
Research Article
J Cardiovasc Disord. 2021; 7(1): 1043.
Galectin-3 in Heart Failure with Preserved Ejection Fraction and Persistent Atrial Fibrillation Versus Sinus Rhythm. Correlation with Left Atrial Volume and N-Terminal Pro B-Type Natriuretic Peptide
Bertoni M¹*, Traini AM², Celli A³, Bini C¹, Bracciali A¹, Foretic M¹ and Di Natale ME¹
¹2ndDepartment of Internal Medicine, Santo Stefano Hospital, Prato, Italy
²Department of Cardiology, Santo Stefano Hospital, Prato, Italy
³Department of Biochemical Chemistry, Santo Stefano Hospital, Prato, Italy
*Corresponding author: Bertoni M, 2nd Department of Internal Medicine, Santo Stefano Hospital, Via Ugo Foscolo 5, 59100-Prato, Italy
Received: May 21, 2021; Accepted:July 02, 2021; Published: July 09, 2021
Abstract
Background: Galectin-3 (Gal-3) is considered both a profibrotic biomarker in Heart Failure with preserved Ejection Fraction (HFpEF) and a biomarker of atrial remodeling in Atrial Fibrillation (AF). The Left Atrial Volume Index (LAVI) is an echocardiographic parameter considered an index of left atrial remodeling. Aim of this study was to analyse the relation of Gal-3 levels with both LAVI and N-Terminal Pro B-Type Natriuretic Peptide (NT-proBNP) in patients with HFpEF and Persistent AF (HFpEF-PAF).
Methods: Serum Gal-3 and NT-proBNP, along with LAVI were measured. A comparison of such parameters between 49 patients with HFpEF-PAF and 53 patients with HFpEF and sinus rhythm (HEpEF-SR) was made.
Results: Galectin-3, NT-proBNP and LAVI were significantly higher in patients with HFpEF-PAF compared to HFpEF-SR (23±7 ng/mL vs 19.5±8.5 ng/mL, p=0.027; 3,406.8±2,321.9 pg/mL vs 1,459.6±1,372 pg/mL, p<0.001; 40.1±11mL/m² vs 28.4±7.7 mL/m², p<0.001, respectively). In HFpEF-PAF, Gal- 3 showed a significant correlation with both NT-proBNP (r=0.40, p=0.0038) and LAVI (r=0.28, p=0.044). We found a significant association between patients with higher levels of Gal-3 >17.8 ng/mL and HFpEF-PAF (p=0.002). Finally, a multivariate logistic regression analysis adjusted for age, sex and traditional clinical AF risk factors showed that Gal-3 >17,8 ng/mL (OR 3.862, 95% CI 1.416 to 10.532, p=0.008) was an independent predictor of PAF.
Conclusions: In patients with HFpEF-PAF Gal-3 was higher and related with both NT-proBNP and LAVI. The latter correlation may be relevant because LAVI is considered an index of left atrial remodeling. Moreover, higher levels of Gal-3>17,8 ng/mL were an independent predictor of PAF.
Keywords: Galectin-3; Left atrial volume index; Heart Failure with preserved Ejection Fraction; Persistent Atrial Fibrillation
Introduction
Atrial Fibrillation (AF) is a key co-morbidity that is not only highly prevalent, but is associated with worse outcomes in Heart Failure with Preserved Ejection Fraction (HFpEF) [1,2]. In recent years, a growing number of studies have focused on the role of Galectin-3 (Gal-3) as a biomarker of both HFpEF and AF. The level of Gal-3 purportedly reflects an ongoing cardiac fibrotic process and has been associated with ventricular remodeling, which is instrumental in the development of HFpEF syndrome [3]. In this regard, it has been suggested that patients with HFpEF have a much stronger correlation with Gal-3 than those with reduced ejection fraction [4]. Moreover, in the sub-study of PARAMOUNT trial on the role of profibrotic biomarkers in HFpEF, Gal-3 levels correlated with severity of disease as indicated by N-Terminal Pro B-Type Natriuretic Peptide (NTproBNP) and Left Atrial Volume (LAV) [5]. Finally, a recent study showed that increasing levels of Gal-3 possibly reflect the progressive course of HFpEF, as classified by the echocardiographic grades of diastolic dysfunction [3].
