reduced CRP by 14.8%. This group attributed the lowering of serum CRP levels to the inhibition of IL-6 production by Vascular Smooth Muscle Cells (VSMC).
Statins inhibit isoprenylation, thus modulating Rho activity. Rho activates NFκB, which in turn triggers a number of inflammatory responses and inhibits eNOS synthesis [35]. NFκB augments inflammatory responses by inducing the transcription of proinflammatory genes [36]. These inflammatory responses include increasing leukocyte adhesion, apoptosis and synthesis of cytokines such as TNF-a, IL-1β, IL-6, and IL-8, which all play a role in systemic inflammation [37]; furthermore, NFκB serves to decrease serum concentrations of anti-inflammatory cytokines such as IL-10. Hence, through inhibition of isoprenylation and Rho activation, statins have a direct anti-inflammatory effect [5].
The key mechanisms contributing to attenuated inflammation include improvement of endothelial function and reduction of oxidative stress, modulation of leukocyte activity and inhibition of isoprenylation, thereby reducing levels of NFκB, C-reactive protein and other inflammatory markers (Figure 2).
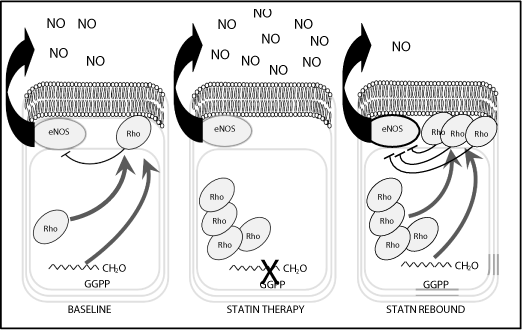
Figure 2: Summary of anti-inflammatory mechanism of Statins. Reproduced
and modified from Jain & Ridker [38].
Effects of statins and smooth muscle cells
Isoprenoids generated in the mevalonate pathway contribute to VSMC proliferation and growth [39], with statin induced inhibition of mevalonate production suppressing VSMC growth. This effect is supported by studies demonstrating that statins inhibit VSMC aggregation and migration in blood vessels [40]. Inhibition of Rho isoprenylation reduces Platelet-Derived Growth Factor (PDGF)- induced VSMC proliferation [41] which is involved in lesion development and plaque rupture. VSMC proliferation and migration is controlled by mediators such as growth factors and cytokines, secreted by leukocytes incorporated in the atherosclerotic lesion [42]. Statins decrease levels of these cytokines and growth factors, thus inhibiting VSMC aggregation to reduce lesion formation and subsequent plaque development.
Statins and platelet function
Platelet aggregation is a key step in both atherosclerotic lesion formation and thrombosis following plaque rupture [17]. Upregulation of eNOS increases NO bioavailability which inhibits platelet adhesion and aggregation [43]. This suppressed platelet accumulation also reduces VSMC exposure to PDGF [44]. Statins evidently also alter the cholesterol content of platelet membranes, modulating membrane fluidity and attenuating their ability to adhere to the endothelium [45]. Thus, by inhibiting platelet adhesion statins potentially reduce vascular atherosclerotic lesion and plaque formation.
Thrombus formation: Platelets are also involved in thrombosis following plaque rupture, which inevitably results in acute ischaemic coronary events such as myocardial infarction, electrical instability or stroke [46]. Plaque rupture exposes platelets to collagen, lipids and VSMCs which stimulates platelet adherence and initiates the coagulation cascade [47]. However, statins lower serum von willebrand factor and coagulation factors V, VII and XII levels [48], and reduce thrombosis by altering the balance between Plasminogen Activator Inhibitor (PAI) and Tissue Plasminogen Activator (TPA) [5].
Therefore the modulation of platelet function caused by statins inhibits both the formation and expansion of atherosclerotic plaques and lesions, and furthermore attenuates adverse cardiac events by attenuating atherothrombosis following plaque rupture.
