Research Article
Austin J Cerebrovasc Dis & Stroke. 2014;1(1): 1002.
An Open Label, One Arm, Dose Escalation Study to Evaluate The Safety of Extremely Low Frequency Magnetic Fields in Acute Ischemic Stroke
Capone F1,2*, Corbetto M1, Barbato C1, Pellegrino G1, Di Pino G1,2, Assenza G1, Setti S3, Cadossi R3 and Di Lazzaro V1,2
1Department of Neurology, Campus Bio-Medico University of Rome, Italy
2Department of Neurology Fondazione Alberto Sordi - Research Institute for Ageing, Italy
3Department of Biomedical Sciences, IGEA Biophysics Laboratory, Italy
*Corresponding author: Capone F, Department of Neurology, Campus Bio-Medico University of Rome, Via Alvaro del Portillo 200, 00128 Rome, Italy.
Received: May 25, 2014; Accepted: June 16, 2014; Published: June 18, 2014
Abstract
There is great interest on the development of novel therapies for acute ischemic stroke, because, to date, thrombolysis is the only approved treatment. Recent evidence suggests that Extremely Low Frequency Magnetic Fields (ELF-MF) could represent an alternative approach for acute ischemic stroke therapy, because of their effects on the main mechanisms of brain ischemic damage and regeneration. Main purpose of this open label, one arm, dose escalation study is the validation of ELF-MF stimulation as non-invasive and safe tool to promote recovery in acute ischemic stroke patients. Nine patients will be treated daily for 5 consecutive days, starting within 48 hours from the onset of stroke. Three dose cohorts of three patients each, will be stimulated with pulsed ELF-MF (75 Hz, 1,8 mT) at duration-increasing daily exposure (45, 120, 240 min). The primary outcome (safety endpoint) will be evaluated by the incidence of adverse events and mortality throughout the stimulation period and after 1-year follow-up. Change from baseline of clinical and radiological scores will be taken as secondary outcomes. This study aims at establishing the safety and tolerability of the ELF-MF stimulation in acute ischemic stroke.
Keywords: Stroke; Brain Ischemia; Extremely low frequency Magnetic fields; Pulsed magnetic fields; ELF; Neuroprotection
Abbreviations
ELF-MF: Extremely Low Frequency Magnetic Fields; MRI: Magnetic Resonance Imaging; NIHSS: National Institutes of Health Stroke Scale; AEs: Adverse Events; SAEs: Severe AEs; CTCAE: Common Terminology Criteria for Adverse Events; mRS: Modified Rankin Scale; BI: Barthel Index; FLAIR: Fluid-Attenuated Inversion Recovery; DWI: Diffusion-Weighted Imaging; ADC: Apparent Diffusion Coefficient; ILV: Ischemic Lesion Volume; TMG: Trial Management Group; PI: Principal Investigator
Introduction
Thrombolysis is the only approved treatment for acute ischemic stroke and so, there is great interest in the development of alternative therapies. Despise, for the last two decades, several neuroprotective drugs have been investigated with clinical trials, none of them proved to be efficacious. Recent evidence suggests that the application of Extremely Low Frequency Magnetic Fields (ELF-MF) could be an alternative approach, because of their effects on the main mechanisms underlying brain ischemic damage and regeneration. Indeed, in-vitro studies have shown that ELF-MF modify genes expression, promote neurite outgrowth [1], reduce apoptosis [2], facilitate neuronal differentiation of stem cells [3] and increase BDNF production [4]. In particular, ELF-MF delivered in pulsed mode can selectively modulate glutamate [5] and adenosine [6] receptors. The neuroprotective potential of pulsed ELF-MF has been also confirmed in animal models of brain ischemia. Grant et al. have evaluated the effects of pulsed ELF-MF on cerebral damage in a rabbit model of transient focal ischemia. They found that pulsed ELF-MF exposure, immediately after the onset of ischemia, attenuated cortical edema on Magnetic Resonance Imaging (MRI) and reduced neuronal damage on histological examination [7]. These results have been recently confirmed in mice by Pena-Philippides et al. who evaluated the effect of pulsed ELF-MF on ischemic infarct size and post stroke inflammation [8]. They found that pulsed ELF-MF reduced the infarct size and changed the profile of inflammatory cytokines, thus unveiling anti-inflammatory and anti-apoptotic effects. Recent evidence suggests that ELF-MF might influence human brain function. These findings are supported by the results of the neurophysiological studies that revealed measurable changes in brain electrical activity following ELF-MF exposure [9]. In particular, Capone et al. demonstrated that pulsed ELF-MF can influence cortical excitability and do not produce side effects in healthy volunteers [10]. Purpose of this study is the validation of pulsed ELF-MF stimulation as non-invasive, safe and effective tool to promote recovery in acute ischemic stroke patients.
