
Review Article
J Cardiovasc Disord. 2021; 7(1): 1042.
Wearable Devices: A Future Useful Tool for Detection of Silent Ischemia in Patients with Diabetes?
Vlachakis PK¹*, Tentolouris A², Kanakakis I¹, Eleftheriadou I² and Alexopoulos D³
¹Department of Clinical Therapeutics, School of Medicine, National and Kapodistrian University of Athens, Alexandra General Hospital, Athens, Greece
²First Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Greece
³2nd Department of Cardiology, Attikon University Hospital, National and Kapodistrian University of Athens Medical School, Athens, Greece
*Corresponding author: Vlachakis PK, Department of Clinical Therapeutics, School of Medicine, National and Kapodistrian University of Athens, Alexandra General Hospital, 80 Vasilissis Sofias Avenue, 11528, Athens, Greece
Received: May 26, 2021; Accepted:June 30, 2021; Published: July 07, 2021
Abstract
As smartphone health care technology continues to evolve, many wearable devices are equipped with Electrocardiographic (ECG) recording. Recently, studies examining the possibility of various wearable devices for continuous ECG recording showed their ability to detect ST-segment alterations. It is known that in almost a quarter of people with diabetes, the presentation of an acute coronary syndrome may be atypical or even asymptomatic (“silent”), and it has been associated with adverse prognosis. The precise mechanisms behind the lack of pain in patients suffering from silent myocardial ischemia remain unknown. The attractive hypothesis that clinicians could use a wearable ECG recording to detect and treat earlier patients suffering from silent myocardial ischemia might change the adverse prognosis of those patients. However, before their clinical application, several obstacles should be overcome in order the physicians to obtain an additional powerful tool in the fight against coronary artery disease in people with diabetes.
Keywords: Silent myocardial ischemia; Diabetes mellitus; Electrocardiogram; Wearable devices; Ambulatory ECG; ST-segment alterations
Abbreviations
ACS: Acute Coronary Syndrome; CAN: Cardiac Autonomic Dysfunction; CAD: Coronary Artery Disease; CV: Cardiovascular; DM: Diabetes Mellitus; ECG: Electrocardiogram; LED: Light- Emitting Diode; LBBB: Left Bundle Branch Block; PET: Positron Emission Tomography; SMI: Silent Myocardial Ischemia; STEMI: ST-Segment Elevation Myocardial Infarction
Introduction
Diabetes Mellitus (DM) is the most common metabolic disorder affecting more than 463 million people worldwide [1]. The presence of DM increases two-fold the risk for Cardiovascular (CV) disease in men as well as three-fold in women [2-4], and CV disease remains the leading cause of morbidity and mortality in people with DM [5]. Atypical presentations of Acute Coronary Syndrome (ACS), such as silent myocardial ischemia (SMI), are more prevalent in subjects with DM and they are associated with worse outcomes [6,7].
It was in 1903, when a bipolar three-channel Electrocardiogram (ECG) was developed and recorded by Einthoven [8]. Since then, the ECG is an essential tool in daily clinical practice for the diagnosis of cardiac diseases. As technology advances in the modern world, portable wearable “smart” devices have already become of utmost importance to everyday life. Nowadays, many of these “smart” devices are used in medicine, and many of them are capable of ECG recording [9]. Several studies have provided evidence regarding the beneficial role of this technology in the detection of arrhythmias, such as atrial fibrillation [10]. Interestingly, some studies have also examined their role in patients suffering from acute Myocardial Infarction (MI) and have provided encouraging data [11,12].
This review aims to consider the tempting hypothesis that wearable devices can detect patients suffering from SMI, including people with DM, through continuous ECG recording. Such an approach may shorten the time of diagnosis and treatment and improve the prognosis of these patients.
Silent Ischemia in People with Diabetes
Angina pectoris is considered the predominant symptom of myocardial ischemia. Nevertheless, angina pectoris might be a poor indicator of myocardial ischemia, particularly in subjects with DM [13]. SMI is defined as the presence of objective findings indicative of myocardial ischemia without angina or anginal equivalent symptoms [14]. In the Framingham Study, approximately one-fourth of the participants with an ACS had SMI or atypical MI [15]. The actual frequency of SMI in that study may have been underestimated since the diagnosis of previous MI was based only on the detection of Q waves on a routine ECG. It is known that asymptomatic MI or asymptomatic myocardial ischemia occurs more frequently in patients with DM [13]. In the Framingham study, the incidence of painless MI was higher in people with DM in comparison with those without DM [16,17].