The profibrotic role of Gal-3 and its implications in the mechanisms of left atrial remodeling and maintenance of AF have recently been studied. With reference to Persistent AF (PAF), two recent studies recently suggested a role of Gal-3 in the maintenance of this arrhythmia. The first one evidenced that Gal-3 levels were significantly higher in PAF patients than in controls with Sinus Rhythm (SR) and were significantly correlated with LAV Index (LAVI), an accepted echocardiographic marker of left atrial remodelling [6]. In the second study, performed in patients with AF and preserved left ventricular function, Gal-3 levels were significantly greater than in controls with SR, significantly higher in patients with PAF than those with paroxysmal AF and, finally, significantly correlated with LAVI [7].
This study primarily aimed at searching a possible increase of Gal-3 levels in patients with HFpEF and PAF (HFpEF-PAF) compared to patients with HFpEF and SR (HFpEF-SR). Secondly, the possible correlation between Gal-3 levels and indices of severity of HFpEF such as LAVI and NT-proBNP was sought in both the aforementioned groups of patients.
Methods
Study design and population characteristics
With regard to the sample size, we considered three main aspects: a) in Europe and the United States the prevalence of heart failure (HF) in over 65-year-olds is about 4% in males and about 3% in females [8]; b) according to the 2014 Italian Institute of Statistics data, the over-65 inhabitants of the province of Prato were about 23,340 males and 30,682 females; c) the Santo Stefano Hospital in Prato is the only hospital of the province. It is therefore conceivable that the expected prevalence values of HF in the Prato population over the age of 65 are about 933 cases in males and about 920 cases in females. Finally, data from a large epidemiological study have shown that the prevalence of HFpEF is about 50% of all cases of HF [9]. Therefore, we planned to include approximately 10% of the target population in the study, i.e. a sample of approximately 100 patients, representative of the reference population and stratified by age and sex.
This study was a mono-centric study conducted at the 2nd Department of Internal Medicine of the Santo Stefano Hospital, Prato, Italy. The research adhered to the principles outlined in the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Area Vasta Toscana Centro to which the Santo Stefano Hospital in Prato belongs. Freely given, written informed consent to participate in the study was obtained from all patients.
The present study incorporated a population subset derived from a patient cohort who attended the outpatient clinic of 2nd Department of Internal Medicine of the Santo Stefano Hospital in Prato, between November 2018 and September 2020. As this was a non-interventional, observational study, diagnostic procedures and treatment plans were not modified.
A total of 102 patients diagnosed with HFpEF were included consecutively. Forty-nine patients diagnosed with non-valvular (mitral and aortic valve) PAF (AF duration longer than one month) were recruited into the HFpEF-PAF group. Fifty-three age-matched patients with HFpEF-SR were recruited into the control group. Both HFpEF and AF diagnosis were made according to the 2016 guidelines of the European Society of Cardiology (ESC) [10,11]. At enrollment, the relevant demographic and clinical data of each patient, including age, gender, Body Mass Index (BMI), Body Surface Area (BSA), heart rate, New York Heart Association (NYHA) functional class, risk factors such as smoke, presence of co-morbidities (diabetes mellitus, hypertension, coronary artery disease, myocardial infarction, chronic obstructive pulmonary disease, kidney dysfunction with estimated Glomerular Filtration Rate (eGFR) >30mL/min/1.73m² (calculated by using the Cockroft-Gault formula), intake of Angiotensin- Converting-Enzyme Inhibitors (ACE-I) or Angiotensin Receptor Blockers (ARB), beta blockers (BB), diuretics, were recorded for all patientsand compiled in a database. Age under 18 years, recent onset acute coronary syndrome (< three months), pulmonary embolism, complex ventricular arrhythmias, idiopathic pulmonary fibrosis, chronic pancreatitis, liver cirrhosis, renal failure with eGFR <30 mL/ min/1.73m² (stages four and five of the Kidney Disease Improving Global Outcomes classification [12]), malignancy any prior blood transfusions, carotid artery disease, were exclusion criteria in this study. Blood samples, collected from all patients, were preserved and processed throughout the study.