Materials and Methods
Design
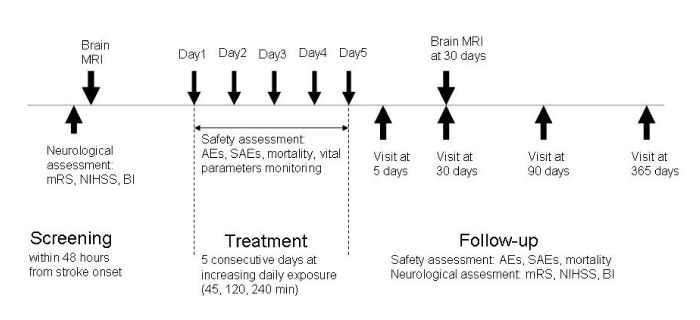
This is an open label, one arm, and dose escalation exploratory study aiming to evaluate the safety of pulsed ELF-MF stimulation in acute ischemic stroke. Subject’s part of the control group will be selected from patients admitted to our clinic for acute ischemic stroke during the last year. The trial consists of a 5-days intervention treatment phase and of a 12-months follow-up phase (Figure1).
Figure 1: Study design. mRS: modified Rankin Scale. NIHSS: National Institutes of Health Stroke Scale. BI: Barthel Index. AEs: adverse events. SAEs: severe adverse events.
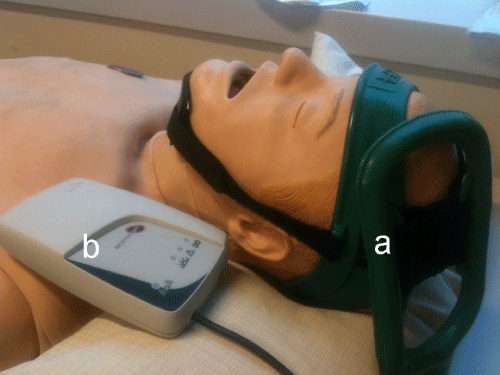
Figure 2: The pulsed ELF-MF stimulation system. (a) Rectangular, flexible coil positioned on the ischemic hemisphere. (b) Pulse generator.
Patient population -inclusion and exclusion criteria
Nine patients will be recruited from in-patient unit of the Neurology Department of Policlinic Campus Bio-Medico of Rome. Inclusion criteria: age>18; first onset, mono-hemispheric ischemic stroke; onset of symptoms within 48 hours; National Institutes of Health Stroke Scale (NIHSS) score >4; patient is alert, medically stable and able to follow simple verbal commands; signed written informed consent.
Exclusion Criteria: acute intracranial hemorrhage; previous ischemic or hemorrhagic stroke; history of seizure; contraindications to transcranial magnetic stimulation (such as implanted metallic parts of implanted electronic devices or other metal in body); life expectancy <3 months; other serious illness or complex disease that may confound treatment assessment; women known to be pregnant, lactating or having a positive or indeterminate pregnancy test; simultaneous participation in another study.
Randomization
This is an open label, non randomized, exploratory study.
Intervention
Within 48 hours from the onset of the stroke, the enrolled patients will undergo clinical and neuroradiological evaluation. Subsequently, while hospitalized, the patients will undergo daily pulsed ELF-MF treatment for 5 consecutive days. The first 3 patients enrolled will be treated for 45 min/day. In the absence of observed Adverse Events (AEs), the following 3 patients will be treated for 120 min/day and, if AEs are still not observed, the last 3 patients for 240 min daily.
The system that will be adopted to deliver pulsed ELF-MF, presented in Capone et al [10]. Exploits a custom-made rectangular, flexible coil (IGEA, Carpi, Italy) kept in place by a Velcro strap, upon the ischemic hemisphere. The coil will be positioned to orient the positive pole of the magnetic field toward the top of skull. The magnetic pulse generator (B-01; IGEA, Carpi, Italy) will supply the coil with a single-pulsed signal at 75±2 Hz, with pulse duration of 1.3 ms. the peak intensity of the magnetic field was 1.8±0.2 mT, while the amplitude of the induced electric field was 3±1 mV (Figure2).
Pulsed ELF-MF stimulation will be delivered by physicians, owning proven expertise in non-invasive brain stimulation. All participants will receive the standard of care for acute ischemic stroke, according to current guidelines.
Primary outcome
The main goal of this exploratory study is to evaluate the safety of pulsed ELF-MF stimulation. The primary outcome will be evaluated by the incidence of AEs, Severe AEs (SAEs) and mortality throughout the stimulation period and along 1-year follow-up. An AE will be defined as any untoward medical occurrence in a subject, independently by the possibility to establish a causal relationship. The Common Terminology Criteria for Adverse Events (CTCAEv4.0) will be used to grade adverse events. During the pulsed ELF-MF stimulation, patients will be continuously monitored by a multimodal monitor that simultaneously assesses and displays ECG and the relevant vital parameters (respiratory rate, heart rate, blood pressure, pulse oximetry). The tolerability of the stimulation will also be measured by the number of subjects requesting to stop treatment sessions.