When the balance between myocardial oxygen supply and demand is disturbed, the resulting mismatch may immediately precipitate a vicious cycle whereby myocardial ischemia is induced. From a pathophysiology perspective, the precise mechanisms of anginal pain and neural pathways are not fully elucidated. Numerous tempting hypotheses have been proposed to shed light on the possible mechanism behind the lack of pain in patients suffering from SMI [14]. Cardiac Autonomic Dysfunction (CAD) is presumed to play a vital role in SMI [18]. CAN is among the most common but least recognized complications of DM [19]. A meta-analysis of 12 studies that included 1460 individuals with DM showed that the prevalence of SMI was higher in DM patients with CAN in comparison with patients without CAN (the pooled prevalence rate risk for SMI was 1.96, 95% CI: 1.53-2.51, p<0.001) [18]. Older studies have speculated that a defective anginal warning system may have a role in the lack of symptoms in SMI patients since it has been reported that cardiac denervation is associated with SMI in patients undergoing cardiac transplantation [20]. In addition, people with CAN may have a higher threshold of pain or even incapability to attain pain threshold during the ischemic episode [14]. The relevant effect of CAN in patients with DM is also suggested by the absence of a peak incidence of myocardial ischemia in the morning hours [21]. In parallel, people with DM with or without signs of autonomic dysfunction have a decreased vagal activity (and therefore a relatively increased sympathetic activity) throughout the night and at the same time of the day during which a higher frequency of CV accidents is observed [22]. Moreover, the ischemic burden might be associated with painless myocardial ischemia. Among 300 patients with established ischemic heart disease and reversible hypoperfusion on exercise sestamibi tomography, those with painless ischemia had less reversible hypoperfusion defects compared to symptomatic patients (mean±SD, 11±7 % vs. 16±10 %, p<0.001, respectively) [23].
B-endorphin, a potent opioid-like peptide, is another factor that is assumed to contribute to this enigmatic pathophysiology. In SMI patients, data from different studies reported that plasma levels of b-endorphin were higher in comparison with symptomatic patients [24]. Predominantly or totally painless myocardial ischemia via generalized defective perception of painful stimuli has also been reported as an alternative underlying mechanism. A possible link between anti-inflammatory cytokines and silent ischemia was evaluated by Mazzone et al. [25]. The investigators noticed that in patients with silent ischemia, the expression of CD11b receptor was lower compared to patients with painful myocardial ischemia. Higher production of anti-inflammatory cytokines was implicated in blocking the pain transmission pathways leading to a higher threshold for nerve activation [25]. Lastly, Rosen et al. tried to examine the possible role of central nervous system in this unresolved equation by using a Positron Emission Tomography (PET) of the brain [26]. In patients with SMI, the activation of the basal frontal anterior and ventral cingulate cortices and the left temporal pole was reduced compared to patients with anginal symptoms [26]. The potential mechanisms that have been described to explain SMI development are summarized in Figure 1.
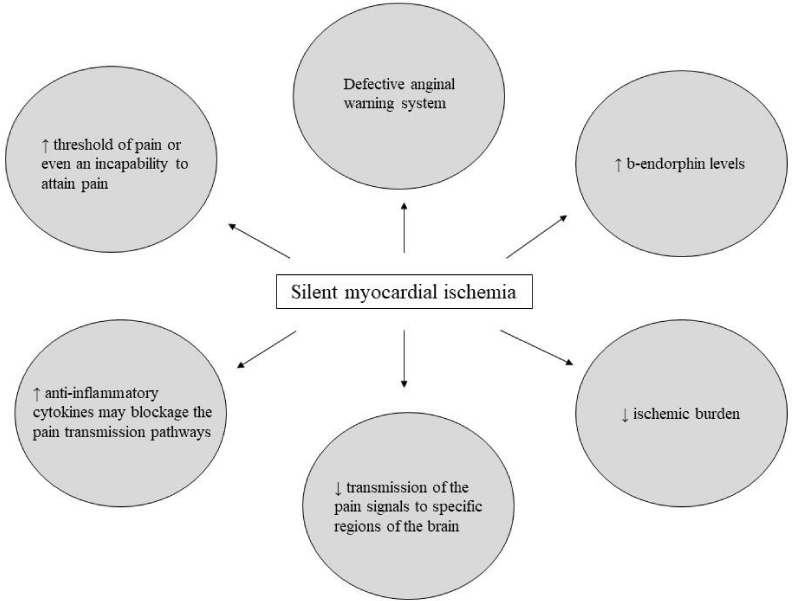
Figure 1: Different mechanisms that have been proposed to explain the development of SMI.