Statins and plaque stability
Statins improve atherosclerotic plaque stability through several mechanisms already discussed previously in this review. Conditions that reduce atherosclerotic plaque stability include; 1) a large necrotic lipid core, 2) thinning of the fibrous cap, and 3) reduced VSMCs and neovascularization [47]. Statins have favourable effects on most of these factors that render a stable plaque vulnerable to rupture. Inhibition of VSMC aggregation and apoptosis caused by statins is detrimental in the context of plaque rupture. These processes however serve to prevent early plaque formation [49]. Statins induce attenuated inflammation, reduce lesion progression and attenuate plaque instability [5]. A randomized trial found that both hydrophilic (rosuvastatin) and lipophilic (simvastatin) statins significantly decrease the necrotic lipid cores size of atherosclerotic plaques [50]. They also modulate macrophage metalloproteinase and proteolytic enzymes secretion [51] that are implicated in fibrous cap thinning and increase plaque vulnerability [28].
Significance of the pleiotropic effects of statins in the perioperative period
Atherosclerotic plaque rupture and atherothrombosis formation are the predominant mechanisms leading to acute coronary events. The anti-atherogenic (lowering LDL cholesterol, improving endothelial function, reducing oxidative stress, attenuating inflammation and inhibiting VSMC proliferation and migration), plaque-stabilisation and anti-atherothrombotic effects (inhibited platelet aggregation, reduction of necrotic lipid core, decreased neovascularisation and inflammation within lesions) of statins provide these compounds with significant cardiovascular protective potential. By simultaneously diminishing atherosclerotic lesion formation and progression, and risk of atherothrombosis caused by plaque rupture, statins appear to be partially responsible for significant improvements in perioperative cardiac outcomes in clinical studies discussed in earlier sections [4,6- 8].
Given the comprehensive risk reduction afforded by perioperative statin therapy, there is growing support for the introduction of perioperative statin therapy [4]. This is particularly relevant for cardiac and vascular procedures where extensive tissue trauma and reperfusion injury induce inflammatory and atherothrombotic effects through increased platelet activation and elevated levels of fibrinogen and stress hormones [5]. These changes predispose patients to potentially fatal postoperative outcomes including myocardial infarction and stroke [13].
Patients subjected to statin withdrawal following cardiac and vascular surgery suffered worsened cardiac outcomes compared to those who had either sustained or no perioperative statin treatment. In some cases the effects of postoperative statin withdrawal were severe enough to serve as an independent predictor of postoperative myonecrosis [12]. This may be explained by the so-called ‘Statin rebound’ phenomenon, in which isoprenylation of Rho and Rac is disinhibited, resulting in eNOS downregulation and reduced NO bioavailability below baseline (pre-treatment) levels (Figure 1) [52]. This reduction would compromise endothelial function [21], increased ROS formation and oxidative stress [24], augmented inflammation [35], increased VSMC proliferation and migration [39], increased platelet aggregation [43] and render the atherosclerotic plaque vulnerable to rupture and atherothrombosis [47]. Since atherosclerosis is the underlying cause of acute coronary events [53], postoperative withdrawal of statin therapy may be inadvisable. Despite growing evidence supporting the cardioprotective effects of statins, it remains difficult to definitively establish whether these benefits are attributable to the so-called pleiotropic or lipidlowering effect of statins [17]. More comprehensive studies should be conducted to resolve these uncertainties.
Acute perioperative statin administration
More than 50% of patients undergoing major vascular surgery and 80% undergoing cardiac surgery [5] are on chronic statin therapy. In addition to improving perioperative outcomes in chronic statin users, studies examining the effects of acute perioperative statin therapy have reported improved postoperative cardiac outcomes in previously statin-naïve, normocholesterolaemic patients [54,55]. These observations are further supported by a study demonstrating that short-term atorvastatin treatment subsequent to vascular surgery significantly reduced postoperative adverse cardiac events in a cohort of both hypercholesterolaemia and normocholesterolaemic patients [56]. These studies suggest that not only chronic, but also acute statin therapy is beneficial in the perioperative period.