Moreover, questionnaires on AEs will be administered daily during the whole hospitalization and, after the discharge, at each outward control.
Secondary outcomes
Although this study has been designed especially to show the safety of the treatment, likely, preliminary evidence on efficacy of pulsed ELF-MF in acute ischemic stroke may be document as well. To this purpose, subject’s part of a control group will be selected from the patients admitted to our clinic for acute ischemic stroke during the last year. Changes from baseline of the clinical and radiological scores, obtained at different time points, will be exploited as secondary outcomes informative of the ELF-MF treatment efficacy
Clinical outcomes
Clinical evaluations will be performed by physicians with specific competence in the management of acute stroke patients, by means of international well validated scales. Clinical outcome measures are:
Changes from baseline in NIHSS score, Modified Rankin Scale (mRS) score and in the Barthel Index (BI), assessed immediately, 30, 90 and 365 days after pulsed ELF-MF treatment.
Differences between the three treated groups in the same clinical outcome measures (NIHSS, mRS, BI).
Differences between treated groups and not-treated stroke patients in the same clinical outcome measures (NIHSS, mRS, BI).
Neuroradiological outcomes
Patients will be examined by MRI using an Achieva 1.5 T scanner (Philips Medical Systems, Best, and the Netherlands) and an 8 channel head phase-array coil with parallel imaging capabilities (SENSE). The following brain MR sequences will be collected: axial plane T1w-SE, Fluid-Attenuated Inversion Recovery (FLAIR), and Diffusion-Weighted Imaging (DWI) with Apparent Diffusion Coefficient (ADC).
The neuroradiologist, blind to all clinical information, will assess the MRI findings and measure the ischemic lesion volume (ILV) through the MRIcro software. The software reconstructs the total ILV from the thickness of single areas of abnormal signal intensity, manually traced by the experimenter on each DWI and FLAIR slice.
Neuroradiological outcome measures are
Change (Δ) in ILV determined by MRI. ΔILV is defined as ILV measured by FLAIR sequence at 30 days after pulsed ELF-MF treatment minus the initial ILV measured by DWI trace sequence before pulsed ELF-MF treatment.
Differences between the three treatment groups in the ΔILV.
Sample size
The total sample size for this study amounts to 9 patients, divided in three cohorts of 3 subjects each. Because of the exploratory nature of this pilot study, the planned sample size is not statistically derived.
Data Monitoring Body
Participant files will be stored in a secure and accessible place and manner and will be maintained in storage for 3 years after completion of the study. Because this is an early phase trial, a formal Data Monitoring Committee will not be convened. This function will be provided by the Trial Management Group (TMG). An interim-analysis is performed on the primary endpoint when each cohort of three patients has completed the first 1-month follow-up. The interim-analysis will be responsibility of the Principal Investigator (PI). The PI will report to the TMG that will discuss the results of the interim-analysis. TMG decides on the continuation of the trial and will report to the local Ethics Committee.
Statistical analyses
This is an open-label study in which a control group will be selected from patients admitted to our clinic for acute ischemic stroke during the last year. Nine control subjects matched for demographic and clinical features (age, sex, NIHSS score) will be used for comparisons.
The primary outcome will be evaluated by the incidence of AEs, SAEs and mortality throughout the 1-year follow-up. Survival and AE profile at 5, 30, 90 and 365 days will be compared with the control patients ‘group. In addition, the tolerability of the stimulation will be measured by the need to stop treatment sessions, as well as the incidence of procedure complications.
The secondary outcomes have to be considered exploratory efficacy end-points and will consist in changes (before and after pulsed ELF-MF treatment) in the evaluated clinical and radiological scores. The secondary end-points will be studied considering both within and between subjects (comparison vs. matched control group) clinical/radiological changes. The statistical test and approach employed will be chosen in order to completely fit the features of the acquired data and fulfill the assumptions of the statistical method. In general, given the small sample size, a non parametric approach will be preferred.
Study organization and funding
This is an independent academic trial. The devices for pulsed ELF-MF stimulation will be provided by IGEA (Carpi, Italy). Ethics approval was granted by the Campus Bio-Medico University Ethics Committee. The trial is registered on clinicaltrials.gov (NCT01941147).