To Revascularize or not to Revascularize: A Dilemma in Patients with SMI
Irrespective of clinical presentation, silent or symptomatic, the physicians should be trying to reduce the burden of myocardial ischemia by using the optimal approach [27]. Data regarding the superiority of invasive treatment compared to the optimal medical treatment alone, and vice versa, are scarce. In the early 1990s, the ACIP trial (Asymptomatic Cardiac Ischemia Pilot Study) tried to evaluate the role of invasive treatment compared to medical treatment in 558 patients suffering from SMI. Regarding medical treatment, patients were randomly assigned by the investigators in two groups: 1) the angina-guided group to relieve symptoms, as 50% of Coronary Artery Disease (CAD) patients with angina have episodes of SMI during ambulatory ECG, and 2) the ischemia-guided group targeting to relieve both angina and ambulatory ECG ischemia. The results showed that the signs of ischemia in the ambulatory ECG were suppressed in 55% of patients in the revascularization group compared to 39% of patients in the angina-guided group and 41% of patients in the ischemia-guided group 12 weeks after the enrollment [28]. Likewise, at 2 years follow up, the invasive approach was associated with reduced mortality (1.1% vs. 4.4% vs. 6.6%, respectively, p<0.02) and lower incidence of death or myocardial ischemia (4.7% vs. 8.8% vs. 12% respectively, p<0.04) in comparison with ischemiaguided and angina-guided strategies [29]. Conversely, in 2007 the COURAGE trial that also compared medical treatment alone with invasive strategy (PCI with bare-metal stents) plus optimum medical treatment in more than 2280 patients with stable CAD who had evidence of myocardial ischemia found no difference between the two approaches concerning the primary endpoint of death from any cause and nonfatal myocardial infraction at 4.6 years (19.0 % vs. .18.5%, respectively, HR 1.05, 95% CI: 0.87-1.27; p=0.62) [30]. Very recently, the ISCHEMIA study also revealed no difference in primary ischemic outcome between invasive and conservative strategy over a median of 3.2 years (cumulative event rate was 16.4% vs. 18.2%, difference=1.9%; 95% CI, 0.8-3.0) in 5179 patients with chronic coronary syndrome and moderate to severe reversible ischemia on imaging testing. It is worth mentioning that among patients who were enrolled in that study, approximately 35% had no angina in the previous 4 weeks [31].
Smartphone Technology to Recognize Ischemic ST-Segment Changes
Health technology industry is thriving, and smart devices have become a part of the daily health life of individuals with several applications aiming to identify a cardiac rhythm abnormality. In the beginning, devices such as smartwatches and wristbands were used bypeople as activity trackers. Later, they were equipped with an integrated system of optical sensors that could detect an irregular rhythm via photoplethysmography with Light-Emitting Diode (LED) lights for the recording of pulse irregularities [32]. The Apple Heart Study and the WATCH AF trial (SmartWATCHes for Detection of Atrial Fibrillation) provided evidence for the feasibility of recognizing pulse irregularity which may unmask asymptomatic atrial fibrillation [33,34]. Recently, few of these devices have been rigged with electrodes to obtain a single-lead ECG. Since data for the detection and diagnosis of arrhythmias via smart devices exist, few investigators have attempted to assess the potential of these devices to identify ST-segment alterations, such as in patients with ST-Segment Elevation Myocardial Infarction (STEMI).