Adverse effects of statins
Notwithstanding compelling evidence for the beneficial effects of statins, there are a number of reported adverse effects that prescribers should be cognizant of when recommending and prescribing statins [4]. The most commonly reported statin-mediated adverse outcome is myopathy or rhabdomyolysis [4]. This condition was however most commonly associated with cerivastatin (3.16 events per million prescriptions), which is no longer manufactured. Statin induced rhabdomyolysis is significantly less apparent in more frequently prescribed statins, with incidence ranging from 0 to 0.19 events per million prescriptions [57]. Myopathic events are significantly more common when using rosuvastatin when compared to other widely used statins [58]. The low incidence of adverse effects caused by rosuvastatin is however inconclusive. In a meta-analysis of the effects of perioperative statins on Creatine Kinase (CK) levels data suggests that the incidence of elevated CK in statin users is only marginally higher than that seen in patients not using statins [60]. It has however been shown that statins significantly increase serum transaminase levels which is a marker of liver damage [61].
Other factors that should also be considered when prescribing statins include: advanced age (>80 years), stature, and chronic illnesses such as renal failure and impaired liver function [59]. Antagonistic medications and chronic alcoholism also augment the risk of statin-associated adverse outcomes [59]. These factors must always be considered prior to initiating statin therapy, whether for chronic or acute use. Despite these often anecdotal adverse outcomes associated with statins, their therapeutic benefits are unquestionable in the majority of statin users [5].
Conclusions
Growing evidence suggests that sustained perioperative statin therapy improves cardiovascular outcomes not only through its primary lipid lowering effects, but also through its pleiotropic effects. These include the prolongation of atherosclerotic lesion formation, plaque development and rupture, and atherothrombosis which will all protect against adverse cardiac events. This cardioprotection is observed with both chronic and acute statin therapy while postoperative statin withdrawal appears to have adverse effects on postoperative cardiac outcomes. These observation suggest that it is advisable that statin therapy be continued as a precautionary measure to mitigate postoperative adverse cardiac events.
References
- World Health Organization, WHO | Obesity and overweight. 2015.
- Chopra V, Wesorick DH, Sussman JB, Greene T, Rogers M, Froehlich JB, et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta-analysis. Arch Surg. 2012; 147: 181-199.
- Kim MC, Ahn Y, Jang SY, Cho KH, Hwang SH, Lee MG, et al. Comparison of Clinical Outcomes of Hydrophilic and Lipophilic Statins in Patients with Acute Myocardial Infarction. Korean Journal of Internal Medicine. 2011; 26: 294-303.
- Fang SY, Roan JN, Luo CY, Tsai YC, Lam CF. Pleiotropic vascular protective effects of statins in perioperative medicine. Acta Anaesthesiologica Taiwanica. 2013; 51: 120-126.
- Le Manach Y, Coriat P, Collard CD, Riedel B. Statin Therapy within the Perioperative Period. The Journal of the American Society of Anesthesiologists. 2008; 108: 1141 -1146.
- Christenson JT. Preoperative lipid-control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABG. European Journal of Cardiothoracic Surgery. 1999; 15: 394-400.
- Poldermans D, Bax JJ, Kertai MD, Krenning B, Westerhout CM, Schinkel AF, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation. 2003; 107: 1848-1851.
- Kuhn EW, Liakopoulos OJ, Stange S, Deppe AC, Slottosch I, Choi YH, et al. Preoperative statin therapy in cardiac surgery: a meta-analysis of 90,000 patients. European Journal of Cardiothoracic Surgery. 2013; 45: 17-26.
- Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke. 2005; 36: 2072.
- Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a Reduced Incidence of Perioperative Mortality after Coronary Artery Bypass Graft Surgery. Circulation. 2004; 110: 45-49.
- Fallouh N, Chopra V. Statin withdrawal after major noncardiac surgery: risks, consequences, and preventative strategies. Journal of Hospital Medicine. 2012; 7: 573-579.
- Le Manach Y, Godet G, Coriat P, Martinon C, Bertrand M, Fléron MH, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg. 2007; 104: 1326-1333.
- Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering is they clinically relevant? European Heart Journal. 2003; 225-248.
- Berg J, Tymoczko J, Stryer L. The Complex Regulation of Cholesterol Biosynthesis takes place at Several Levels, in Biochemistry 5th Edition. WH Freeman: New York. 2002.
- Edwards J, A Moore. Statins in hypercholesterolaemia: A dose-specific metaanalysis of lipid changes in randomised, double blind trials. BMC Family Practice. 2003. 4.