Echocardiographic assessment
Only one cardiologist, blinded to patient clinical history, performed and interpreted al echocardiograms, and verified left ventricular volumetric analysis. The Left Ventricular Ejection Fraction (LVEF) was calculated by using the modified biplane Simpson’s method [13]. Measurements were obtained as the mean value from the apical 4- and 2-chamber views. The LAV was calculated by using the biplane method of discs (modified Simpson’s rule) by using the apical 4- and 2-chamber views at end diastole of the atria. Measurements were obtained as the mean value from the apical 4- and 2-chamber views. The LAVI was then calculated as LAV divided by BSA [13].
Laboratory analysis
Blood samples were collected from all patients (at rest) at a single assessment time point upon study inclusion by venepuncture with serum monovettes and centrifuged at 2,500 g at 20°C for ten minutes. The aliquoted samples were cooled down in liquid nitrogen before being stored at -80°C for further analysis. After thawing, the samples were gently mixed by inverting and centrifuged at 2,500 g for ten minutes at 20°C.
Determination of Gal-3 level was prospectively completed by using the VIDAS Galectin-3 kit (BioMérieux, Marcy-l’Etoile, France) which is a quantitative, one-step immunoassay sandwich assay with fluorescence detection. The kit measuring range is 3.3-100 ng/mL. Briefly the system measures Gal-3 in human serum or plasma (200 μL) by using the Enzyme-Linked Fluorescent Assay (ELFA) technique in 20 minutes. All stages of the assay are performed automatically by the instrument, calculating the concentration of Gal-3 relative to a stored calibration curve. This test has already been validated in HF patients [14].
For Gal-3, there are Food and Drug Administration (FDA) approved partition values that can be used in risk assessment analyses to predict morbid and mortal outcomes. When Gal-3 is >17.8 ng/mL, there is an increase in risk [15]. Serum Gal-3 levels were also measured in a control group of 26 subjects of age, sex, and race distribution similar to this study population, without histories of any disease, who underwent routine screening visits in the outpatient clinics.
The serum level of NT-proBNP, used as a reference biomarker, was measured using an NT-proBNP II assay on a VIDAS analyzer (BioMérieux) using the ELFA technique. The limit of detection for this dosage was 10 pg/mL. Serum creatinine concentrations were measured using the Creatinine Jaffe Gen. 2 test on an AU5800 analyzer (Beckman Coulter). The following blood tests were also performed: cystatin-C (Quantikine Human Cystatin C Immunoassay, R&D Systems, Minneapolis, MN, USA), blood count (including neutrophil/lymphocyte ratio-N/LR-), platelet count, high sensitive C-reactive protein (hs-CRP), thyroid stimulating hormone, low density lipoprotein-cholesterol, all of them by the common commercial kits used in the analysis laboratory of the Santo Stefano Hospital in Prato.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (interquartile range) when appropriate. Categorical variables are expressed as percentages. To compare parametric continuous variables the Student’s t-test was used; to compare nonparametric continuous variables the Mann-Whitney U-test was used. To compare categorical variables the chi-square-test was used. The Pearson and Spearman correlation coefficient were used to respectively determine parametric and nonparametric measure of statistical dependence between two variables. Multivariate logistic regression analysis adjusted for age, sex and traditional clinical AF risk factors was used to examined the relation of AF with high levels of Gal-3 (>17.8 ng/mL). A 2-tailed p-value of less than 0.05 was considered to indicate statistical significance. The statistical analyses were performed using software (SPSS 25.0.0.0, SPSS Inc, Chicago, IL).