Discussion
This project aims at providing an innovative neuroprotective strategy, in which unconventional non-invasive brain stimulation will be tested as an alternative approach to drugs. Recent evidence suggests that ELF-MFs might influence human brain activity. These findings are supported by the results of the neurophysiological studies that revealed measurable changes in brain electrical activity following ELF-MF exposure. Moreover, the experimental data at cellular and tissue level, showing the effects on cell membrane receptors and intracellular signaling, suggest possible mechanisms for ELF-MF action on the brain [9].
An interesting field of research is the effect of ELF-MFs on glutamate and adenosine receptors because of the ascertained role of these molecules in the pathophysiology of ischemic stroke [11- 12]. In hippocampal glutamatergic synapsis, Wieraszko [5] showed that pulsed ELF-MFs enhanced neurotransmission by an increase of glutamate concentration in synaptic cleft. Varani et al. found that pulsed ELF-MFs (75 Hz; 1,8mT; 30 min) increased density and functionality of A2A adenosine receptors expressed by rat neuronal cells [6]. Interestingly, Capone et al [10]. Using ELF-MF with identical field characteristics, found an increase in intracortical facilitation produced by paired pulse TMS, which is a cortical phenomenon known to be largely mediated by NMDA receptors. These findings suggest that the modulation of neurotransmitters receptors such as adenosine and glutamate could represent possible neurophysiological substrates for the effect of ELF-MF.
The main concerns of this proposal are the lack of a sham treated group and the small size of the enrolled sample of patients. To overcome this issue, we considered previously treated stroke patients as control subjects, although it could lead to a risk of arbitrariness and selection bias. Despite the preliminary nature of this trial, in the case that a mild effect will be achieved by our intervention, the 3-patients sample size could be too small to obtain data about efficacy. We are well aware of these limits, however both the small size of the sample and the absence of the control group are in line with the aim of demonstrating the safety of pulsed ELF-MF stimulation. This pilot trial is justified especially given the low cost and non-invasive nature of the treatment and the promising evidence arising from animal studies [7-8].
Conclusion
In conclusion, ELF-MFs have been shown to modulate the main mechanisms of brain ischemic damage and regeneration in in-vitro and in-vivo animal models. Preliminary results in humans suggest that ELF-MF stimulation is safe. This pilot study is designed to investigate the safety, tolerability and effectiveness of pulsed ELF-MF in patients with acute ischemic stroke. Patients will be monitored for AEs, neurological outcomes and lesion size on MRI up to 1 year from onset of the treatment.
References
- McFarlane EH, Dawe GS, Marks M, Campbell IC. Changes in neurite outgrowth but not in cell division induced by low EMF exposure: influence of field strength and culture conditions on responses in rat PC12 pheochromocytoma cells. Bioelectrochemistry. 2000; 52: 23-28.
- Oda T, Koike T. Magnetic field exposure saves rat cerebellar granule neurons from apoptosis in vitro. Neurosci Lett. 2004; 365: 83-86.
- Piacentini R, Ripoli C, Mezzogori D, Azzena GB, Grassi C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J Cell Physiol. 2008; 215: 129-139.
- Di Loreto S, Falone S, Caracciolo V, Sebastiani P, D'Alessandro A, Mirabilio A, et al. Fifty hertz extremely low-frequency magnetic field exposure elicits redox and trophic response in rat-cortical neurons. J Cell Physiol. 2009; 219: 334-343.
- Wieraszko A. Amplification of evoked potentials recorded from mouse hippocampal slices by very low repetition rate pulsed magnetic fields. Bioelectromagnetics. 2004; 25: 537-544.
- Varani K, Vincenzi F, Targa M, Corciulo C, Fini M, Setti S, et al. Effect of pulsed electromagnetic field exposure on adenosine receptors in rat brain. Bioelectromagnetics. 2011.
- Grant G, Cadossi R, Steinberg G. Protection against focal cerebral ischemia following exposure to a pulsed electromagnetic field. Bioelectromagnetics. 1994; 15: 205-216.
- Pena-Philippides JC, Yang Y, Bragina O, Hagberg S, Nemoto E, Roitbak T, et al. Effect of Pulsed Electromagnetic Field (PEMF) on Infarct Size and Inflammation After Cerebral Ischemia in Mice. Transl Stroke Res. 2014.
- Di Lazzaro V, Capone F, Apollonio F, Borea PA, Cadossi R, Fassina L, et al. A consensus panel review of central nervous system effects of the exposure to low-intensity extremely low-frequency magnetic fields. Brain Stimul. 2013; 6: 469-476.
- Capone F, Dileone M, Profice P, Pilato F, Musumeci G, Minicuci G, et al. Does exposure to extremely low frequency magnetic fields produce functional changes in human brain? J Neural Transm. 2009; 116: 257-265.
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008; 55: 363-389.
- Dai SS, Zhou YG. Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Rev Neurosci. 2011; 22: 231-239.