- Paraskevas KI, Mikhailidis DP, Veith FJ. Optimal statin type and dosage for vascular patients. Journal of Vascular Surgery. 2011; 53: 837-844.
- Liao JK, Laufs U. Pleiotropic Effects of Statins. Annual Review of Pharmacology and Toxicology. 2005; 45: 89-118.
- Heeschen C, Hamm CW, Laufs U, Snapinn S, Böhm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002; 105: 1446-1452.
- Omori H, Nagashima H, Tsurumi Y, Takagi A, Ishizuka N, Hagiwara N, et al. Direct in vivo evidence of a vascular statin: a single dose of cerivastatin rapidly increases vascular endothelial responsiveness in healthy normocholesterolaemic subjects. British Journal of Clinical Pharmacology. 2002; 54: 395-399.
- Burma O, Onat E, Uysal A, Ilhan N, Erol D, Ozcan M, et al. Effects of rosuvastatin on ADMA, rhokinase, NADPH oxidase, caveolin-1, hsp 90 and NFkB levels in a rat model of myocardial ischaemia-reperfusion. Cardiovascular Journal of Africa. 2015; 25: 212-216.
- Förstermann U, T Münzel. Endothelial Nitric Oxide Synthase in Vascular Disease. Circulation. 2006; 113: 1708-1714.
- Chen M, Li H, Wang Y. Protection by atorvastatin pretreatment in patients undergoing primary percutaneous coronary intervention is associated with the lower levels of oxygen free radicals. Journal of Cardiovascular Pharmacology. 2013; 62: 320-324.
- Jantzen F, Könemann S, Wolff B, Barth S, Staudt A, Kroemer HK, et al. Isoprenoid depletion by statins antagonizes cytokine-induced downregulation of endothelial nitric oxide expression and increases NO synthase activity in human umbilical vein endothelial cells. Journal of physiology and pharmacology. 2007; 53.
- Soccio M, Toniato E, Evangelista V, Carluccio M, De Caterina R. Oxidative stress and cardiovascular risk: the role of vascular NAD(P)H oxidase and its genetic variants. European Journal of Clinical Investigation. 2005; 35: 305- 314.
- Szocs K. Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. General Physiology and Biophysics. 2005; 23: 265-295.
- Raedschelders K, Ansley DM, Chen DD. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacology & Therapeutics. 2011; 133: 230-255.
- Wagner AH, Köhler T, Rückschloss U, Just I, Hecker M. Improvement of nitric oxide- dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation endothelial superoxide anion formation. Arteriosclerosis Thrombosis Vascular Biology. 2000; 20: 61-9.
- Pasqui A, Bova G, Maffei S, Auteri A. Immune factors in atherosclerosis. Ann Ital Med Int. 2005; 20: 81-89.
- Willerson J, Ridker PM. Inflammation as a Cardiovascular Risk Factor. Circulation. 2004; 109: 1.
- Giroux LM, Davignon J, Naruszewicz M. Simvastatin inhibits the oxidation of low- density lipoproteins by activated human monocyte-derived macrophages. Biochimica et Biophysica Acta. 1993; 1165: 335-338.
- Torzewski J, Bowyer DE, Waltenberger J, Fitzsimmons C. Processes in atherogenesis: complement activation. Atherosclerosis. 1997; 132: 131-138.
- Stalker TJ, Lefer AM, Scalia R. A new HMG-CoA reductase inhibitor, rosuvastatin, exerts anti-inflammatory effects on the microvascular endothelium: the role of mevalonic acid. British Journal of Pharmacology. 2001; 133: 406-412.
- Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C- reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. New England Journal of Medicine, 2001; 344: 1959-1965.
- Ikeda U, Ito TA, Shimada K. Statins and C-reactive protein. The Lancet. 1999; 353: 1274-1275.
- Perona R, Montaner S, Saniger L, Sánchez-Pérez I, Bravo R, Lacal JC. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes & Development. 1997; 11: 463-475.
- Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annual Review of Immunology. 1996; 14: 649-683.
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. Journal of Clinical Investigation. 2001; 107: 7-11.
- Jain M, Ridker PM. Anti-Inflammatory Effects of Statins: Clinical Evidence and Basic Mechanisms. Nature Reviews Drug Discovery. 2005; 4: 977-987.