Results
Baseline characteristics
As we can see in the participant flow diagram of Figure 1, fortynine patients with HFpEF-PAF and 53 patients with HFpEF-SR were included in the study. Of the 60 potentially enrolled HFpEFPAF patients, five were excluded because of renal failure with eGFR <30 mL/min/1.73m², three because of chronic pancreatitis and one because of liver cirrhosis. Two additional patients refused to undergo laboratory tests. Of the 59 potentially enrolled HFpEFSR patients, four were excluded because of renal failure with eGFR <30 mL/min/1.73m² and two additional patients refused to undergo laboratory tests.
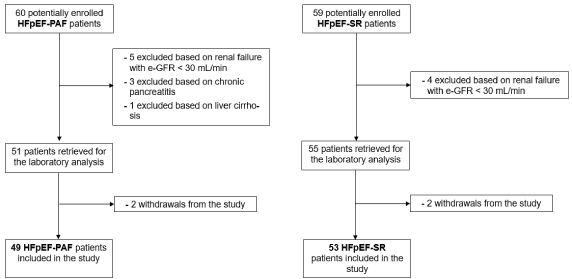
Figure 1: Participant flow diagram. Disposition of patients through the study. HFpEF-PAF: Heart Failure with Preserved Ejection Fraction and Persistent Atrial Fibrillation; HFpEF-SR: Heart Failure with Preserved Ejection Fraction and Sinus Rhythm.
Baseline demographic, clinical and laboratory characteristics of the study population are listed in Table 1. Compared with the HFpEF-SR group, in the HFpEF-AF the mean age was slightly higher (82.5 ± 7 years vs 79.1 ± 8 years, p=0.022) and there was both a prevalence of female patients (69.4 % vs 45.3 %, p=0.024), and a slightly higher heart rate (76.6 ± 17 beats/min vs 70.5 ± 9.3 beats/min, p=0.004). There were no significant differences between the groups in terms of baseline clinical characteristics, including BMI, BSA, NYHA functional class, comorbidity burden, and treatment. Likewise, there were no significant differences in the conventional laboratory findings, particularly in the determination of two inflammatory markers, namely N/LR and hs-CRP.
HFpEF-PAF
(n = 49)HFpEF-SR
(n = 53)p value
Age, yrs
82.5 ± 7
79.1 ± 8
0.022*
Female, %
34 (69.4)
24 (45.3)
0.024^
Body mass index, kg/m2
27.2 ± 5.4
28.3 ± 4.7
0.278*
Body surface area, m2
1.8 ± 0.2
1.8 ± 0.2
0.082*
Heart rate, beats/min
76.6 ± 17
70.5 ± 9.3
0.004*
NYHA functional class II, III
31/18 (63.3/36.7)
42/11 (79.2/20.8)
0.087 ^
Risk factors
Smoke
8 (16.3)
10 (18.9)
0.736 ^
Comorbidities, %
History of diabetes mellitus
History of hypertension
History of CAD
History of myocardial infarction
History of COPD
History of kidney dysfunction
(stages 1,2,3 of KDIGO Classification)
15 (30.6)
36 (73.5)
6 (12.2)
3 (6.1)
4 (8.2)
3/10/36
(6.1/20.4/73.5)
21 (39.6)
44 (83)
7 (13.2)
4 (7.5)
6 (11.3)
4/18/31 (7.5/34/58.5)0.341 ^
0.241 ^
0.884 ^
0.776 ^
0.592 ^
0.265 ^Medications at enrollment, %
ACEI/ARB
Beta-blockers
Diuretics
33 (67.3)
45 (91.8)
48 (97,9)
41 (77.4)
40 (75.5)
49 (92.4)
0.257 ^
0.051 ^
0.198 ^Laboratory values
Creatinine, mg/dL
eGFR, mL/min/1.73 m2
Cystatin-C, mg/L
Hemoglobin, g/dL
NLR
hs – CRP, mg/dL
LDL-cholesterol, mg/dL
TSH, mUI/L
1.1 ± 0.3
52.8 ± 19.1
2 ± 0.7
12.5 ± 1.9
4.2 ± 2.9
2.3 (0.1 – 12.6)
110.5 ± 34.4
2.1 (0.1-7.8)
1.1 ± 0.7
58.3 ± 20.7
1.7 ± 0.6
12.5 ± 2
3.8 ± 2.6
2,7 (0.1 – 10.7)
120.3 ± 37
1.8 (0.1-6.4)
0.207 *
0.198 *
0.103 *
0.903 *
0.435 *
0.681 #
0.184 *
0.865 #Values are mean ± standard deviation (SD), n (%): or median (interquartile range). Student’s t-test (*), chi-square-test (^), and Mann-Whitney U-test (#) and are used.