The first attempt to record a 3-lead Smartwatch ECG (Apple Watch 4, Apple Inc, Cupertino, CA) and compare it with the conventional 12-lead ECG was performed by Avila in two cases of patients presenting with STEMI in the emergency department. As the author adjusted the smartwatch position and a 3-lead ECG was obtained (Einthoven lead I, II, and III), he found good comparability between the Apple Watch ECG and the standard ECG in detecting the ST-segment elevation [35]. In the same direction, Samol et al. also found a good agreement between a 6-channel smartwatch ECG (Einthoven lead I-III, and Wilson-like chest leads V1, V4, and V6) and a standard ECG in two STEMI patients [36]. Of note, the investigators mentioned that the cardiologists recruited to obtain and read the smartwatch ECG assumed correctly the location of the culprit lesion, even if they characterized the smartwatch ECG quality as moderate.
The first trial that tested the possible-feasible role of smartphone “12-lead-equivalent” ECG in patients with symptoms suggestive of ACS was the ST LEUIS trial (The Smartphone ECG for Evaluation of ST-Segment Elevation Myocardial Infarction Study) [11]. In this multicenter, non-randomized, open trial, a total of 204 patients complaining of chest pain were recruited; a single lead ECG was recorded from a portable smartphone-based technology [2-wires with alligator clips (modified version of the AliveCorTMHeart Monitor)] andwas paired with a 5th generation iPod Touch device]. The recordings were compared with the standard 12-lead ECG device. An expert reading panel was used to assess the quality of smartphone ECG. A total of 87.4% of the smartphone ECG readings were characterized as good, while 0.5% of the ECG readings were characterized as poor or uninterpretable. Side-to-side comparisons were not attempted in 14 pairs because of unreadable technical quality in the smartphone ECG (n=13) or because of ventricular pacing in the 12-lead ECG (n=1). A side-by-side comparison of the smartphone compared to conventional ECG revealed a good agreement, since the diagnosis of STEMI, Left Bundle Branch Block (LBBB), and Not- STEMI were established in 22.5%, 5.4%, and 71.6% of patients with the conventional ECG compared to 29.4%, 5.4% and 58.5% with smartphone ECG, respectively. Of importance, smartphone ECG was associated with high sensitivity and negative predictive values for the diagnosis of STEMI/LBBB (0.89 and 0.95, respectively) and also very high specificity and positive predictive values for Not-STEMI cases (0.89 and 0.95, respectively) [11].
Moreover, the potential use of ambulatory ECG monitoring such as a commercially available wearable in patients with ACS was examined by the SMARTAMI Trial (Multichannel Electrocardiograms Obtained by a Smartwatch for the Diagnosis of ST-Segment Changes) [12]. In this study, the investigators obtained leads I, II, III, V1, V2, V3, V4, V5, and V6 with a smartwatch (Apple Watch Series 4 smartwatch-all ECGs were collected using heath app of an iPhone Series 11 Pro smartphone), positioned in the posterior sensor of the watch in different standardized body positions, in 100 participants (54 patients had STEMI, 27 had non-STEMI and 19 healthy patients admitted as control group) and compared the ECG alterations with a standard 12-lead-ECG device. A sufficient concordance between smartwatch and standard ECGs for the identification of normal ECG [Cohen’s kappa (k) 0.90, 95% CI, 0.78-1.00], ST-segment elevation (k 0.88, 95% CI, 0.78-0.97) and non-ST segment-elevation alterations (k 0.85, 95% CI, 0.74-0.96) was demonstrated. A good capability of smartwatch to identify the localization of ST- alterations (anterior, inferior, and lateral [k 0.66 95% CI (0.79-0.96)]) was revealed. The sensitivity and specificity of smartwatch ECG for the diagnosis of the normal ECG were 84% (95% CI, 60-97%) and 100% (95% CI, 95%-100%), respectively. Likewise, for the patients with ST-segment elevation the sensitivity was 93% (95% CI, 82- 99%) and specificity was 95% (95% CI, 85-99%) and for those with non ST-segment elevation ECG alteration, sensitivity was 94% (95% CI, 81- 99%) and specificity was 92% (95% CI, 83-97%). Authors suggested that a possible applicability of ECG recording by smartwatch could be useful under specific situations such as when the standard ECG is not available or during pandemics such as coronavirus disease of 2019 (COVID-19) where system delays jeopardize the principle of “time is muscle” [12,37]. Table 1 summarizes the data from ST LEUIS and SMARTAMI trials.