- Raiteri M, Arnaboldi L, McGeady P, Gelb MH, Verri D, Tagliabue C, et al. Pharmacological control of the mevalonate pathway: effect on arterial smooth muscle cell proliferation. J Pharmacol Exp Ther, 1997; 281: 1144-1153.
- Axel DI, Riessen R, Runge H, Viebahn R, Karsch KR. Effects of cerivastatin on human arterial smooth muscle cell proliferation and migration in transfilter cocultures. J Cardiovasc Pharmacol. 2000; 35: 619-629.
- Yang Z, Kozai T, van der Loo B, Viswambharan H, Lachat M, Turina et al. HMG-CoA reductase inhibition improves endothelial cell function and inhibits smooth muscle cell proliferation in human saphenous veins. Journal of the American College of Cardiology. 2000; 36: 1691-1697.
- Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Medica Indonesiana. 2007; 39: 86-93.
- Emerson M, Momi S, Paul W, Alberti PF, Page C, Gresele P. Endogenous nitric oxide acts as a natural antithrombotic agent in vivo by inhibiting platelet aggregation in the pulmonary vasculature. Thrombosis and Haemostasis. 1999; 81: 961-966.
- Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, et al. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994; 23: 1121-1131.
- Le Quan Sang KH, Mazeaud M, Astarie C, Duranthon V, Driss F, Devynck MA. Plasma lipids and platelet membrane fluidity in essential hypertension. Thromb Haemost. 1993; 69: 70-76.
- Willerson JT, Golino P, Eidt J, Campbell WB, Buja LM. Specific platelet mediators and unstable coronary artery lesions. Experimental evidence and potential clinical implications. Circulation. 1989; 80: 198-205.
- Fuster V, Stein B, Ambrose JA, Badimon L, Badimon JJ, Chesebro JH. Atherosclerotic plaque rupture and thrombosis. Evolving concepts. Circulation. 1990; 82: 47-59.
- Ray KK, Cannon CP. The Potential Relevance of the Multiple Lipid- Independent (Pleiotropic) Effects of Statins in the Management of Acute Coronary Syndromes. Journal of the American College of Cardiology. 2005. 46: 1425–1433.
- Erl W. Statin-induced vascular smooth muscle cell apoptosis: a possible role in the prevention of restenosis? Curr Drug Targets Cardiovasc Haematol Disord. 2005; 5: 135-144.
- Hong MK, Park DW, Lee CW, Lee SW, Kim YH, Kang DH, et al. Effects of statin treatments on coronary plaques assessed by volumetric virtual histology intravascular ultrasound analysis. JACC Cardiovasc Interv. 2009; 2: 679-688.
- Izidoro-Toledo TC, Guimaraes DA, Belo VA, Gerlach RF, Tanus-Santos JE. Effects of statins on matrix metalloproteinases and their endogenous inhibitors in human endothelial cells. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2011; 383: 547-554.
- Endres M, Laufs U. Effects of statins on endothelium and signaling mechanisms. Stroke. 2004; 35: 2708-2711.
- Badimon L, Padro T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care. 2012; 1: 60-74.
- Billings FT, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury following cardiac surgery. J Cardiothorac Vasc Anesth. 2010; 24: 913-920.
- Dotani MI, Elnicki DM, Jain AC, Gibson CM. Effect of preoperative statin therapy and cardiac outcomes after coronary artery bypass grafting. Am J Cardiol. 2000; 86: 1128-1130.
- Durazzo AE, Machado FS, Ikeoka DT, De Bernoche C, Monachini MC, Puech-Leão P, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg, 2004; 39: 967-975.
- Sethi M, Collard C. Perioperative statin therapy: are formal guidelines and physician education needed? Anesthaesia and Analgaesia. 2007; 104: 1322- 1324.
- Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH, et al. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005; 111: 3051-3057.
- Grundy SM. The Issue of Statin Safety. Circulation. 2005; 111: 3016-3019.
- Kapoor AS, Kanji H, Buckingham J, Devereaux PJ, McAlister FA. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ. 2006; 333: 1149.
- Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006; 114: 2788-2797.