Abbreviations: HFpEF-PAF: Heart Failure with Preserved Ejection Fraction and Persistent Atrial Fibrillation; HFpEF-SR: Heart Failure with Preserved Ejection Fraction and Sinus Rhythm; CAD: Coronary Arterial Disease; COPD: Chronic obstructive pulmonary disease; KDIGO: Kidney Diseases Improving Global Outcome; ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blocker; NYHA: New York Heart Association; eGFR: estimated Glomerular Filtration Rate; NLR: Neutrophil/Lymphocyte Ratio; hs-CRP: high sensitive C Reactive Protein; LDL-cholesterol: Low Density Lipoprotein-cholesterol; TSH: Thyroid Stimulating Hormone.
Table 1: Baseline demographic, clinical, and laboratory characteristics of the study population (n=102).
Biomarker dosage
Median values for serum Gal-3 levels of the control group were firstly compared qualitatively with referent control values, proving to be completely similar (Table 2). Median Inter Quartile Range (IQR) referent control data for serum Gal-3 were taken from a previously published study in which 1092 subjects of age, sex, and race distribution similar to the control group population were examined [16]. Secondly, serum Gal-3 mean values ± standard deviation of the control group (13 ± 4.7 ng/mL) were compared both with those of the HFpEF-PAF group (23 ± 7 ng/mL), and those of the HFpEF-SR group (19.5 ± 8.5 ng/mL), resulting significantly lower than each of them (p<0.001). Finally, serum Gal-3 levels of the HFpEF-PAF group were significantly higher compared with those of the HFpEF-SR group (p=0.027) (Table 2).
HFpEF-PAF group
HFpEF-SR group
Control group^
Referent controls
Mean ± SD
Median (IQR)
Mean ± SD
Median (IQR)
Mean ± SD
Median (IQR)
Median (IQR)*
Galectin-3 (ng/mL)
23±7
21.8 (13.7-43.3)
19.5±8.5
17.2 (10-49.6)
13±4.7
13.1 (3.5-17.1)
12 (9-15)
p = 0.027
p < 0.001
p < 0.001
NTproBNP (pg/mL)
3,406.8±2,321.9
2750 (663-11,420)
1,459.6±1,372
850 (125-5247)
-
-
-
p < 0.001
Abbreviations: HFpEF-PAF: Heart Failure with Reserved Ejection Fraction and Persistent Atrial Fibrillation; HFpEF-SR: Heart Failure with Preserved Ejection Fraction and Sinus Rhythm; ^control group of 26 subjects of age, sex, and race distribution similar to this study population, without histories of any disease. SD: Standard Deviation; IQR: Interquartile Range; *referent control median (IQR) values taken from ref. 16.
Table 2: Biomarker data.