Study (Year)
Patients
Device
Comparison
Results
ST LEUIS
trial
(2019)(11)204 patients:
92 STEMI patients and 112 patients presenting in the ED complaining chest pain.AliveCorTM Heart Monitor pair with a 5th generation iPod Touch device.
Smartphone ECG compared with the standard 12-lead ECG device in patients which STEMI protocol was activated and in patients presenting in the emergency department complaining chest pain.
Smartphone ECG vs Conventional ECG
STEMI: 29.4% vs 22.5%
LBBB: 5.4% vs 5.4%
Not-STEMI: 58.5% vs 71.6%Smartphone ECG technical quality was rated as good or fair in 183 participants and poor or unreadable in 21 participants.
Smartphone ECG:
- STEMI/LBBB diagnosis sensitivity: 0.89
- STEMI/LBBB diagnosis negative predictive values: 0.95
- Not-STEMI diagnosis specificity: 0.89
- Not-STEMI diagnosis positive predictive values: 0.95
SMARTAMI trial
(2020)(12)100 patients:
54 STEMI patients, 27 NSTEMI patients and 19 healthy individuals.Apple Watch Series 4 smartwatch-all ECGs were collected using heath app of an iPhone Series 11 Pro smartphone.
Smartwatch ECG compared with the standard 12-lead ECG device.
Concordance between Smartwatch ECG and Conventional ECG:
- identification of normal ECG [Cohen’s kappa (k) 0.90, 95% CI, 0.78-1.00]
- identification of STEMI (k 0,88, 95% CI, 0.78-0.97)
- identification of NSTEMI alterations (k 0.88, 95% CI, 0.74-0.96).
Smartwatch ECG:
- Normal ECG diagnosis sensitivity: 84% (95% CI, 60-97 %)
- Normal ECG diagnosis specificity: 100% (95% CI, 95-100 %)
- STEMI sensitivity: 93% (95% CI, 82-99 %)
- STEMI specificity: 95% (95% CI, 85-99%)
- NSTEMI sensitivity: 94% (95% CI, 81-99 %)
- NSTEMI specificity: 92% (95% CI, 83-97 %)
Abbreviations: ECG: Electrocardiography; ED: Emergency Department; CI: Confidence Interval; LBBB: Left Bundle Branch Block; NSTEMI: non-ST segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction.
Table 1: Data from ST LEUIS and SMARTAMI studies for the applicability of smartphone-based ECG in patients with ST-segment alterations.
Despite the favorable outcomes, there are several limitations in the aforementioned studies. Both trials were designed to identify patients suffering from STEMI. However, whether those findings could have been extrapolated in patients with other ischemic changes such as ST-segment depression or T-wave inversion remains questionable, as they were not systematically recorded with these devices [11,12]. Moreover, technical considerations such as the need to remove the smartwatch from the wrist in order to record the ECG and the absence of leads aVR, aVL, and aVF for better localization of acute MI were pointed out by the investigators of the SMARTAMI study [12]. Furthermore, the compared groups were not matched for the confounding factors of gender and age [12]. Additionally, the ST LEUIS study hinted at the selection of trained clinical researchers to interpret the smartphone tracing recordings because the primary aim was to develop a smartphone ECG technology that could be used by all physicians in daily clinical practice [11].
A countable proportion of patients, especially those with concomitant DM, suffering from an ischemic attack may have an atypical presentation or be asymptomatic [19,38]. Data from the two studies as mentioned earlier revealed the feasibility of smartphone technology, such as wearables, to detect ST-segment alterations with reliable outcomes when compared to the conventional 12-leads ECG. As early therapeutic approach is crucial, these new devices may eliminate system and patient-related delays in diagnostic evaluation with favorable morbidity and mortality outcomes. However, as the mean age of the patients in both aforementioned studies were 60 years old, appropriate use of a smartwatch for ECG recording in older diabetic patients, which are more prone to SMI, may be challenging due to difficulties in the use of the device or mobile application, the different positioning of the smartwatch, or even a potential inability to lie still in a supine position due to comorbidities like Parkinson’s disease. In addition, this continuous electrocardiographic equipment could help clinicians achieve more rapidly the optimum long-term treatment dose titration, such as beta-blockers. Moreover, they could also be used as secondary prevention to identify malignant ventricular arrhythmias since patients with STEMI are more prone to them in the first 1 to 2 years after the acute coronary event and, therefore, are at greater risk for sudden cardiac death [39].