Following the FDA approved partition values for Gal-3 in which a level >17.8 ng/mL is related to an increased risk for HF hospitalization and death for cardiovascular diseases, a chi-square test showed that there was a significant association between patients with Gal-3 >17.8 ng/mL and patients with HFpEF-PAF (p=0.002) (Figure 2). Serum NT-proBNP levels of the HFpEF-PAF group were significantly higher with respect to those of the HFpEF-SR group (3,406.8 ± 2,321.9 pg/ mL vs 1,459.6 ± 1,372 pg/mL, p<0.001) (Table 2).
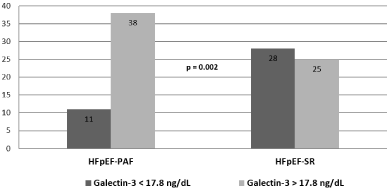
Figure 2: Significant association between the proportion of patients with
serum levels of Gal-3 >17.8 ng/mL and patients with HFpEF-PAF (p=0.002).
HFpEF-PAF: Heart Failure with Preserved Ejection Fraction and Persistent
Atrial Fibrillation; HFpEF-SR: Heart Failure with Preserved Ejection Fraction
and Sinus Rhythm.
Echocardiographic assessment
There were no significant differences between the groups regarding LVEF, while LAVI was significantly higher in patients with HFpEF-PAF compared to patients with HFpEF-SR (41.1 ± 11 mL/m² vs 28.4 ± 7.7, p<0.001) (Table 3).
Echocardiographic parameters
HFpEF-PAF
(n = 49)HFpEF-SR
(n = 53)p value
LVEF, %
53.5 ± 4.5
55.1 ± 4.7
0.07*
LAVI, mL/m2
41.1 ± 11
28.4 ± 7.7
<0.001*
Values are mean ± SD. Student’s t-test (*) is used.
Abbreviations: HFpEF-PAF: Heart Failure with Preserved Ejection Fraction and Persistent Atrial Fibrillation; HFpEF-SR: Heart Failure with Preserved Ejection Fraction and Sinus Rhythm; LVEF: Left Ventricular Ejection Fraction; LAVI: Left Atrial Volume Index.
Table 3: Echocardiographic assessment of the study population (n = 102).
Correlation analysis between serum Galectin-3 levels and laboratory/echocardiographic data
In the HFpEF-PAF group serum Gal-3 levels significantly and directly correlated with both serum NT-proBNP (r=0.40, p=0.0038) and LAVI (r=0.28, p=0.044) (Figures 3 and 4). No correlation was observed with other laboratory findings. In the HFpEF-SR group there were also significant correlations both between serum Gal-3 levels and serum NT-proBNP levels (r=0.50, p<0.001, and between serum Gal-3 levels and LAVI (r=0.29, p=0.037).
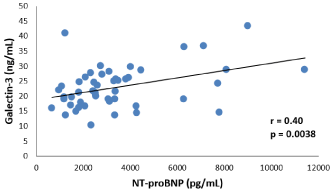
Figure 3: Correlation analysis between serum Gal-3 levels and serum NTproBNP
levels in patients with HFpEF-PAF.
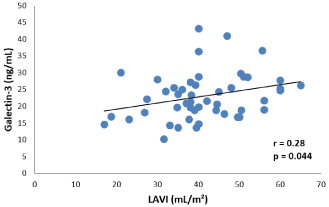
Figure 4: Correlation analysis between serum Gal-3 levels and LAVI in
patients with HFpEF-PAF.
Association of PAF with Gal-3
We performed a multivariate logistic regression analysis on the overall study cohort, adjusted for age, sex, clinical PAF risk factors (diabetes mellitus, high blood pressure, smoking status and history of myocardial infarction), to examine the relation of AF and high levels of Gal-3. These covariates were selected based on a prior study which examined a risk prediction model for AF using five epidemiological cohorts [17]. These association was significant after adjustment for these covariates (Table 4).
OR
CI (95%)
p value
Age, sex, PAF clinical risk factors and drug assumption of ACE-I, ARB and BB
3.862
1.416-10.532
0.008
Abbreviations: OR: Odds Ratio; CI: Confidence Interval; PAF: Persistent Atrial Fibrillation; clinical risk factors: diabetes mellitus, high blood pressure, smoking status and history of myocardial infarction.
Table 4: Laboratory values at hospital admission.
Discussion
In this observational study, we demonstrated that both serum Gal-3 levels, and serum NT-proBNP levels and, finally, LAVI were significantly elevated in patients with HFpEF-PAF compared with patients with HFpEF-SR. Moreover, taking into account the well established decisional Gal-3 cut-offs identifying HF higher risk categories, we found a significant association between the group of patients with HFpEF-PAF (but not the one with HFpEF-SR) and the Gal-3–associated HF higher risk categories.
We also demonstrated a significant correlation between Gal- 3 levels and indices of severity of HFpEF such as NT-proBNP and LAVI in patients with HFpEF-PAF. To the best of our knowledge, Gal-3 levels have so far not been evaluated in patients with HFpEFPAF, especially with reference to NT-proBNP and LAVI. Finally, multivariate regression analysis, adjusted for age, sex and traditional clinical AF risk factors, demonstrated that partition values of Gal-3 >17.8 ng/mL, used in risk assessment analyses to predict HF morbid and mortal outcomes, were an independent predictor of PAF in HFpEF patients.
Galectin-3 is a novel profibrotic molecule. In experimental studies, Gal-3 expression has been shown to induce fibroblast proliferation and type I fibrillar collagen production [18]. Two previous large cohort studies showed that higher Gal-3 levels were associated with increased risk of incident AF in age- and gender-adjusted analyses [19,20]. The results of our study showed that Gal-3 levels were elevated in patients with HFpEF-PAF according to previous studies performed in patients with AF and preserved LVEF [7]. This could imply that Gal-3 might be a biomarker of the prominent structural remodeling of left atrium in patients with PAF. However, this finding should be evaluated with caution, because patients with paroxysmal AF may also have underlying left atrium structural changes [21,22].
In our study we demonstrated a statistically significant correlation between Gal-3 and LAVI in patients with HFpEF-PAF. Though LAVI is considered a marker of left atrial remodelling, we can presume that in HFpEF-PAF patients the mechanisms underlying the fibrotic and remodeling processes are more intensified with respect to HFpEF-SR patients.
Moreover, it is worth stressing that, with reference to HFpEF-SR patients, in those with HFpEF-PAF significantly higher levels of both the biomarker of myocardial fibrosis Gal-3, and the biomarker of ventricular parietal stretching NT-proBNP (synthesized and released in response to pressure and volume overload) have been identified. On the whole, such biomarkers should provide the guidance of echocardiographic measurements such as LAVI in HFpEF-PAF patients compared with HFpEF-SR patients.
A recent statement of the Heart Failure Association of ESC has been published, in which both a new diagnostic algorithm and a score for HFpEF have been proposed [23]. Th e reassessment of our cases on the basis of the criteria proposed by the aforementioned declaration, allowed us to confirm the diagnosis of HFpEF in all cases with a score ≥ of five points. Moreover, in patients with HFpEF the same paper suggests distinct diagnostic thresholds for natriuretic peptides and LAVI in SR vs AF, based on existing literature and consensus [18]. In this regard, our findings concerning the assessment of Gal-3 could furtherly help a diagnostic distinction between SR vs AF patients with HFpEF.
Limitations
There were some limitations to the study. First, the lack of histologic correlation, because we did not perform a histopathological examination of atrial tissue. Second, circulating Gal-3 concentrations are not specific to the cardiovascular system and could potentially reflect other fibrotic conditions. About this, we made any effort to rule out patients affected with such conditions. Finally, this was an observational study performed on limited number of patients and revealed only an association rather than causal relation.