As previously mentioned, the limitations of the wearable ECG are several according to the available data so far. The collaboration of technicians and physicians is of importance in order to upgrade the available software and to design randomized studies that could demonstrate the diagnostic efficacy of these new devices and the feasibility in clinical practice to identify patients suffering from SMI, such as people with diabetes.
Conclusion
As individuals with DM and SMI are carrying an increased hazard for ischemic events, it is essential to develop strategies to optimize outcomes and mitigate risk. The “futurist” idea of using the smartphone technology such as smartwatches to detect early patients with signs of silent ischemia is tempting. The first available data for detecting ST-segment alterations is encouraging. However, as several considerations remain unanswered, further artificial intelligence studies are needed to improve the available software of these devices in order physicians to obtain another powerful “weapon” in the armamentarium against coronary artery disease.
References
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes research and clinical practice. 2019; 157: 107843.
- Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979; 59: 8-13.
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes care. 1993; 16: 434-444.
- Tentolouris A, Eleftheriadou I, Athanasakis K, Kyriopoulos J, Tsilimigras DI, Grigoropoulou P, et al. Prevalence of diabetes mellitus as well as cardiac and other main comorbidities in a representative sample of the adult Greek population in comparison with the general population. Hellenic journal of cardiology : HJC=Hellenike kardiologike epitheorese. 2020; 61: 15-22.
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999; 100: 1134-1146.
- El-Menyar A, Zubaid M, Sulaiman K, AlMahmeed W, Singh R, Alsheikh-Ali AA, et al. Atypical presentation of acute coronary syndrome: a significant independent predictor of in-hospital mortality. Journal of cardiology. 2011; 57: 165-171.
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Hansen JF. Prevalence and prognostic significance of daily-life silent myocardial ischaemia in middleaged and elderly subjects with no apparent heart disease. European heart journal. 2005; 26: 1402-1409.
- Einthoven W, Fahr G, De Waart A. On the direction and manifest size of the variations of potential in the human heart and on the influence of the position of the heart on the form of the electrocardiogram. American heart journal. 1950; 40: 163-211.
- Vardas P, Cowie M, Dagres N, Asvestas D, Tzeis S, Vardas EP, et al. The electrocardiogram endeavour: from the Holter single-lead recordings to multilead wearable devices supported by computational machine learning algorithms. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2020; 22: 19-23.
- Zungsontiporn N, Link MS. Newer technologies for detection of atrial fibrillation. Bmj. 2018; 363: k3946.
- Muhlestein JB, Anderson JL, Bethea CF, Severance HW, Mentz RJ, Barsness GW, et al. Feasibility of combining serial smartphone single-lead electrocardiograms for the diagnosis of ST-elevation myocardial infarction. American heart journal. 2020; 221: 125-35.
- Spaccarotella CAM, Polimeni A, Migliarino S, Principe E, Curcio A, Mongiardo A, et al. Multichannel Electrocardiograms Obtained by a Smartwatch for the Diagnosis of ST-Segment Changes. JAMA cardiology. 2020.
- Chiariello M, Indolfi C. Silent myocardial ischemia in patients with diabetes mellitus. Circulation. 1996; 93: 2089-2091.
- Deedwania PC, Carbajal EV. Silent myocardial ischemia. A clinical perspective. Archives of internal medicine. 1991; 151:2373-82.
- Boland LL, Folsom AR, Sorlie PD, Taylor HA, Rosamond WD, Chambless LE, et al. Occurrence of unrecognized myocardial infarction in subjects aged 45 to 65 years (the ARIC study). The American journal of cardiology. 2002; 90: 927-931.