Conclusion
Based on our results, we suggest that the assessment of Gal-3 could support a diagnostic distinction between in SR vs AF patients with HFpEF. Moreover, in patients with HFpEF-PAF our data may point us towards a role of Gal-3 in initiating the atrial remodeling process.
Acknowledgement
We wish to thank for numerous individuals assisting in preparation of this article. The authors are fully responsible for editorial decisions and suggestion during the stages of article submission and publishment.
Funding Sources
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Oluleye OW, Rector TS, Win S, McMurray JJV, Zile MR, Komajda M, et al. History of atrial fibrillation as a risk factor in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014; 7: 960-966.
- Patel RB, Vaduganathan M, Shah SJ, Butler J. Atrial fibrillation in heart failure with preserved ejection fraction: insights into mechanisms and therapeutics. Pharmacol Ther. 2017; 176: 32-39.
- Ansari U, Behnes M, Hoffmann J, Natale M, Fastner C, El-Battrawy I, et al. Galectin-3 reflects the echocardiographic grades of left ventricular diastolic dysfunction. Ann Lab Med 2018; 38: 306-315.
- de Boer RA, Lok DJ, Jaarsma T, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011; 43: 60-68.
- Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, et al. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: data from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction study. Circ Heart Fail 2016; 9:e002551.
- Sonmez O, Ertem FU, Vatankulu MA, Erdogan E, Tasal A, Kucukbuzcu S, et al. Novel fibro-inflammation markers in assessing left atrial remodeling in non-valvular atrial fibrillation. Med Sci Monit. 2014; 20: 463-470.
- Gurses KM, Yalcin MU, Kocyigit D, Canpinar H, Evranos B, Yorgun H, et al. Effects of persistent atrial fibrillation on serum galectin-3 levels. Am J Cardiol. 2015; 115: 647-651.
- Alla F, Zannad F, Filippatos G. Epidemiology of acute failure syndrome. Heart Fail Rev. 2007; 12: 91-95.
- Owan TE, Hodge DO, Herges, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355: 251-259.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891-975.
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18: 1609-1678.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 Clinical practice guideline update for the dagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney International Supplements. 2017; 7: 1-59.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277-314.
- Meijers WC, van der Velde AR, de Boer RA. The ARCHITECT galectin-3 assay: comparison with other automated and manual assays for the measurement of circulating galectin-3 levels in heart failure. Expert Rev Mol Diagn. 2014; 14: 257-266.
- Van der Velde AR, Gullestad L, Ueland T, Aukrust P, Guo Y, Adourian A, et al. Prognostic value of changes in Galectin-3 levels over time in patients with heart failure. Data From CORONA and COACH. Circ Heart Fail. 2013; 6: 219-226.
- Christenson RH, Duh SH, Wu AH, Smith A, Abel G, deFilippi CR, et al. Multi-center determination of galectin-3 assay performance characteristics: anatomy of a novel assay for use in heart failure. Clin Biochem. 2010; 43: 683-690.
- Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2: e000102.
- Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004; 110: 3121-3128.
- Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012; 60: 1249-1256.
- Fashanu OE, Norby FL, Aguilar D, Ballantyne CM, Hoogeveen RC, Chen LY, et al. Galectin-3 and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2017; 192: 19-25.
- Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial bio.psies in patients with lone atrial fibrillation. Circulation. 1997; 96: 1180-1184.
- Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, et al. Longterm effects of catheter ablation for lone atrial fibrillation: progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm. 2012; 9: 473-480.
- Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019; 40: 3297-3317.
Citation: Bertoni M, Traini AM, Celli A, Bini C, Bracciali A, Foretic M, et al. Galectin-3 in Heart Failure with Preserved Ejection Fraction and Persistent Atrial Fibrillation Versus Sinus Rhythm. Correlation with Left Atrial Volume and N-Terminal Pro B-Type Natriuretic Peptide. J Cardiovasc Disord. 2021; 7(1): 1043.