- Margolis JR, Kannel WS, Feinleib M, Dawber TR, McNamara PM. Clinical features of unrecognized myocardial infarction--silent and symptomatic. Eighteen year follow-up: the Framingham study. The American journal of cardiology. 1973; 32: 1-7.
- Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. The New England journal of medicine. 1984; 311: 1144-1147.
- Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes care. 2003; 26: 1553-1579.
- Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes care. 2017; 40: 136-154.
- Cohn PF. Silent myocardial ischemia in patients with a defective anginal warning system. The American journal of cardiology. 1980; 45: 697-702.
- Zarich S, Waxman S, Freeman RT, Mittleman M, Hegarty P, Nesto RW. Effect of autonomic nervous system dysfunction on the circadian pattern of myocardial ischemia in diabetes mellitus. Journal of the American College of Cardiology. 1994; 24: 956-962.
- Bernardi L, Ricordi L, Lazzari P, Solda P, Calciati A, Ferrari MR, et al. Impaired circadian modulation of sympathovagal activity in diabetes. A possible explanation for altered temporal onset of cardiovascular disease. Circulation. 1992; 86: 1443-1452.
- Marcassa C, Galli M, Baroffio C, Campini R, Giannuzzi P. Ischemic burden in silent and painful myocardial ischemia: a quantitative exercise sestamibi tomographic study. Journal of the American College of Cardiology. 1997; 29: 948-954.
- Hikita H, Etsuda H, Takase B, Satomura K, Kurita A, Nakamura H. Extent of ischemic stimulus and plasma beta-endorphin levels in silent myocardial ischemia. American heart journal. 1998; 135: 813-818.
- Mazzone A, Cusa C, Mazzucchelli I, Vezzoli M, Ottini E, Pacifici R, et al. Increased production of inflammatory cytokines in patients with silent myocardial ischemia. Journal of the American College of Cardiology. 2001; 38: 1895-1901.
- Rosen SD, Paulesu E, Nihoyannopoulos P, Tousoulis D, Frackowiak RS, Frith CD, et al. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Annals of internal medicine. 1996; 124: 939-949.
- Kaski JC, Cosin Sales J, Arroyo Espliguero R. Silent myocardial ischaemia: clinical relevance and treatment. Expert opinion on investigational drugs. 2005; 14: 423-434.
- Bourassa MG, Pepine CJ, Forman SA, Rogers WJ, Dyrda I, Stone PH, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study: effects of coronary angioplasty and coronary artery bypass graft surgery on recurrent angina and ischemia. The ACIP investigators. Journal of the American College of Cardiology. 1995; 26: 606-614.
- Davies RF, Goldberg AD, Forman S, Pepine CJ, Knatterud GL, Geller N, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year followup: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation. 1997; 95: 2037-2043.
- Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. The New England journal of medicine. 2007; 356: 1503-1516.
- Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. The New England journal of medicine. 2020; 382: 1395-1407.
- Ip JE. Wearable Devices for Cardiac Rhythm Diagnosis and Management. Jama. 2019; 321: 337-338.
- Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. The New England journal of medicine. 2019; 381: 1909-1917.
- Dorr M, Nohturfft V, Brasier N, Bosshard E, Djurdjevic A, Gross S, et al. The WATCH AF Trial: SmartWATCHes for Detection of Atrial Fibrillation. JACC Clinical electrophysiology. 2019; 5: 199-208.
- Avila CO. Novel Use of Apple Watch 4 to Obtain 3-Lead Electrocardiogram and Detect Cardiac Ischemia. The Permanente journal. 2019; 23.
- Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S. Single-Lead ECG Recordings Including Einthoven and Wilson Leads by a Smartwatch: A New Era of Patient Directed Early ECG Differential Diagnosis of Cardiac Diseases? Sensors. 2019; 19.
- Vlachakis PK, Tentolouris A, Kanakakis I. Concerns for management of STEMI patients in the COVID-19 era: a paradox phenomenon. Journal of thrombosis and thrombolysis. 2020; 50: 809-813.
- Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes care. 2010; 33: 434-441.
- Morrow DA. Cardiovascular risk prediction in patients with stable and unstable coronary heart disease. Circulation. 2010; 121: 2681-2691.